Abstract
Exposure to environmental pollutants tightly impacts on the male fertility. In the present study, we examined the toxic effects of lead acetate (Pb) on testicular structure and the possible effect of quercetin on mitigating these effects. The apoptotic changes in the testes were also studied by the TUNEL assay and changes in apoptosis-related gene (Bax, Bcl-2, and caspase-3) expression. Twenty-one male mice were randomly divided into 3 groups of control, Pb, and lead acetate + quercetin. Testicular weight, both absolute and relative, was higher in Pb-exposed mice in comparison with the control and Pb-quercetin groups. The increase in size of testis was related to the lumen and connective tissue in this group. Lead acetate induced different patterns in testicular cell number; as spermatogonia, spermatocyte, and Sertoli cells number did not affect in lead acetate exposed group, while total number of round spermatids and long spermatids significantly reduced. In addition, Bcl-2 expression was downregulated, and Bax expression was upregulated in Pb-treated group in comparison with the control and Pb + quercetin groups. The TUNEL assay revealed that the number of apoptotic cells in Pb-treated group were increaed significantley in comparison to other groups. In conclusion, Pb administration adversely impacted on the cellular organization and activation of the apoptotic pathways in the testis; on the other hand, quercetin co-administration with lead partially ameliorated these adverse effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hazardous chemicals pollute the environment through natural events or anthropogenic activities, causing adverse effects on animals and human species [1]. Excessive accumulation of metals, whether essential or not, in the body can exert many side effects on the general health and wellbeing of the affected individuals [2]. Illegal drugs such as opium plus contaminated food and water as the result of metal dissolution into the ecosystem are among the primary sources of lead exposure in recent years [3]. Reports reveal the intentional addition of heavy metals of various kinds such as lead to pure opium by salesmen and smugglers with the intention of incrementing its weight and, hence, profitability, as Aghaee-Afshar et al. have reported the presence of Pb in ten opium samples collected in Southeast Iran [4, 5].
Heavy metals bear different oral bioaccessibility features and can impose serious health risks on the consumers. Fish consumed as food represents another concerning source of heavy metals, the level of which in different fish organs and tissues rely on a range of biotic as well as abiotic elements like ecological requirements, sex, age, size, life cycle and background, feeding behaviors, habitat preferences, and water parameters such as acidity, duration of contact to heavy metals, and homeostatic regulation activity. On top of that, oil industry is attributed as one prominent producer of heavy metals found in marine animals. The presence of heavy metals, such as lead (Pb), cadmium (Cd), and vanadium (V) in vast water spreads, is an indication of oil pollution [6].
Heavy metals, such as arsenic (As), cadmium (Cd), lead (Pb), and mercury (Hg), are highly toxic and may cause irreversible effects on the male reproductive system [7, 8]. The human activities within the recent centuries have caused 1000-fold increase in environmental lead level, which has become a global concern nowadays [9]. Lead, as a non-biodegradable and toxic heavy metals family, is one of the most frequent divalent metals that induces toxicity on the organs and cells in the body [10,11,12]. Lead, due to its persistency and bioaccumulative properties, has been of controversial concern worldwide [13, 14].
The reproductive organs are very sensitive to stressful conditions and may directly or indirectly affected by any toxic element [15, 16], with testicular morphology being directly impacted on by lead [17]. Chowdhury et al. [18] reported that heavy metals affected the sperm characteristics, such as the sperm motility, morphology, concentration, and disrupted the enzymatic function. It has been suggested that lead indirectly affects the male reproductive function via disruption of the endocrine system [19]. Male infertility is a complex condition, and its underlying physiological and biochemical bases are still unclear [20].
Several mechanisms have been proposed to explain the lead-induced toxicity. One possible mechanism is the disruption in pro-oxidant/antioxidant balance and production of the reactive oxygen species (ROS) that activate the cell death signaling (apoptosis and necroptosis) pathways [21]. The currently approved treatment for lead poisoning is the use of the chelating agents, which form an insoluble complex with lead. It can shield it from biological targets, thus reducing its toxicity too [22, 23]. Recently, more attentions have been paid to the protective effects of natural antioxidants against toxicities induced by chemicals [24].
Quercetin is the most abundant flavonoid in vegetables and fruits, with antioxidants activities [25, 26]. It was shown that quercetin can exert multiple biological effects as a potent natural antioxidant through free radical scavenging, xanthine oxidase blockage, and vitamin C absorption [27,28,29]. Quercetin, a phenolic compound, is also considered as a metal-chelating agent with high affinity for Pb ions [30]. It has numerous effects on the biological systems through its antioxidant activities [31, 32].
Although there are extensive researches about lead acetate effects on the male reproductive system, conversely, little is known about the testes mechanisms involved in Pb toxicity and the possible direct effect(s) of Pb on the testicular function. In addition, it is unclear whether co-administration of quercetin would be helpful in alleviating the effect of lead toxicity in males. Therefore, this study was undertaken to determine the possible protective effect(s) of quercetin on testicular structure and apoptosis-related biomarkers in mice that were administered with lead acetate.
Materials and Methods
Experimental Animals
Twenty-one NMRI strain male mice (10–12 weeks old) were purchased from Royan Institute (Karaj, Iran) and kept under a 12:12-h light-dark schedule at controlled temperature (22 ± 2 °C) and humidity of 40–50%. Standard rodent chow (Behparvar®, Tehran, Iran) and water were available ad libitum [33]. All procedures were approved by the Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.,1398.484).
Experimental Design
The animals were divided into 3 groups (n = 7 for each group) including lead acetate group receiving 150 mg/kg lead acetate (Merck, 6080-56-4), lead acetate (150 mg/kg) + quercetin (Sigma, Q4951; 75 mg/kg) group receiving them at 3-h intervals, and the control group receiving deionized water. Lead acetate and quercetin were dissolved in distilled water and were administered daily by gavage for one cycle of the seminiferous epithelium. Each spermatogenic cycle in mice lasts 8.7 days, and each seminiferous epithelial cycle consists of four spermatogenic cycles; hence, the duration of one cycle of the seminiferous epithelium was 34.5 days [34].
After treatment, the mice were anesthetized, using ketamine (100 mg/kg) and xylazine (10 mg/kg) [35] by intramuscular injection, and were sacrificed by cervical dislocation. The left testis was dissected out and fixed in 10% buffered formaldehyde for stereological measurements and TUNEL assay. The right testis was immediately frozen in liquid nitrogen and stored at − 70 °C until RNA extraction.
Estimation of Testicular Volume
Testis volume was measured according to Scherle [36]; briefly, a beaker was filled with isotonic saline and placed on a precision scale and weighed. Then, the testis was suspended by a thin thread in the beaker. Weight and volume were recorded in grams and mm3, respectively.
Stereological Measurement: Tissue Processing
The fixed testes were dehydrated in graded ethanol, cleared in xylene, and embedded in paraffin wax. Serial sections were prepared using Cavalieri method [37] in a series of equal parallel planes (5- and 20-μm thickness). Microscopic descriptive analysis was performed by point counting on sections stained with hematoxylin (Sigma, 517-28-2) and eosin (Merck, 1159350025) and mounted on coverslips.
Estimation of the Epithelial, Luminal, and Connective Tissue Volumes
The volume density of various testicular structures is evaluated by randomly placing the point probe on each field (Fig. 1a). Volumes of the seminiferous tubules and interstitial tissue were estimated using the following equation (Howard and Reed, 2004), in which, “ΣPStructure” is the total counted points in each part and Σ point total is counted in systematic random sampling.
The Cavalieri volume was estimated using the equation by Howard and Reed (2004). In this equation, Σp is the total points that hit the cut surface in all sections, and area per points is the area associated with each point and estimated by the following formula (Δx and ΔY are real distances between two points and M is linear magnification of the image).
The absolute volume was obtained by multiplying the density by the Cavalieri volume as shown below:
Estimation of Seminiferous Tubule Length
The length density (Lv) of the seminiferous tubules is determined by covering a fair frame for measuring the number randomly on the monitor at a final magnification of × 180 (Fig. 1b). The length density of the tubule was determined using the following formula [37], where Lv is the length density of the tubule, a/f is the area of a frame at the final magnification (μm2), Σp is the number of frame-associated points, p is hitting the reference space, and Q is the number of profiles sampled by the frame. The total length of seminiferous tubules was estimated by multiplying the length density by the Cavalieri volume.
Estimation of the Testicular Total Number of the Cells
Total number of Sertoli cells, Leydig cells, spermatogonia, spermatocytes, round spermatids, and long spermatids were calculated, using the following formula by Howard [37]. An optical dissector probe was used for cell counting the cells in 20 μm sections at a × 60 magnification [38]. In the formula, Nv = the estimate of numerical density, a/f = area of frame, h = dissector height, ΣQ = sum of particles counted, and Σp = sum of the frame associated points hitting the reference space. The total number of cells was estimated by multiplying the numerical density by the Cavalieri volume.
The TUNEL Assay
The TUNEL assay revealed DNA fragmentation occurring in the last phase of apoptosis, and is based on labeling of the enzyme terminal deoxynucleotide transferase, which binds deoxynucleotides to the 3′-hydroxyl terminus of the DNA breaks. TUNEL staining was done according to the manufacturer’s protocol. Briefly, the sections were de-waxed and rehydrated with descending concentration of ethanol and washed with phosphate-buffered saline (PBS). The specimens were then incubated in 50 μL proteinase K solution (1 μL proteinase K and 50 μL apoptosis grade water) for 10 min. Finally, the sections were washed in PBS and incubated with 50 μL of labeling reaction mixture (TUNEL enzyme solution and labeling solution, 1:10) for 2 h at 37 °C in a humid chamber and darkness [39].
Real-Time Quantitative PCR
Testicular total RNA was extracted using a commercial kit (Cinnagen Inc., Iran) according to the manufacturer’s instructions. A NanoDrop™ 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA) was used for the assessment of RNA quality, and quantity reversed transcribed to complementary DNA (cDNA) was synthetized by RevertAid™ First Strand cDNA Synthesis kit (Fermentas Inc.). Selected genes expression was carried out by quantitative real-time PCR (qPCR) using the Applied Biosystems StepOne and the Real Plus 2x Master Mix Green (Amplicon Inc.). The qPCR conditions were set for 10 min at 94 °C followed by 40 cycles of 15 s at 94 °C, 60 s at 60 °C, and final extension of 7 min at 72 °C.The primers were designed based on the mouse DNA sequences, found in the GenBank Primer–BLAST online program [40]. The real-time PCR trials were performed in triplicate. The relative expression was calculated and normalized relative to β-actin, using the comparative threshold cycle method. The primer sequences are presented in Table 1.
Statistical Analyses
Data were analyzed using the PROC GLM in SAS, and the means were compared at significant level < 0.05 using the Tukey’s multiple range test (SAS, 2010). The data were expressed as mean ± standard deviation (SD). Tests of data normality and homogeneity of variance showed that there was no need for data transformation. Final body weight was analyzed using a model containing the initial body weight as the covariate, while weight of testis was used as the covariate in analysis of quantitative testicular measurements. Charts were drawn using the Prism Graph Pad software (version 8).
Results
Body Weight (g) and Testicular Total Volume (cm3) and Weight (g)
The live body weight is greatest for the control and lowest for the lead acetate and lead acetate + quercetin mice (Table 2). Lead acetate treatment increased the testicular weight and relative weight of the testis compared with other treatments (P < 0.05). Testicular volume numerically, but not significantly, is higher in lead acetate–treated mice (Table 2).
Total Volume of the Epithelia (mm3), Lumen (mm3), and Connective Tissue (mm3)
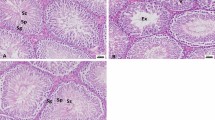
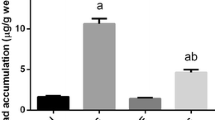
The stereological study of testicular tissue revealed no significant effect of the treatment on the epithelial volume; however, the volume of lumen was higher in the lead acetate group than the lead acetate + quercetin or the control mice (P < 0.05). The mean volume of connective tissue is highest for the lead acetate, lowest for the control group, and intermediate for lead acetate + quercetin-treated mice (Figs. 2 and 3).
Photomicrographs of the testicular histology and structures in mice. a Tissue from control. b Histopathological changes in lead acetate–treated group. c Testicular architecture in mice treated with lead acetate + quercetin. Normal spermatogenesis with no evidence of inflammatory cell infiltration or necrosis
Total Length of Seminiferous Tubules (m)
The seminiferous tubule length is not significantly affected (Fig. 4), but it was numerically shorter in the lead acetate group.
Total Number of Testicular Cells
Number of spermatogonia (Fig. 5a), spermatocytes (Fig. 5b), Sertoli cells (Fig. 5e), and Leydig cells (Fig. 5f) are not significantly different among the treatments. The total number of round spermatids (Fig. 5c) and long spermatids (Fig. 5d) is highest in the control, intermediate in lead acetate + quercetin, and the lowest in lead acetate mice.
Histopathology
The seminiferous tubules were enclosed by thin basement membrane, normal spermatogenesis with spermatid maturation in all 3 groups. The Sertoli cells were present between seminiferous tubules. There was no evidence of tissue inflammation or necrosis.
Apoptosis Assay
The apoptotic cells (assessed by TUNEL) increase significantly in the lead acetate–treated group when compared to the control and lead acetate + quercetin mice (Fig. 6). Furthermore, the rate of apoptosis in quercetin-administered group was significantly lower than in lead acetate mice, approaching the rate in the control group.
The testicular TUNEL assay in the control (a, d, g), lead acetate (b, e, h), and lead acetate plus quercetin (c, f, i) treated mice. a, b, and c show the TUNEL staining. d, e, and f demonstrate the cell nuclei in the seminiferous epithelial tissue when stained with DAPI (× 40). g, h, and i denote to the merged pictures
Expression of Bax, Bcl-2, and Caspase-3 Gene
The relative mRNA expression levels of Bax and Caspase-3 genes were not different between the treatments. The relative mRNA expression level of the Bcl-2 in lead acetate–treated mice was significantly lower than other groups, showing the mitigating effect of quercetin on downregulation of this gene. Bax/Bcl-2 ratio was numerically higher in lead acetate–treated mice (Fig. 7).
Discussion
The negative effect of lead acetate on fertility is of great concern. Indeed, there are evidences indicating irreversible lead toxicity effects on fertility [41].
This study indicated that body weight was lower in lead acetate–treated mice, which is in agreement with Kang et al. [42]. They attributed the decrease in body weight to the reduction in the phospholipid hydroperoxidase glutathione peroxidase mRNA level caused by malnutrition in rats. In line with these findings, El-Nekeety et al. [43] reported a reduction in feed intake in lead acetate–treated rats. Hasanein et al. [44] did not find any changes in the body weight in the lead-treated rats but recorded a reduction in testicular weight which they hypothesized to be due inhibition in spermatogenesis and the decreased level of testosterone.
We found an increasing in the testicular weight and relative testicular weight, but not in testicular volume, in lead acetate-treated mice. These findings are in accordance with the results of Graca et al. [45] and Dkhil et al. [46] who reported a significant increase in the weight of lead-treated groups which can be because of testis atrophy and interstitial edema. Acharya et al. [47] and Ommati et al. [48] also showed significant reductions in the testicular weight and relative testicular weight in Pb-treated mice and rats. The increased testicular weight in the Pb-exposed animals might be related to interstitial edema resulting from fluid accumulation, thickening of the basement membrane, and atrophy [46, 49]. Massanyi et al. [50] reported that intraperitoneal administration of lead resulted in interstitial dilated blood capillaries. Our findings indicated no differences in overall testicular volume or volume density in various testicular structures between the control and quercetin-administered mice. Taepongsorat et al. [51] showed an improvement in the testicular weight in a dose-dependent manner in quercetin-administered rats. Moreover, in the present study, a non-significant decrease was observed in the volume of the seminiferous tubules in lead acetate and lead acetate + quercetin-treated mice. These findings are in agreement with Asadpour et al. [52] who showed a decrease in proportions of seminiferous tubules, seminiferous tubule diameter (non-significant), seminiferous tubule epithelial height, seminiferous tubule epithelium volume density, and Leydig cell percentage, but a significant increase in Sertoli cell percentage in rats treated with lead acetate. It is worth mentioning that coefficient of error (CE) in this study was less than 5% [53].
The present study showed that lead acetate reduced the seminiferous tubule length. Mustafa [54] reported that the seminiferous tubule shrinkage in the lead-treated rats was due to contraction of myoid cells.
Although no significant difference was found in the total number of spermatogonia, spermatocytes, and Sertoli cells in the present experiment, the number of these cells in the lead acetate–treated group was numerically higher than the control and quercetin co-administered mice. According to Pinon-Lataillade et al. [55], the germ cells from undifferentiated spermatogonia to preleptotene spermatocytes were numerically higher in lead-treated than the control rats. They also reported a non-significant decrease in the number of pachytene spermatocytes until late spermatids. In contrast, Ayuba et al. [49] found a dose-dependent degeneration of spermatogonium in rats.
In the current study, total number of round and long spermatids was reduced in the lead acetate–treated mice in comparison with other groups. Kaushal et al. [56] showed a significant reduction in proportions of the young spermatids to the pachytene spermatocytes and the mature spermatids to young spermatids in lead-treated rats. The cell kinetic study by Batra et al. [57] showed a significant decrease in various cell populations [preleptotene, pachytene, young (step 7) spermatids, and mature (step 19) spermatids] in lead-exposed rats. One suggested underlying mechanism is increased cell proliferation duo to attenuation of apoptosis through the activation of p38/MAPK and PI3K/Akt/mTOR signaling pathways [58].
In the lead acetate–treated mice, testicular weight (absolute and relative) and volume of connective tissue were higher, but Leydig cell number was not affected; therefore, it seems that the increased connective tissue volume was not related to Leydig cell numbers, because testosterone level was the lowest in this group [59].
The result of TUNEL assay showed that lead acetate induced apoptosis in the treated mice; this is in agreement with Wang et al. [60] in mice. Moreover, Bcl-2 as an antiapoptotic gene was downregulated, while the expression of BAX gene, as a pro-apoptotic gene, was not changed in the lead acetate–exposed mice. Also, the Bax/Bcl-2 ratio was numerically highest in this group. Our data were in line with Jin and Feng [61] who reported a significant downregulation in Bcl-2 and upregulation in Bax and Caspase-3 in lead-treated mice. Jang et al. [62] showed that the increased in Bax/bcl-2 ratio led to an increased susceptibility of cells to apoptosis. Increased ratio of Bax/Bcl2 leads to stimulation of mitochondria-mediated pathway involved in apoptosis [63]; therefore, it can be assumed that lead toxicity caused mitochondrial-related apoptosis. Interestingly, most of testicular apoptotic cells were found in the basal compartment of lead acetate–treated mice. These findings were in line with the results of El-Shafai et al. [64] and Adhikari et al. [65] who found basal part of the epithelium of in mouse and rat testes affected by lead toxicity. Lead acetate administration differently impacted on various cells in the testes. This could be considered as the most probable reason for the disordered arrangement of the cells in the basal compartment and observed difference in the cells counts. This may be due to toxic effect of lead on the mitochondrial ATP synthesis through PGC-1α/SIRT3/ROS signaling and the activation of apoptotic pathways [66]. In turn, production of the reactive oxygen substances (ROS) is known to play a critical role in testicular injury and induced germ cell apoptosis, consequently leading to a reduction in the sperm number [67, 68]. Quercetin, by free radical-regulated process, inhibits the apoptotic pathway activation [69]. Quercetin, as a natural antioxidant and metal chelator, protects most of the organs against lead-induced oxidative stress and apoptosis in a dose-dependent manner [70, 71].
As you know, different methods in each research study give us various opportunities. One of the limitations of this study is that the quantification of apoptotic markers in protein level did not done which can be considered as a future line to evaluate. The other limitation of study is that in in vivo study sample collection more limited because usually involving the sacrifice of animals. Further research is needed to explain the precise apoptosis pathways during lead acetate toxicity and the molecular mechanisms of reconstruction of testicular tissue in farm animals and human.
Conclusions
In summary, the finding of the present study clearly showed that lead acetate administration in male mice adversely impacted the reproductive function through disturbances in testicular histology, cellular organization, and structure. It seems that lead acetate affected the testes through apoptotic pathways. It was also shown that quercetin co-administered with lead acetate could partially alleviate the adverse effects of lead acetate on the testes.
References
Nateghian Z, Aliabadi E (2020) Aspects of environmental pollutants on male fertility and sperm parameters. J Environ Treat Tech 8(1):299–309
Jain J, Gauba P (2017) Heavy metal toxicity-implications on metabolism and health. Int J Pharm Bio Sci 8:452–460
Negi R, Maurya A (2015) Heavy metal concentrations in tissues of major carp and exotic carp from Bhagwanpur fish pond, India. J Fish Aquat Sci 10(6):543
Aghaee-Afshar M, Khazaeli P, Behnam B, Rezazadehkermani M, Ashraf-Ganjooei N (2008) Presence of lead in opium. Arch Iran Med 11(5):553–554
Aghababaei R, Javadi I, Nili-Ahmadabadi A, Parsafar S, Ahmadimoghaddam D (2018) Occurrence of bacterial and toxic metals contamination in illegal opioid-like drugs in Iran: a significant health challenge in drug abusers. DARU J Pharm Sci 26(1):77–83
Heshmati A, Karami-Momtaz J, Nili-Ahmadabadi A, Ghadimi S (2017) Dietary exposure to toxic and essential trace elements by consumption of wild and farmed carp (Cyprinus carpio) and Caspian kutum (Rutilus frisii kutum) in Iran. Chemosphere 173:207–215
Alaee S (2018) Air pollution and infertility–a letter to editor. J Environ Treat Tech 6(4):72–73
Mansoor A, Roghaye D (2018) Adsorptive performance of Iminodiacetic acid functionalized nanoporous carbon for removal of Pb (II), Cu (II) and Cd (II) ions in aqueous system. J Environ Treat Tech 6(2):26–32
National Academy of Sciences-National Research Council, W., DC (1993) Measuring lead exposure in infants, children, and other sensitive populations. ERIC Clearinghouse, Ohio
Reyes JL, Molina-Jijón E, Rodríguez-Muñoz R, Bautista-García P, Debray-García Y, Namorado MC (2013) Tight junction proteins and oxidative stress in heavy metals-induced nephrotoxicity. Biomed Res Int 2013:1–14
Wani A, Ara A, Usmani J (2015) Lead toxicity: a review. Interdiscip Toxicol 8(2):55–64
Azadi S, Karimi-Jashni A, Talebbeydokhti N, Khoshbakht R, Haghighi AB (2020) Industrial composting of commingled municipal solid waste: a case study of Shiraz City, Iran. J Environ Treat Tech 8(4):1292–1303
Johnson F (1998) The genetic effects of environmental lead. Mutat Res Rev Mutat Res 410(2):123–140
Patra R, Swarup D, Dwivedi S (2001) Antioxidant effects of α tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology 162(2):81–88
Pinon-Lataillade G, Thoreux-Manlay A, Coffigny H, Masse R, Soufir J (1995) Reproductive toxicity of chronic lead exposure in male and female mice. Hum Exp Toxicol 14(11):872–878
Abiodun Emokpae M, Adeleye Moronkeji M (2020) Association of copper-to zinc ratio with sperm concentration among males investigated for infertility. J Infertil Reprod Biol 8(3):49–52
Hamadouche NA, Sadi N, Kharoubi O, Slimani M, Aoues A (2013) The protective effect of vitamin E against genotoxicity of lead acetate intraperitoneal administration in male rat. Arch Biol Sci 65(4):1435–1445
Chowdhury AR (2009) Recent advances in heavy metals induced effect on male reproductive function-a retrospective. Al Ameen J Med Sci 2(2):37–42
Balabanič D, Rupnik M, Klemenčič AK (2011) Negative impact of endocrine-disrupting compounds on human reproductive health. Reprod Fertil Dev 23(3):403–416
Beeram E (2019) Hormonal effect on male fertility and stem cell survival. J Infertil Reprod Biol 7(1):4–7
Redza-Dutordoir M, Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta Mol Cell Res 1863(12):2977–2992
Flora SJ, Saxena G, Mehta A (2007) Reversal of lead-induced neuronal apoptosis by chelation treatment in rats: role of reactive oxygen species and intracellular Ca2+. J Pharmacol Exp Ther 322(1):108–116
Flora S, Saxena G, Gautam P, Kaur P, Gill KD (2007) Response of lead-induced oxidative stress and alterations in biogenic amines in different rat brain regions to combined administration of DMSA and MiADMSA. Chem Biol Interact 170(3):209–220
Mehana E, Meki ARM, Fazili KM (2012) Ameliorated effects of green tea extract on lead induced liver toxicity in rats. Exp Toxicol Pathol 64(4):291–295
Liu C-M, Sun Y-Z, Sun J-M, Ma J-Q, Cheng C (2012) Protective role of quercetin against lead-induced inflammatory response in rat kidney through the ROS-mediated MAPKs and NF-κB pathway. Biochim Biophys Acta Gen Subj 1820(10):1693–1703
Neisy A, Zal F, Seghatoleslam A, Alaee S (2019) Amelioration by quercetin of insulin resistance and uterine GLUT4 and ERα gene expression in rats with polycystic ovary syndrome (PCOS). Reprod Fertil Dev 31(2):315–323
Akhlaghi M, Foshati S (2017) Bioavailability and metabolism of flavonoids: a review. Int J Nutr Sci 2(4):180–184
Bolouki A, Zal F, Alaee S (2020) Ameliorative effects of quercetin on the preimplantation embryos development in diabetic pregnant mice. J Obstet Gynaecol Res 46(5):736–744
Nabavi SF, Russo GL, Daglia M, Nabavi SM (2015) Role of quercetin as an alternative for obesity treatment: you are what you eat! Food Chem 179:305–310
Flora SJ (2009) Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxidative Med Cell Longev 2:191–206
Sirotkin AV, Hrabovszká S, Štochmaľová A, Grossmann R, Alwasel S, Harrath AH (2019) Effect of quercetin on ovarian cells of pigs and cattle. Anim Reprod Sci 205:44–51
Song X, Wang Y, Gao L (2020) Mechanism of antioxidant properties of quercetin and quercetin-DNA complex. DNA 3:5
Council NR (2010) Guide for the care and use of laboratory animals. National Academies Press, Washington, D.C.
Oakberg EF (1956) Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat 99(3):507–516
Davis JA (2008) Mouse and rat anesthesia and analgesia. Curr Protoc Neurosci 42(1):A. 4B. 1–A. 4B. 21
Scherle W (1970) A simple method for volumetry of organs in quantitative stereology. Mikroskopie 26:57–60
Howard V, Reed M (2004) Unbiased stereology: three-dimensional measurement in microscopy. Garland Science, New York, NY
Noorafshan A (2014) Stereology as a valuable tool in the toolbox of testicular research. Ann Anat 196(1):57–66
Kheirabad MK, Khodabandeh Z, Rahmanifar F, Tamadon A, Jahromi BN, Owjfard M, Koohi-Hosseinabadi O (2016) Testicular germ cells apoptosis following exposure to chronic stress in rats. Asian Pac J Reprod 5(5):371–375
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL (2012) Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13(1):134
Landrigan PJ, Boffetta P, Apostoli P (2000) The reproductive toxicity and carcinogenicity of lead: a critical review. Am J Ind Med 38(3):231–243
Kang JK, Sul D, Kang JK, Nam S-Y, Kim H-J, Lee E (2004) Effects of lead exposure on the expression of phospholipid hydroperoxidase glutathione peroxidase mRNA in the rat brain. Toxicol Sci 82(1):228–236
El-Nekeety AA, El-Kady AA, Soliman MS, Hassan NS, Abdel-Wahhab MA (2009) Protective effect of Aquilegia vulgaris (L.) against lead acetate-induced oxidative stress in rats. Food Chem Toxicol 47(9):2209–2215
Hasanein P, Fazeli F, Parviz M, Roghani M (2018) Ferulic acid prevents lead-induced testicular oxidative stress and suppressed spermatogenesis in rats. Andrologia 50(1):e12798
Graça A, Ramalho-Santos J, de Lourdes Pereira M (2004) Effect of lead chloride on spermatogenesis and sperm parameters in mice. Asian J Androl 6(3):237–241
Dkhil MA, Moneim AEA, Al-Quraishy S (2016) Indigofera oblongifolia ameliorates lead acetate-induced testicular oxidative damage and apoptosis in a rat model. Biol Trace Elem Res 173(2):354–361
Acharya U, Acharya S, Mishra M (2003) Lead acetate induced cytotoxicity in male germinal cells of Swiss mice. Ind Health 41(3):291–294
Ommati MM, Jamshidzadeh A, Heidari R, Sun Z, Zamiri MJ, Khodaei F, Mousapour S, Ahmadi F, Javanmard N, Yeganeh BS (2019) Carnosine and histidine supplementation blunt lead-induced reproductive toxicity through antioxidative and mitochondria-dependent mechanisms. Biol Trace Elem Res 187(1):151–162
Ayuba Y, Ekanem AU, Garba SH (2017) Effect of oral administration of lead acetate exposure on the histology of the testis and testicular sperm concentration in Wistar Albino Rats. App Med Sci 5(6D):2337–2344
Massanyi P, Lukac N, Makarevich AV, Chrenek P, Forgacs Z, Zakrzewski M, Stawarz R, Toman R, Lazor P, Flesarova S (2007) Lead-induced alterations in rat kidneys and testes in vivo. J Environ Sci Health A 42(5):671–676
Taepongsorat L, Tangpraprutgul P, Kitana N, Malaivijitnond S (2008) Stimulating effects of quercetin on sperm quality and reproductive organs in adult male rats. Asian J Androl 10(2):249–258
Asadpour R, Shahbazfar A, Kianifard D, Azari M, Zaboli N (2013) Comparison of the protective effects of garlic (Allium sativum L) extract, vitamin E and N acetyl cystein on testis structure and sperm quality in rats treated with lead acetate. Rev Med Vet 164(1):27–41
Gundersen HJG, Jensen E (1987) The efficiency of systematic sampling in stereology and its prediction. J Microsc 147(3):229–263
Mustafa HN (2015) Potential alleviation of Chlorella vulgaris and Zingiber officinale on lead-induced testicular toxicity: an ultrastructural study. Folia Biol 63(4):269–278
Pinon-Lataillade G, Thoreux-Manlay A, Coffigny H, Monchaux G, Masse R, Soufir J (1993) Effect of ingestion and inhalation of lead on the reproductive system and fertility of adult male rats and their progeny. Hum Exp Toxicol 12(2):165–172
Kaushal D, Bansal M, Bansal M (1996) Cell kinetics of the rat seminiferous epithelium following lead acetate treatment. J Trace Elem Exp Med 9(2):47–56
Batra N, Nehru B, Bansal M (2004) Reproductive potential of male Portan rats exposed to various levels of lead with regard to zinc status. Br J Nutr 91(3):387–391
Mancuso F, Arato I, Lilli C, Bellucci C, Bodo M, Calvitti M, Aglietti MC, dell'Omo M, Nastruzzi C, Calafiore R (2018) Acute effects of lead on porcine neonatal Sertoli cells in vitro. Toxicol in Vitro 48:45–52
Dolati P, Khodabandeh Z, Zamiri MJ, Jamhiri I, Mehrabani D (2020) The effect of lead acetate and quercetin on the tight and gap junctions in the mouse testis. Biol Trace Elem Res 198(2):535–543
Wang C, Zhang Y, Liang J, Shan G, Wang Y, Shi Q (2006) Impacts of ascorbic acid and thiamine supplementation at different concentrations on lead toxicity in testis. Clin Chim Acta 370(1–2):82–88
Jin L, Feng C (2011) Effects of lead on testicular cells apoptosis and expression of caspase-3, Bcl-2 and Bax genes in mouse. J Anhui Norm Univ (Nat Sci) 6:1–8
Jang M-H, Shin M-C, Shin H-S, Kim K-H, Park H-J, Kim E-H, Kim C-J (2002) Alcohol induces apoptosis in TM3 mouse Leydig cells via bax-dependent caspase-3 activation. Eur J Pharmacol 449(1–2):39–45
Sharma V, Anderson D, Dhawan A (2012) Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 17(8):852–870
El Shafai A, Zohdy N, El Mulla K, Hassan M, Morad N (2011) Light and electron microscopic study of the toxic effect of prolonged lead exposure on the seminiferous tubules of albino rats and the possible protective effect of ascorbic acid. Food Chem Toxicol 49(4):734–743
Adhikari N, Sinha N, Narayan R, Saxena D (2001) Lead-induced cell death in testes of young rats. J Appl Toxicol Int J 21(4):275–277
Li Z, Liu X, Wang L, Wang Y, Du C, Xu S, Zhang Y, Wang C, Yang C (2016) The role of PGC-1α and MRP1 in lead-induced mitochondrial toxicity in testicular Sertoli cells. Toxicology 355:39–48
Wang L, Xu T, Lei W-w, Liu D-m, Li Y-j, Xuan R-j, Ma J-j (2011) Cadmium-induced oxidative stress and apoptotic changes in the testis of freshwater crab, Sinopotamon henanense. PLoS One 6(11):e27853
Aderemi AS, Samson OB, Akomaye AJ, Gabriel IA (2019) Spermatogenic and steroidogenesis functions of rat testis following exposure to Alafia barteri leaf extracts. J Infertil Reprod Biol 7(2):11–16
Korkina LG, Afanas’ Ev IB (1996) Antioxidant and chelating properties of flavonoids. Advances in Pharmacology 38:151–163
Liu C-M, Ma J-Q, Sun Y-Z (2010) Quercetin protects the rat kidney against oxidative stress-mediated DNA damage and apoptosis induced by lead. Environ Toxicol Pharmacol 30(3):264–271
Hu P, Wang M, Chen W-H, Liu J, Chen L, Yin S-T, Yong W, Chen J-T, Wang H-L, Ruan D-Y (2008) Quercetin relieves chronic lead exposure-induced impairment of synaptic plasticity in rat dentate gyrus in vivo. Naunyn Schmiedeberg's Arch Pharmacol 378(1):43–51
Acknowledgments
The authors would like thank Mr. Argasi at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for his invaluable assistance in editing this article.
Funding
This study was financially supported by the Stem cells Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran (Grant Number: 1397.869).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khodabandeh, Z., Dolati, P., Zamiri, M.J. et al. Protective Effect of Quercetin on Testis Structure and Apoptosis Against Lead Acetate Toxicity: an Stereological Study. Biol Trace Elem Res 199, 3371–3381 (2021). https://doi.org/10.1007/s12011-020-02454-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02454-8