Abstract
Selenium (Se) mainly performs its function through Se-containing proteins. Selenoprotein W (SelW), one member of the selenoprotein family, plays important roles in the normal function of the heart. To investigate the possible relationship between Se and SelW for the regulation of oxidative damage in chicken embryo myocardial cells, we treated myocardial cells with Se and H2O2. Then, the levels of lactate dehydrogenase (LDH) and 3,4-methylenedioxyamphetamine in the culture media, levels of SelW, inflammatory genes NF-κB, tumor necrosis factor (TNF)-α, p53, and the cell cycle were analyzed. Furthermore, the correlation between SelW and the levels of these factors was determined. The results indicated that Se treatment increased the expression of SelW (P < 0.05) and caused a downregulation of p53, NF-κB, and TNF-α (P < 0.05). In contrast, H2O2 increased the expression of p53, NF-κB, TNF-α, and LDH (P < 0.05) and induced early cell apoptosis, which was alleviated by treatment with Se. In addition, SelW had a positive correlation with the levels of inflammatory genes investigated. Taken together, our findings suggested that SelW is sensitive to Se levels and oxidative stress, and may play a role in the protective function of Se against oxidative damage and inflammation in chicken myocardial cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is recognized as an essential nutritional trace element for organisms. It plays a vital role in the normal physiology of a wide range of species, including birds. Numerous lines of evidence suggest Se has an important role in many aspects of health, including chemopreventive effects [1, 2], muscle metabolism [3], oxidant defense [4], neurobiology [5], aging [6], and reproduction [7]. Se deficiency causes degenerative muscle diseases in humans, cattle, pigs, turkey, sheep, ducklings, and chicks [8–10] that are characterized by the degeneration and necrosis of skeletal and cardiac muscle. Therefore, Se is essential for the health of animals and cells lines. Se performs its biological functions through the incorporation of the amino acid selenocysteine (Sec) into a unique class of proteins called selenoproteins. Approximately 25 families of selenoprotein genes have been identified in animals, and a variety of beneficial biological functions of selenoproteins on health have been described [10–14].
Selenoprotein W (SelW), one member of the selenoprotein family, is highly expressed in the brain of chickens [15] and in proliferating C2C12 myoblasts [16]. In a previous study, we sequenced the complementary DNA (cDNA) of SelW from chicken cerebral tissue [15] and observed that the structure of chicken SelW was similar to that of other mammals. Our prior studies showed that chicken SelW has an antioxidative function in types of cell lines [17]. In addition, SelW promotes a sensitive response to supplemental Se treatment in chickens [18]. However, there have been few reports of the role of SelW in chicken myocardial cells; therefore, the specific role of SelW requires further study.
Oxidative stress occurs when intracellular oxidative factors exceed or damage the cell antioxidant protection system. H2O2 is an oxidant that enhances the inflammatory response and the expression of inflammatory factors, tumor necrosis factor (TNF)-α, and NF-κB. However, oxidative damage may alleviate by antioxidative enzymes [19]. Cells can defend against excessive reactive oxygen species by superoxide dismutase, catalase, and other antioxidative selenoproteins such as the Gpx family members, Gpx1, Gpx2 Gpx3, and Gpx4; Txnrd family members, Txnrd1, Txnrd2, and Txnrd3 [19, 20]; and other identified or possible antioxidative selenoproteins, SelW, Selk, and Sepn1. In addition, Se also has an antioxidative function in cells. Se reduces apoptosis induced by H2O2 [21]. Thus, as the executor of Se, selenoprotein may be directly related to the redox regulatory role of Se. SelW is an important antioxidative selenoprotein in the heart and might be related to the oxidative regulatory role of Se and other oxidative factors. However, the possible relationship between Se and SelW during oxidative damage in chicken embryo myocardial cells is unclear. Whether SelW is involved in the process of Se protection against oxidative damage is unknown. In the present study, we investigated the effect of Se on oxidative injury biomarkers, inflammatory responses, and the expression of SelW in chicken embryo myocardial cells treated with Se and H2O2 and analyzed the correlation between SelW and oxidative injury and inflammatory gene expression.
Materials and Methods
Isolation and Identification of Primary Chick Embryo Myocardial Cells
Primary culture of chicken embryo-driven myocardial cells was prepared as described by Yablonka and Sato with some modifications [22, 23]. Briefly, cells were isolated from the heart of 12-day-old chicken embryos, minced, and digested with 0.12 % collagenase type II (Invitrogen, Carlsbad, CA, USA). To release single cells, the suspension was triturated by gentle pipetting and filtered to remove large debris. Then, the supernatant was collected every 5 min. The cells were harvested by 800 cycles of centrifugation and by differential adherence two times (1.5 and 1 h, respectively). Non-adherent cells were grown after counting. Primary chick embryo myocardial cells were seeded in six-well plates (Jet, China) coated at a density of 2 × 105 cells/cm2 with DMEM/F12 at 37 °C in humidified 95 % air—5 % CO2.
Effects of Se on the Expression of SelW
Real-time PCR was used to detect the expression of SelW. The cells were treated with 10−9–10−5 M sodium selenite for 24, 48, and 72 h, respectively. Cell morphology was observed. Total RNA was isolated from cells and the supernatant using TRIzol reagent according to the manufacturer’s instructions (Invitrogen). The dried RNA pellets were resuspended in 50 μL of diethyl-pyrocarbonate-treated water. The concentration and purity of the total RNA were determined spectrophotometrically at 260/280 nm. First-strand cDNA was synthesized from 5 μg of total RNA using oligo dT primers and Superscript II reverse transcriptase according to the manufacturer’s instructions (Invitrogen). Synthesized cDNA was diluted five times with sterile water and stored at −80 °C before use. Reaction mixtures were incubated in the ABI PRISM 7500 real-time PCR system (Applied Biosystems, USA). The primers used in this study were SelW: forward: gcaggaggtcacgggatggt and reverse: acgggagggcgacttggat; β-actin: forward: ccgctctatgaaggctacgc and reverse: ctctcggctgtggtggtgaa. Reactions consisted of the following: 10 μL of 2× SYBR Green I PCR Master Mix (Takara, China), 2 μL of either diluted cDNA, 0.4 μL of each primer (10 μM), 0.4 μL of 50× ROX reference Dye II, and 6.8 μL of PCR-grade water. The PCR program for SelW was 1 cycle at 94 °C for 3 min, 40 cycles at 95 °C for 30 s, and then 60 °C for 30 s. The melting curve analysis showed only one peak for each PCR product. Electrophoresis was performed with the PCR products to verify primer specificity and product purity. The messenger RNA (mRNA) relative abundance was calculated according to the method of Pfaffl, accounting for gene-specific efficiencies and was normalized to the mean expression of actin.
Cell Viability Assay
Cell viability was measured by the MTT method. Briefly, cells were cultured in a 96-well plate with a flat bottom in a final volume of 100 μL/well culture medium. The cells were treated with 0, 1, 5, 10, 50, 100, or 200 μM H2O2 for 2, 4, or 24 h, respectively. MTT was added to each well, and the cells were cultured for an additional 2.5 h. The absorbance of samples was measured at a wavelength of 450 nm using a microtiter plate reader (Sunrise Remote/Touch screen, Columbusplus, Austria).
Protection Assay
Cells were seeded into six-well plates at appropriate densities and cultured overnight. Then, the cells were divided into four groups: 1, normal cell group; 2, sodium selenite pretreatment group; 3, sodium selenite pretreatment plus H2O2 injury group; and 4, H2O2 injury group. Cells of the sodium selenite pretreatment group and the sodium selenite pretreatment plus H2O2 injury group were treated with different concentrations of 107 M of sodium selenite for 48 h, and then the cells in the sodium selenite pretreatment plus H2O2 injury group and the H2O2 injury group were treated with 50 μM H2O2 for 2 h. Finally, the following indicators were measured for each group:
-
A.
LDH content in the supernatant from each group was measured by Nanjing Jiancheng kit. Separated culture media was stored at 2–8 °C, and assays were completed within 8 h.
-
B.
Cell cycle assay in each group. (1) The cell density was adjusted to 106/mL, and the cells were seeded to 6-well plates, with each well containing 1.0 mL cells; (2) cells were grouped and treated as above; (3) cells were harvested and washed with ice-cold phosphate-buffered saline (PBS); (4) cells were fixed with 1 mL 70 % ice-cold ethanol for 2 h at 4 °C and then centrifuged at 1000×g for 5 min. Next, the cells were washed and resuspended in 500 μL PBS containing 25 μL propidium iodide (PI, 2.5 mg/mL) and 10 μL RNase A (2.5 mg/mL) for 30 min at 37 °C in the dark; (5) cells were immediately observed by flow cytometry.
-
C.
Real-time PCR assay. Real-time PCR was used to detect the expression of inflammatory factors. The primers used in this study were p53: forward: gagatgctgaaggagatgaatgag and reverse: gtggtcagtccgagcctttt; TNF-α: forward: gcccttcctgtaaccagatg and reverse: acacgacagccaagtcaacg; NF-κB: forward: tcaacgcaggacctaaagacat and reverse: gcagatagccaagttcaggatg.
-
D.
Western blotting assay. Briefly, protein extracts were subjected to SDS-polyacrylamide gel electrophoresis under reducing conditions on 15 % gels. Separated proteins were then transferred to nitrocellulose membranes using a tank transfer for 2 h at 100 mA in Tris-glycine buffer containing 20 % methanol. Membranes were blocked with 5 % skim milk for 1 h and incubated overnight with diluted primary antibodies (SelW, p53, TNF-α, NF-κB) followed by a horseradish peroxidase conjugated secondary antibody rabbit IgG (1:1000, Santa Cruz Biotechnology, USA) or goat (1:1000, Santa Cruz Biotechnology). To verify equal loading of samples, the membrane was incubated with a monoclonal β-actin antibody (1:1000, Santa Cruz Biotechnology), followed by a horseradish peroxidase conjugated goat anti-mouse IgG (1:1000). The signal was detected by an X-ray film (Trans Gen Biotech Co., Beijing, China).
Statistical Analyses
Statistical analysis of Se concentration and mRNA levels were performed using SPSS statistical software for Windows (version 13; SPSS Inc., Chicago, IL, USA). The effect on mRNA levels in chickens was assessed by one-way analysis of variance. Data were presented as the mean ± SD. Differences were considered to be significant when P < 0.05.
Results
Primary Chick Embryo Myocardial Cell Separation and Identification
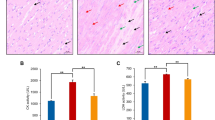
As shown in Fig. 1, we isolated primary chick embryo myocardial cells. Fibroblasts were removed using a differential speed adherent culture method, and the remaining cells began to adhere at 24 h, spread morphologically at 48 h, and individual cells showed rhythmic contractions after 72 h, and all cells formed a connection and displayed rhythmic contractions as a whole. We also identified these cells through hematoxylin-eosin staining (not shown in here) and smooth muscle actin staining. Taken together, these studies indicated the isolated cells were primary chick embryo myocardial cells.
Effects of Se on the Expression of SelW
To investigate the roles of Se, we studied the effects of selenite on SelW expression. Compared with control group, there was a significant increase in SelW expression at 48 h treated with different concentration of selenite (P < 0.05 Fig. 2), indicating selenite promotes SelW expression and that SelW might play an important role in protection against selenite. However, our results showed that the protein expression of SelW was significantly decreased at 72 h (P < 0.05); thus, we considered whether the phenomenon of selenium poisoning existed. We examined the level of LDH in the supernatant of cells cultured with different selenium concentrations at 24, 48, and 72 h. The results in Table 1 show that the amount of LDH was increased with an increase of incubation time and concentration of selenium. The level of LDH was significantly elevated at 72 h in the 10−7 M selenium group (P < 0.05) and was higher in the 10−5 M Se group compared with the 10−7 M selenium group. These results suggested that the phenomenon of selenium poisoning exists. When the cells were treated with 10−7 M selenite for 48 h, SelW was maximally increased. Therefore, this condition was used for the protection test.
H2O2 Inhibition of Myocardial Cells
As shown in Figs. 3 and 4, there was a significant inhibition of H2O2 on the cell viability of the myocardial cells. Morphologically, we observed that cell contraction was increased, myocyte volume was smaller, the bridge structure was reduced, and the gap increased between cells at 50–200 μM, 2 h. When the cells were treated with 50 μM of H2O2 for 2 h, cell growth was suppressed; therefore, this condition was used for the protection test.
The Protective Effects of Se
To study the specific function of Se, we found that LDH content from the selenite pretreatment group was significantly lower than in the H2O2 injury group (P < 0.05) and that the G0/G1 ratio in the selenite pretreatment group was significantly lower than in the H2O2 injury group (P < 0.05) (Tables 2 and 3). These data suggested in myocardial cells, Se was protective against H2O2-induced damage.
Effects of Selenite on the Expression of SelW and Inflammatory Genes
To further demonstrate the effect of SelW, SelW mRNA expression in myocardial cells during the protection assay was examined (Fig. 5). The results confirmed that the expression of SelW decreased after H2O2 treatment (P < 0.05, vs. control). However, in the group with Se and H2O2, a greater increase of SelW mRNA expression was observed (P < 0.05, vs. H2O2 treatment).
We examined the roles of cell signaling pathway-associated proteins after selenite and H2O2 treatment. At the mRNA level, p53, TNF-α, and NF-κB transcription-related factors in the Se pretreatment group were significantly lower than in the H2O2 injury group (P < 0.05) (Fig. 6). Furthermore, the change of protein level was similar to the gene expression (Fig. 7). These results suggest that selenite protects cells from H2O2 damage by inhibited activating genes, which regulate the cell cycle, which was activated by p53. Calculations of the Pearson correlation coefficients (Table 4) for inflammatory genes and SelW indicated a correlation (r < −0.40; P < 0.05), and the correlation (r > 0.90; P < 0.05) between inflammatory gene expressions was high indicating there may be interaction between these inflammatory genes.
Effects of Selenite on the relevant signaling pathway proteins by Western blotting assay (a and b). SelW, p53, NF-κB, and TNF-α were quantified by laser scanning densitometry. Results were normalized for β-actin and the data are presented as fold stimulations. *P < 0.05 vs. control; # P < 0.05 vs. 10−7 M Se treatment + H2O2 (50 μM)
Discussion
Se plays an important role in normal physiology in a wide range of species, including birds. Poultry diets lacking Se results in slow growth and development, reduced egg production, decreased hatchability, pancreatic degeneration, nutritional muscular dystrophy, and necrotic lesions in the liver, muscle, and heart [24–26]. Se plays important roles in the regulation of oxidative injury in mammalian cells [27]. In a previous study, Gu et al. [28] indicated Se was necessary for the normal differentiation of oligodendrocyte lineage cells. The effect of Se deprivation on oligodendrocyte differentiation was caused by the influence of Se on the activity of GSH-Px or other Se-containing enzymes, which resulted in oxidative stress. In this study, we described the quantity-dependent effects of Se in oxidative damage and protection. We demonstrated that Se at moderate concentrations might protect cardiomyocytes against inflammation injury induced by reactive oxygen radicals.
H2O2 induces oxidative damage in several cell lines and induces inflammatory responses [29, 30]. Yasuo et al. [29] indicated that H2O2 at low concentrations caused premature senescence in human keratinocytes by activating the p53-p21 signaling pathway, and that NF-κB activation caused premature senescence in primary keratinocytes. Similar to these prior studies, the results in the present study showed that H2O2 also induced oxidative damage in chicken embryo myocardial cells. H2O2 increased the apoptosis rate and expression of an oxidative injury marker, LDH. In addition, inflammatory factors, p53, NF-κB, and TNF-α were also increased by H2O2 in chicken embryo myocardial cells. Thus, an oxidative injury model in chicken myocardial cells was successfully established. We then treated the cells with different concentrations of Se and observed that Se treatment reversed the effect of H2O2 in chicken embryo myocardial cells. Thus, Se has a protective role against oxidative damage in chicken embryo cardiomyocytes. As indicated in previous studies, the NF-κB gene is the primary activator of inflammatory responses [31, 32]. Following the activation of NF-κB signaling by pro-inflammatory molecules such as TNF-α [33], downstream genes including iNOS, COX-2, and PTGES were also induced [34]. The aberrant expression of these genes have important roles in the pathogenesis of inflammatory diseases and different cancers [35]. Therefore, the reversed expression of these genes in Se-treated cells showed that Se preserves cellular functions by regulating inflammatory responses in chicken myocardial cells. However, in this process, we found that high concentrations of Se injured cardiomyocytes, suggesting the phenomenon of Se poisoning. Compared with some prior studies, the present study investigated a larger number of genes and showed a new effect of Se on injury-related genes.
However, how Se influenced the expression of these genes is unclear. As the functional mediators of Se, selenoproteins may provide important crosstalk between Se and target genes. In vitro experiments found that Se depletion reduced SelW mRNA levels in human intestinal Caco-2 cells and a neuronal cell line (SH-SY5Y), while additional Se caused an increase of SelW mRNA levels [36, 37], indicating that SelW expression is also sensitive to Se content alterations in vitro. To investigate the relationship of SelW and oxidative stress regarding Se in chicken myocardial cells, we also examined the level of SelW following treatment with Se and H2O2. The level of SelW mRNA was increased when myocardial cells were treated with additional Se, but deceased after 72 h incubation. Thus, these data suggest Se is involved in the regulation of SelW gene levels in chicken myocardial cells, and 10−7 M Se is the optimal concentration for SelW mRNA expression in chicken myocardial cells. This indicated that SelW gene expression is increased by an appropriate Se supply rather than excessive or deficient expression. Thus, SelW plays an important antioxidative role in many cell types [37–39]. In our prior study, we demonstrated SelW was a highly expressed selenoprotein in myoblasts and had a crucial antioxidative function. When the level of SelW was altered, the mRNA levels of other selenoproteins were influenced, which may depend on the levels of reactive oxygen species [40]. Although the antioxidative roles of SelW in different cells have been indicated, the possible role of SelW in chicken myocardial cells has been rarely reported. In addition, whether the inflammatory response induced by oxidative stress was related to SelW is unknown. In the present study, we found that Se treatment increased the expression of SelW and the Pearson correlation coefficients showed that SelW mRNA levels were negatively correlated with p53, NF-κB, and TNF-α. These results indicated that similar to other mammalian cell lines, SelW is sensitive to Se levels in chicken embryo myocardial cells and that the protective role of Se may be related to the levels of SelW. Thus, high SelW expression might play an important role in chicken embryo myocardial cells and may have a relationship with inflammatory responses.
In summary, our findings indicate that Se might reduce oxidative damage induced by H2O2 and by influencing the levels of p53, NF-κB, and TNF-α. Thus, Se regulates inflammatory injury in chicken myocardial cells. In addition, in this process, SelW levels might be related to the protective role of Se and have an important role in the redox regulation in chicken myocardial cells. The present study provides initial points for further study of chicken SelW.
References
Combs Jr GF, Clark LC, Turnbull BW (2001) An analysis of cancer prevention by selenium. Biofactors 14:153–159
Li JL, Gao R, Li S, et al (2010) Testicular toxicity induced by dietary cadmium in cocks and ameliorative effect by selenium. Biometals 23:695–705
Chariot P, Bignani O (2003) Skeletal muscle disorders associated with selenium deficiency in humans. Muscle Nerve 27:662–668
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241
Schweizer U, Schomburg L, Savaskan NE (2004) The neurobiology of selenium: lessons from transgenic mice. J Nutr 134:707–710
Kryukov GV, Gladyshev VN (2000) Selenium metabolism in zebrafish: multiplicity of selenoprotein genes and expression of a protein containing 17 selenocysteine residues. Genes Cells 5:1049–1060
Kaur P, Bansal MP (2005) Effect of selenium-induced oxidative stress on the cell kinetics in testis and reproductive ability of male mice. Nutrition 21:351–357
Peng X, Cui Y, Cui W, et al (2009) The decrease of relative weight, lesions, and apoptosis of bursa of fabricius induced by excess dietary selenium in chickens. Biol Trace Elem Res 131:33–42
Green DE, Albers PH (1997) Diagnostic criteria for selenium toxicosis in aquatic birds: histologic lesions. J Wildl Dis 33:385–404
Vega L, Rodriguez-Sosa M, Garcia-Montalvo EA, et al (2007) Non-optimal levels of dietary selenomethionine alter splenocyte response and modify oxidative stress markers in female mice. Food Chem Toxicol 45:1147–1153
Bekaert B, Cooper ML, Green FR, et al (2008) Effect of selenium status and supplementation with high-selenium yeast on plasma homocysteine and B vitamin concentrations in the UK elderly. Mol Nutr Food Res 52:1324–1333
Kryukov GV, Castellano S, Novoselov SV, et al (2003) Characterization of mammalian selenoproteomes. Science 300:1439–1443
Kryukov GV, Gladyshev VN (2004) The prokaryotic selenoproteome. EMBO Rep 5:538–543
Castellano S, Lobanov AV, Chapple C, et al (2005) Diversity and functional plasticity of eukaryotic selenoproteins: identification and characterization of the SelJ family. Proc Natl Acad Sci U S A 102:16188–16193
Zhang Y, Fomenko DE, Gladyshev VN (2005) The microbial selenoproteome of the Sargasso Sea. Genome Biol 6:R37
Papp LV, Lu J, Holmgren A, et al (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9:775–806
Han YH, Zhang ZW, Su J, et al (2012) Effects of chicken selenoprotein W on H2O2-induced apoptosis in CHO-K1 cells. Biol Trace Elem Res 147:395–402
Wu Q, Yao HD, Zhang ZW, et al (2012) Possible correlation between selenoprotein W and myogenic regulatory factors in chicken embryonic myoblasts. Biol Trace Elem Res 150:166–172
Ruan H, Zhang Z, Wu Q, et al (2012) Selenium regulates gene expression of selenoprotein W in chicken skeletal muscle system. Biol Trace Elem Res 145:59–65
Lescure A, Rederstorff M, Krol A, et al (2009) Selenoprotein function and muscle disease. Biochim Biophys Acta 1790:1569–1574
Papp LV, Holmgren A, Khanna KK (2010) Selenium and selenoproteins in health and disease. Antioxid Redox Signal 12:793–795
Lobanov AV, Hatfield DL, Gladyshev VN (2009) Eukaryotic selenoproteins and selenoproteomes. Biochim Biophys Acta 1790:1424–1428
Pappas AC, Zoidis E, Surai PF, et al (2008) Selenoproteins and maternal nutrition. Comp Biochem Physiol B Biochem Mol Biol 151:361–372
Walter ED, Jensen LS (1963) Effectiveness of selenium and non-effectiveness of sulfur amino acids in preventing muscular dystrophy in the turkey poult. J Nutr 80:327–331
Scott ML, Olson G, Krook L, et al (1967) Selenium-responsive myopathies of myocardium of smooth muscle in the young poult. J Nutr 91:573–583
Cantor AH, Tarino JZ (1982) Comparative effects of inorganic and organic dietary sources of selenium on selenium levels and selenium-dependent glutathione peroxidase activity in blood of young turkeys. J Nutr 112:2187–2196
Tang J, Tan W, Zhu Y, et al (2012) Effect of selenium on the protection of myocardial cells from injuries induced by overloaded reactive oxygen species, and on the expression of actin in myocardial cells. Wei Sheng Yan Jiu 41(2–5):12
Gu J, Royland JE, Wiggins RC, et al (1997) Selenium is required for normal upregulation of myelin genes in differentiating oligodendrocytes. J Neurosci Res 47:626–635
Ido Y, Duranton A, Lan F, et al (2012) Acute activation of AMP-activated protein kinase prevents H2O2-induced premature senescence in primary human keratinocytes. PLoS One 7:e35092
Cao C, Lu S, Kivlin R, et al (2009) SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J Cell Mol Med 13:3632–3643
Nagaraju K, Casciola-Rosen L, Lundberg I, et al (2005) Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum 52:1824–1835
Chen YW, Nagaraju K, Bakay M, et al (2005) Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology 65:826–834
Baudy AR, Saxena N, Gordish H, et al (2009) A robust in vitro screening assay to identify NF-kappaB inhibitors for inflammatory muscle diseases. Int Immunopharmacol 9:1209–1214
Qi WN, Chaiyakit P, Cai Y, et al (2004) NF-kappaB p65 involves in reperfusion injury and iNOS gene regulation in skeletal muscle. Microsurgery 24:316–323
Tanabe T, Tohnai N (2002) Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat 68-69:95–114
Pagmantidis V, Bermano G, Villette S, et al (2005) Effects of Se-depletion on glutathione peroxidase and selenoprotein W gene expression in the colon. FEBS Lett 579:792–796
Kim YJ, Chai YG, Ryu JC (2005) Selenoprotein W as molecular target of methylmercury in human neuronal cells is down-regulated by GSH depletion. Biochem Biophys Res Commun 330:1095–1102
Lag M, Refsnes M, Lilleaas EM, et al (2005) Role of mitogen activated protein kinases and protein kinase C in cadmium-induced apoptosis of primary epithelial lung cells. Toxicology 211:253–264
Yi JH, Park SW, Kapadia R, et al (2007) Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage. Neurochem Int 50:1014–1027
Yao HD, Liu W, Zhao WC, et al (2014) Different responses of selenoproteins to the altered expression of selenoprotein W in chicken myoblasts. RSC Adv 4:64032
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31272626), the International (Regional) Cooperation and Exchange Projects of National Natural Science Foundation of China (31320103920), the laboratory opening project of Key Laboratory of Myocardial Ischemia Chinese Ministry of Education of Harbin Medical University (KF201009), National Natural Science Foundation of China (31402267), Foundation for Young Talents in Higher Education of Heilongjiang China (UNPYSCT-2015009) and “Young Talents” Project of Northeast Agricultural University (14QC20). All authors have read the manuscript and agreed to submit it in its current form for consideration for publication in the Journal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Liu, W., Yao, H., Zhao, W. et al. Selenoprotein W was Correlated with the Protective Effect of Selenium on Chicken Myocardial Cells from Oxidative Damage. Biol Trace Elem Res 171, 419–426 (2016). https://doi.org/10.1007/s12011-015-0529-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0529-7