Abstract
It is well known that excess iodide can lead to thyroid colloid retention, a classic characteristic of iodide-induced goiter. However, the mechanism has not been fully unrevealed. Iodide plays an important role in thyroid function at multiple steps of thyroid colloid synthesis and transport among which sodium/iodide symporter (NIS) and pendrin are essential. In our study, we fed female BALB/c mice with different concentrations of high-iodine water including group A (control group, 0 μg/L), group B (1500 μg/L), group C (3000 μg/L), group D (6000 μg/L), and group E (12,000 μg/L). After 7 months of feeding, we found that excess iodide could lead to different degrees of thyroid colloid retention. Besides, NIS and pendrin expression were downregulated in the highest dose group. The thyroid iodide intake function detected by urine iodine assay and thyroidal 125I experiments showed that the urine level of iodine increased, while the iodine intake rate decreased when the concentration of iodide used in feeding water increased (all p < 0.05 vs. control group). In addition, transmission electron microscopy (TEM) indicated a reduction in the number of intracellular mitochondria of thyroid cells. Based on these findings, we concluded that the occurrence of thyroid colloid retention exacerbated by excess iodide was associated with the suppression of NIS and pendrin expression, providing an additional insight of the potential mechanism of action of excess iodide on thyroid gland.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid colloid retention is a pathological phenomenon of goiter, mostly resulting from overproduced colloid or damaged transportation towards thyroid cells. Multiple factors are involved in the pathogenesis of thyroid colloid including both genetic and environmental factors. Among the many environmental factors that have been suggested to influence the development of goiter, iodine intake may be the most important one. Many studies have shown an increase in the prevalence of overt hypothyroidism, subclinical hypothyroidism, and autoimmune thyroiditis associated with high-iodine intake [1–7].
The function of the thyroid gland is to synthesize colloid, which is predominantly composed of thyroglobulin, a large glycoprotein that serves as the scaffold for thyroid hormone synthesis. It is constituted of specialized cells (thyrocytes) organized into follicles. As we already know, iodide (I−), the negatively charged (anionic) form of the iodine atom, is essential for the synthesis of thyroid colloid [8]. Iodide is actively transported by the sodium/iodide symporter (NIS) at the basolateral membrane of the thyrocytes [9] and diffuses by an exchanger, known as pendrin (solute carrier family 26 or anion exchanger, member 4, SLC26A4), to the lumen at the apical membrane [10]. However, the mechanism of how NIS and pendrin lead to thyroid colloidal retention by uptaking excess iodide remains unknown.
In the current study, we hypothesize that NIS and pendrin play novel roles in the development of thyroid colloid retention. NIS is an integral plasma membrane glycoprotein localized at the basolateral plasma membrane of thyrocytes [11]. This protein plays an essential role in thyroid physiology by mediating uptake of iodide into the thyrocytes, a key step in thyroid hormone synthesis which cotransports two sodium ions along with one iodide ion and with the sodium gradient serving as the driving force [12]. The energy required to produce the sodium gradient is provided by the ouabain-sensitive Na+/K+-ATPase [13]. The mechanisms by which chronic high-concentration iodide uptake regulate NIS transcription and biosynthesis in female mouse are still unclear. Recent studies suggest that iodide regulates iodide accumulation by modulating NIS activity via transcriptional and posttranscriptional mechanisms [14]. Pendrin is capable of mediating I− transport in a number of heterologous expression systems, including Xenopus oocytes as well as mammalian cell systems [11, 15]. Researchers have found that pendrin is expressed at the apical membrane in thyroid follicular cell [13, 16], which can mediate iodide efflux [11]. On the other hand, defect in iodide organification has been observed in patients with Pendred syndrome [17, 18]. In fisher rat thyroid cells (FRTL-5), thyroid-stimulating hormone (TSH) does not significantly modify pendrin gene expression [13]. Interestingly, thyroglobulin has been shown to upregulate SLC26A4 mRNA levels in FRTL-5 cells while suppressing expression of several thyroid-specific genes including TSH receptor, NIS, thyroid peroxidase (TPO), and thyroglobulin (Tg) [13]. Most of the studies have focused on the effect of iodide excess on NIS, while little is known about the effect of iodide excess on pendrin expression [19]. It is worth to demonstrate whether excess iodide couple also be associated with altered pendrin expression levels in female mouse.
Although previous studies have demonstrated that chronic excess iodine intake could induce goiter formation in people or animals of thyroid disorders, few studies have been performed on subjects with normal thyroid function without thyroid disease. Therefore, in this study, we fed different concentrations of iodine-containing water using healthy female mice and investigated the effect of NIS and pendrin on thyroid colloid retention developed by excess iodide.
Materials and Methods
Animals and Treatments
Eighty female BALB/c mice (3–4 weeks old with body weight 15–17 g) were randomly divided into five groups. Mice in experimental groups B, C, D, and E were fed different concentrations of iodine-containing water (1500, 3000, 6000, and 12,000 μg/L of potassium iodate, respectively). Mice in control group A were fed on tap water. The animals used in the experiment were approved by the Guangdong Province Medical Laboratory Animal Center, China, and housed in temperature (22 ± 2 °C) and light-controlled (12-h light/12-h dark cycle; lights on at 7 am) conditions with free access to food and water. To observe the effect of long-term treatment with different doses of iodide on thyroid based on our unpublished pilot study, we set up 7 months as the final time point for thyroid phenotype readout. Animal handling and experimental procedures in this study were approved by the Animal Experimental Ethics Committee of Guangzhou Medical University (Guangzhou, China).
Thyroid Tissue Morphology Study
The thyroid glands of experimental and control groups were dissected and weighted. Part of the thyroid gland was removed and fixed overnight in 4 % paraformaldehyde. Paraffin-embedding method was used in tissue processing, and HE staining and light-microscopy were used to study thyroid morphology. Another part of the thyroid gland of each mouse was fixed overnight in 3 % glutaraldehyde and cut into ultrathin sections. Acetic acid uranium-lead citrate dyeing was used for transmission electron microscopy (TEM) study of thyroid ultrastructure.
Total RNA Extraction and Quantitative PCR (qPCR)
Thyroid glands were dissected and frozen in liquid nitrogen, and then stored at −80 °C. Total RNA was prepared using the TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. Total RNA was reverse transcribed into cDNA using PrimeScript RT reagent kit (Applied Biosystems, USA). The forward and reverse primers for mouse NIS (Slc5a5) gene were TGCCAACACTTCCAGAGGGA and TGGTCAAAGTACCCAGAGCCC, respectively; pendrin (Slc26a4) were TAGAGACGGTCGCTCGCATT and GGAAGCAAGTCTACGCATGGC, respectively. Real-time quantitative polymerase chain reaction (qPCR) amplifications were carried out in the ABI Prism 7500 (Applied Biosystems) machine. The amplification reactions were performed in 96-well plates in 25 μL final volume that contained 2 μL of 12.5 μL diluted cDNA, 12.5 μL of 2× Power SYBR® Premix Ex Taq™ (Applied Biosystems), and 10 nM each of forward and reverse primers. The amplification program was 55 °C for 2 min, 95 °C for 5 s followed by 40 cycles of 60 °C for 30 s. The NIS and pendrin genes expression levels were normalized to that of β-actin. The forward and reverse primers for mouse β-actin were CATCCGTAAAGACCTCTATGCCAAC and ATGGAGCCACCGATCCACA, respectively.
The relative quantities of NIS and pendrin expression were determined by the comparative CT method expressed using the formula 2−(ΔΔCt), where CT referred to threshold cycle and was determined for each plate by the 7500 Real-Time PCR System Sequence Detection Software (Applied Biosystems).
Western Blot
Thyroid glands were dissected and frozen in liquid nitrogen then stored at −80 °C. Thyroid glands were homogenized in radioimmunoprecipitation assay (RIPA) buffer (Beyotime, China). Subsequently, the vials were centrifuged and the supernatant was collected. The protein concentration was determined using a BCA protein assay kit (Pierce, Rockford, IL, USA). Proteins (30 μg/lane) were denatured by heating homogenates at 100 °C for 5 min in the loading buffer containing 0.6 mL 1 mol/L Tris-HCl (pH 8.8), 2 mL 10 % SDS, 5 mL 50 % glycerol, 0.5 mL 2-mercaptoethanol, 1 mL 1 % bromophenol blue, and 0.9 mL ultrapure water. Proteins were separated on 20 % SDS-PAGE and transferred to a nitrocellulose membrane (Hybond ECL; Amersham Biosciences, Roosendaal, Netherlands). Membranes were blocked with 5 % nonfat dry milk in phosphate-buffered saline (PBS) containing TBST buffer for 2 h at RT and then incubated overnight at 4 °C either with an anti-NIS antibody (GTX37599, GeneTex, 1:500 dilution), an anti-pendrin antibody (sc-50346, Santa Cruz Biotechnology, CA, USA, 1:200 dilution), or with an anti-β-actin antibody (sc-130301, mouse monoclonal, Santa Cruz Biotechnology, 1:500 dilution) on the same membrane. Membranes were washed, incubated with secondary anti-rabbit IgG HRP-linked (#7074S, CST, 1:1500 dilution) for 1 h at RT. After incubation with the second antibody, membranes were exposed to ECL solution (Perkin-Elmer, USA). Signals were detected by Fluorchem SP gel imaging analytical system (Alpha) and quantified by AlphaEase software (Alpha). β-Actin was included as a loading control. All extracts were prepared in duplicate, and at least three independent experiments were conducted.
Urine Iodine Assay
The morning urine was collected at the time of 7 months. Urine concentrations were measured by a urine iodine quantitative detection kit (Wuhan ZhongSheng Biochemical Technology Ltd., China) as instructed by manufacture’s manual (www.whzssh.com). This kit determined the urine iodine quantity by using catalytic spectrophotometric method based on the Sandell-Kolthoff reaction.
Thyroid Radioactivei Uptake (RAIU) Measurement
Iodide uptake was measured in the thyroid. According to the metabolism of mice, after the administration of 0.5 mCi I125 for 20 min and 2 h, all mice were sacrificed. The thyroids were weighted and the radioactive counts were examined at both time points.
Statistical Analysis
All results are expressed as mean ± standard deviation (SD). Statistical analysis was performed using two-tailed Student’s t test for unpaired parameters, and ANOVA with Fisher’s protected test was used in the comparison when the group number is more than 3. Logarithmic transformation of data was performed when SDs varied by more than 20-fold. A two-tail P value less than 0.05 was considered as significantly different.
Results
Thyroid Gland Morphology, Thyroid Organ Coefficient, and Thyroid Colloid Retention
Under light microscopy, the thyroid colloid and epithelial cells were stained pale pink and purple in HE staining, respectively. In the experimental groups, the follicle area increased and a small amount of follicular cavity fused together, forming a large region of follicle cavity with thyroid cells becoming flat. This phenomenon became more prominent as iodine intake dose in feeding water increased (Fig. 1a). The thyroid organ coefficient judged by thyroid weight/body weight of experimental C, D, and E groups was significantly elevated compared with the control group (A). Furthermore, higher iodine concentration was associated with a greater thyroid organ coefficient (Fig. 1b). These results suggested that thyroid colloid retention existed and thyroid tissue trended to be goiter which was relevant to the increased amount of iodine concentration in the feeding water.
Thyroid morphology and thyroid organ coefficient in the experimental model of excess iodine intake. Thyroid morphology (a) in views of H&E-stained sections from thyroid glands of BALB/c mice. Scale bars = 200 μm. The thyroid colloid and epithelial cells were stained pale pink and purple with H&E, respectively, In the iodine-water fed groups, the follicle area were increased and a small amount of follicular cavity had even fused, forming a large region of follicle cavity. Thyroid cells became flat. This phenomenon was more obvious as iodine intake increased. Thyroid organ coefficient (b) was equal to the thyroid gland weight (mg) divided by mice mouse body weight (g). Results are expressed by mean ± SD; n = 10 per group. Thyroid organ coefficient of experimental groups in addition to group B were significantly elevated compared with control group A. *P < 0.05 vs. control; ***P < 0.001 vs. control
The Expression Levels of NIS and Pendrin Were Downregulated in Thyroid Glands
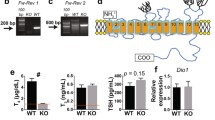
To determine whether there was redundancy in the expression of NIS and pendrin in the thyroid gland, we measured the mRNA levels and protein expression using qPCR and Western blot in the mice of the control group and group E. As shown in Fig. 2, NIS and pendrin expression of mice in group E was significantly decreased on both mRNA and protein levels compared with those of the control group, indicating that excessive iodine suppressed NIS and pendrin expression.
Gene and protein expression of NIS and pendrin. Gene expression of NIS and pendrin by quantitative PCR. a, b Expression of NIS and pendrin mRNAs in relation to beta-actin ratio in arbitrary units. Genes that had ≥|2|-fold change were identified as significantly differential expression; n = 10 per group. c, d Western blot analysis of NIS (85 KD) (c) and pendrin (85 KD) (d). Group A includes samples 1, 2, and 3; group E includes samples 4, 5, and 6. NIS and pendrin protein expression in the samples of group E were downregulated
Urine Iodine Value Increased
As a sensitive index, urine iodine could be used as a measure of total level of iodine intake. When iodine from food and water is absorbed by the human body, more than 90 % of iodine will be eventually discharged into the urine. By applying the same principle in our model, we found that urine iodine value of the experimental groups increased significantly compared with that of the control group, consistent with level of iodine intake (Fig. 3a).
Urine iodine (μg/L) (a). Results are expressed by mean ± SD; n = 10 per group. The urine iodine level of each experimental group was notably higher than those in the control group; the differences were statistically significant. ***P < 0.001 vs control. Thyroidal 125I intake rate (b). All mice were given 125I at the beginning of the experiment, with its level of radioactivity measured after both 20 min and 2 h. Measurements of thyroidal radioactivity are expressed as percent of the median 125I uptake; n = 10 per group. *P < 0.001 vs. control, ***P < 0.001 vs. control. The ultrastructure of thyroid glands under transmission electron microscopy (TEM) (c). The black arrows point to mitochondria. In the control group, thyroid cells are rich in mitochondria. However, it was hard to see mitochondria in group E
Thyroid Iodine Uptake Function Was Inhibited with Excessive Iodide
In thyroidal 125I uptake experiment, we set up two time points to monitor the change of thyroid iodine uptake rate, namely 20 min and 2 h (Fig. 3b). After 20 min, the percent of 125I uptake by thyroid in each iodine-water feeding group was lower than that of the control group. However, after 2 h, the percent of 125I uptake of the thyroid in each iodine-water feeding group, particularly groups C and D, was notably lower than that of the control group. For both shorter and longer time points, thyroid iodine uptake function was most severely inhibited under conditions of the most excessive amount of iodine intake, which was associated with decreased of NIS and pendrin expression.
Thyroid Cell Ultrastructure Was Injured Using TEM
TEM indicated a reduction in the number of intracellular mitochondria of thyroid cells in group E, indicating cell damage. In contrast, thyroid cells were rich in mitochondria in the control group (Fig. 3c), suggesting that excess iodide might have caused mitochondrial injury in this model.
Discussion
The classic characteristic of the mouse goiter induced by excessive iodide intake is thyroid colloid retention. Our study confirmed that over a long period of time, excess iodide intake could lead to thyroid colloid retention in which NIS and pendrin might play a vital role.
Regulation of NIS is complex, occurring at the transcriptional, translational, and post-translational levels [12]. Thus, discrepancy often exists between NIS mRNA and protein levels [20, 21]. Excess of iodide has a profound influence on the thyroid gland [22–26]. In vitro studies using rat FRTL-5 thyroid cells have shown that an excess amount of iodide suppresses the uptake of radioactive iodide [27]. Previous study also has shown that an excess amount of iodide decreased NIS gene expression at transcriptional level and the suppression of NIS promoter activity was associated with an iodide-specific decrease in the binding of transcription factors to DNA [28].
Functional studies performed in heterologous cells, including polarized cells [14, 29–32], along with the iodide organification defects were found in patients with Pendred syndrome [17, 18], suggested that pendrin could mediate apical iodide efflux in thyrocytes. In rat FRTL-5 cells, TSH did not significantly modify SLC26A4 gene expression [13]. Interestingly, thyroglobulin has been shown to upregulate SLC26A4 mRNA levels in FRTL-5 cells while suppressing the expression of several thyroid-specific genes including TSH receptor, NIS, TPO, and Tg genes. Our data showed decreased pendrin mRNA levels in response to iodide excess in female mice, which differs from those results obtained by Calil-Silveira et al. [19] in rat thyroid. In the study of Calil-Silveira et al., researcher showed the increase in pendrin mRNA expression occurred when animals were treated with an acute high dose of iodide from 30 min to 48 h [19].The thyroid gland could exhibit the capacity of Wolff-Chaikoff effect [22] when being acutely exposed to high amounts of iodide, although such effect was short. The increased pendrin expression enhanced efflux of iodide from the thyroid cell to the follicular lumen. This could also reduce the intracellular iodide concentration, contributing to the escape from the Wolff-Chaikoff [23]. However, in our study, chronic high doses of iodide were treated in mice for 7 months, a relatively long period of time. Since thyroid iodine uptake function was inhibited for a long time, we speculated that this situation cannot be reversed. Therefore, pendrin expression was suppressed in our study. Besides, there have been studies suggesting that rat and mouse present different characteristics in this aspect through unknown mechanisms.
Studies have shown that NIS and pendrin are iodide transporters [11, 12, 15], and a decline in the expression of either can hinder iodide transport. Transport of iodide into cells is inhibited when iodide levels get too high and excess iodine can inhibit the expression of both NIS and pendrin, thus, reducing the number of NIS transporters and pendrin available to import and outflow iodide. This process allows the thyroid cell to regulate the amount of iodide to enter the cell and lumen and to maintain appropriate levels for colloid production. Sometimes the mechanisms for regulating intrathyroidal iodide can be impaired or defective. When this happens, the thyroid cells are not able to maintain optimal levels of iodide. If such inhibition continues longer than body could adjust, intrathyroidal iodide levels will be too low such that the effect of low iodide becomes apparent. The urine iodine assays and thyroidal 125I experiment in the current study demonstrated that the thyroid was in a state of iodine deficiency. When NIS is suppressed, iodide intake is blocked. Consequently, excess iodide builds up in the blood and is finally excreted through urine. In contrast, when pendrin is inhibited, iodide cannot successfully enter the lumen to attach to the tyrosine in the thyroglobulin protein (Tg organification). According to this theory, we thought that the combination of these conditions generated a false iodine deficiency signal in our model, leading thyroid follicular epithelial cells to constantly produce excessive colloid which ultimately accumulated in the lumen. As a result, thyroid colloid retention developed.
Mitochondria are used to synthesize ATP, providing energy for various intracellular physiological activities. When damage occurs, it leads to a decrease in energy generation. We know that the processes of NIS and pendrin transferring iodine require energy, and therefore, a decline in mitochondria may also influence the expression of NIS and pendrin. Conversely, mitochondrial damage or decrease would hinder iodine transportation (Fig. 4). These conditions exacerbate thyroid colloid retention. However, the mechanism linking excess iodide and mitochondrial dysfunction has not been fully demonstrated. Several published studies could provide us with some clues including oxidative stress and metallothioneins [33, 34]. Since mitochondrial is an important organism that has thyroid hormone receptors and also produces reactive oxygen species, Joanta et al. demonstrated that high-iodide diet induces alterations in prooxidant/antioxidant levels in thyroid hormone target tissues, indicating a possibly link between excess iodide and mitochondrial function [33]. Recently-published study by Zhang et al. showed that metallothionein-I/II deficiency showed enhanced mitochondrial superoxide production with elevated expression level of peroxiredoxin 3 when challenged with excess iodide [34]. The over-production of superoxide products further damaged the thyroid tissue which had already impaired by excess iodide, providing one potential mechanism that could relate excess iodide and thyroid dysfunction through oxidative stress. However, further evidence is still needed to fulfill the mechanistic gap between excess iodide and oxidative stress.
The role of NIS and pendrin in mediating thyroid colloid retention developed by excess iodide intake, a schematic representation. The excess iodide inhibits expression of the NIS and pendrin, leading to iodide intake decrease. Over a long period of time, this situation becomes more serious, such that iodide deficiency becomes apparent and colloid accumulates in the lumen. As a result, thyroid colloid retention develops. Moreover, the processes of NIS and pendrin transferring iodine consume energy, and therefore, a decline in mitochondria may influence the expression of NIS and pendrin. Conversely, mitochondrial damage or decrease will hinder iodine transportation and aggravate excessive colloid formation
On the other hand, mild iodine deficiency not only shares some similarity with iodine over intake but also has distinguished features in histology and thyroid function. First of all, both iodine deficiency and over intake could lead to increased thyroid tissue weight, although the change of prior should be more significantly than the latter. Second, iodine deficiency usually leads to decreased colloid secretion while excessive iodine intake is usually associated with colloid retention [5, 35]. Third, iodine deficiency is characterized by enhanced proliferation of thyroid cells histologically while iodine over intake always associated with thyroid hypotrophy. Lastly, iodine deficiency is negatively correlated with enhanced iodine intake, while iodine overdose usually leads to diminished iodine intake [5, 35].
In summary, results obtained from the current study found that NIS and pendrin expression levels were significantly decreased by excess iodine and, thus, exacerbated thyroid colloid retention, refining our understanding of the mechanisms underlying iodide homeostasis in the thyroid gland.
References
Braverman LE (1994) Iodine and the thyroid: 33 years of study. Thyroid 4:351–356
Kahaly GJ, Dienes HP, Beyer J, Hommel G (1998) Iodide induces thyroid autoimmunity in patients with endemic goitre: a randomised, double-blind, placebo-controlled trial. Eur J Endocrinol 139:290–297
Laurberg P, Pedersen KM, Hreidarsson A, Sigfusson N, Iversen E, Knudsen PR (1998) Iodine intake and the pattern of thyroid disorders: a comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. J Clin Endocrinol Metab 83:765–769
Laurberg P, Bulow Pedersen I, Knudsen N, Ovesen L, Andersen S (2001) Environmental iodine intake affects the type of nonmalignant thyroid disease. Thyroid 11:457–469
Roti E, Uberti ED (2001) Iodine excess and hyperthyroidism. Thyroid 11:493–500
Li Y, Teng D, Shan Z, Teng X, Guan H, Yu X, Fan C, Chong W, Yang F, Dai H, Gu X, Yu Y, Mao J, Zhao D, Li J, Chen Y, Yang R, Li C, Teng W (2008) Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab 93:1751–1757
Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, Pedersen IB, Carle A (2010) Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab 24:13–27
Kohn LD, Saji M, Kosugi M, Ban T, Giuliani C, Hidaka A, Shimura H, Shimura Y, Okajima F (1993) The synthesis and secretion of thyroid hormones: regulation by multiple hormones and signals which can be subverted by autoantibodies to the thyrotropin receptor, thyroid diseases: basic science, pathology. Clin and Lab Diagn 1993:59–118
Carrasco N (1993) Iodide transport in the thyroid. Biochim Biophys Acta 1154:65–82
Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED (1997) Pendred syndrome is caused by mutations in a putative sulphate transport gene (PDS). Nat Genet 17:411–422
Scott DA, Wang R, Kreman TM, Sheffield VC, Karniski LP (1999) The Pendred syndrome gene encodes a chloride-iodide transport protein. Nat Genet 21:440–443
Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N (2003) The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr Rev 24:48–77
Royaux IE, Suzuki K, Mori A, Katoh R, Everett LA, Kohn LD, Green ED (2000) Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology 141:839–845
Bizhanova A, Kopp P (2009) The sodium-iodide symporter NIS and pendrin in iodide homeostasis of the thyroid. Endocrinology 150:1084–1090
Gillam MP, Sidhaye AR, Lee EJ, Rutishauser J, Stephan CW, Kopp P (2004) Functional characterization of pendrin in apolarized cell system. Evidence for pendrin-mediated apical iodide efflux. J Biol Chem 279:13004–13010
Bidart JM, Mian C, Lazar V, Russo D, Filetti S, Caillou B, Schlumberger M (2000) Expression of pendrin and the Pendred syndrome (PDS) gene in human thyroid tissues. J Clin Endocrinol Metab 85:2028–2033
Fraser GR, Morgans ME, Trotter WR (1960) The syndrome of sporadic goitre and congenital deafness. Q J Med 29:279–295
Kopp P (1999) Pendred’s syndrome: clinical characteristics and molecular basis. Current Opin Endocrinol Diabetes 6:261–269
Calil-Silveira J, Serrano-Nascimento C, Nunes MT (2012) Iodide treatment acutely increases pendrin (SLC26A4) mRNA expression in the rat thyroid and the PCCl3 thyroid cell line by transcriptional mechanisms. Mol Cell Endocrinol 350:118–124
Kogai T, Endo T, Saito T, Miyazaki A, Kawaguchi A, Onaya T (1997) Regulation by thyroid-stimulating hormone of sodium/iodide symporter gene expression and protein levels in FRTL-5 cells. Endocrinology 138:2227–2232
Suzuki K, Mori A, Saito J, Moriyama E, Ullianich L, Kohn LD (1999) Follicular thyroglobulin suppresses iodide uptake by suppressing expression of the sodium/iodide symporter gene. Endocrinology 140:5422–5430
Wolff J, Chaikoff IL (1948) Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem 174:555–564
Wolff J, Chaikoff IL, Goldberg RC, Meier JR (1949) The temporary nature of the inhibitory action of excess iodine on organic iodine synthesis in the normal thyroid. Endocrinology 45:504–513
Braverman LE, Ingbar SH (1963) Changes in thyroidal function during adaptation to large doses of iodide. J Clin Invest 42:1216–1231
Chang DC, Wheeler MH, Woodcock JP, Curley I, Lazarus JR, Fung H, John R, Hall R, McGregor AM (1987) The effect of preoperative Lugol’s iodine on thyroid blood flow in patients with Graves’ hyperthyroidism. Surgery 102:1055–1061
Arntzenius AB, Smit LJ, Schipper J, van der Heide D, Meinders AE (1991) Inverse relation between iodine intake and thyroid blood flow: color Doppler flow imaging in euthyroid humans. J. Clin. Endocrinol Metab 73:1051–1055
Grollman EF, Smolar A, Ommaya A, Tombaccini D, Santisteban P (1986) Iodine suppression of iodide uptake in FRTL-5 thyroid cells. Endocrinology 118:2477–2482
Suzuki K, Kimura H, Wu H, Kudo N, Kim WB, Suzuki S, Yoshida A, Caturegli P, Kohn LD (2010) Excess iodide decreases transcription of NIS and VEGF genes inrat FRTL-5 thyroid cells. BBRC 393:286–290
Riedel C, Levy O, Carrasco N (2001) Post-transcriptional regulation of the sodium/iodide symporter by thyrotropin. J Biol Chem 276:21458–21463
Taylor JP, Metcalfe RA, Watson PF, Weetman AP, Trembath RC (2002) Mutations of the PDS gene, encoding pendrin, are associated with protein mislocalization and loss of iodide efflux: implications for thyroid dysfunction in Pendred syndrome. J Clin Endocrinol Metab 87:1778–1784
Yoshida A, Taniguchi S, Hisatome I, Royaux IE, Green ED, Kohn LD, Suzuki K (2002) Pendrin is an iodide-specific apical porter responsible for iodide efflux from thyroid cells. J Clin Endocrinol Metab 87:3356–3361
Yoshida A, Hisatome I, Taniguchi S, Sasaki N, Yamamoto Y, Miake J, Fukui H, Shimizu H, Okamura T, Okura T, Igawa O, Shigemasa C, Green ED, Kohn LD, Suzuki K (2004) Mechanism of iodide/chloride exchange by pendrin. Endocrinology 145:4301–4308
Joanta AE, Filip A, Clichici S, Andrei S, Daicoviciu D (2006) Iodide excess exerts oxidative stress in some target tissues of the thyroid hormones. Acta Physiol Hung Dec 93(4):347–359
Zhang N, Wang L, Duan Q, Lin L, Ahmed M, Wang T, Yao X (2015) Metallothionein-I/II knockout mice aggravate mitochondrial superoxide production and peroxiredoxin 3 expression in thyroid after excessive iodide exposure. Oxidative Med Cell Longev 2015:267027. doi:10.1155/2015/267027 Epub 2015 May 25
Zimmermann MB, Boelaert K (2015) Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. Apr 3(4):286–295
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 81373038) and the Doctor Starting Foundation of Guangzhou Medical University (grant number 2006GD059). The authors wish to acknowledge Mr. Hui-qiu Zhang and Yong-Jian Zhang for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Chen, Xy., Lin, Ch., Yang, Lh. et al. The Effect on Sodium/Iodide Symporter and Pendrin in Thyroid Colloid Retention Developed by Excess Iodide Intake. Biol Trace Elem Res 172, 193–200 (2016). https://doi.org/10.1007/s12011-015-0580-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0580-4