Abstract
The biological function of selenium (Se) is mainly elicited through Se-containing proteins. Selenoprotein W (SelW), one member of the selenoprotein family, is essential for the normal function of the skeletal muscle system. To investigate the possible relationship of Se in the process of differentiation in chicken myoblasts and the expression of SelW, the cultured chicken embryonic myoblasts were incubated with sodium selenite at different concentrations for 72 h, and then the mRNA levels of SelW and myogenic regulatory factors (MRFs) in myoblasts were determined at 12, 24, 48, and 72 h, respectively. Furthermore, the correlation between SelW mRNA expression and MRF mRNA expression was assessed. The results showed that the sodium selenite medium enhanced the mRNA expression of SelW, Myf-5, MRF4, and myogenin in chicken myoblasts. The mRNA expression levels of MRFs were significantly correlated with those of SelW at 24, 48, and 72 h. These data demonstrate that Se is involved in the differentiation of chicken embryonic myoblasts, and SelW showed correlation with MRFs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential nutritional trace element and involved in many aspects of health such as chemoprevention [1, 2], neurobiology [3], immune function [4, 5], reproduction [6], aging [7], carcinogenesis [8], and muscle metabolism [9, 10]. It has been reported that Se deficiency causes degenerative muscle diseases in humans, cattle, pigs, turkey, sheep, ducklings, and chicks [11–13] that are characterized by the degeneration and necrosis of skeletal and cardiac musculature [11, 14]. It has been demonstrated that Se controls the development of several cell lines [15, 16]. However, the role of Se in the differentiation of chicken myoblasts is not yet fully understood.
The process of myogenesis, which includes the specification and differentiation of myoblasts, also produces muscle fibers [17]. Myogenesis is initiated with the cell cycle arrest of myoblasts, which contribute to cell fusion and the formation of multinucleated myotubes [18]. This process is regulated by several muscle-specific transcriptional regulatory factors including Myf-5, MyoD, myogenin, and MRF4 [19]. These transcription regulatory factors are the members of the myogenic regulatory factor (MRF) family, which is required for fiber formation and which cannot be replaced by other factors [20–22]. The factors which influence the expression of MRFs also influence the development of skeletal muscles.
The biological functions of Se are mainly elicited through its incorporation of selenocysteine (Sec) into the formation of Se-containing proteins [23]. Approximately 25 kinds of selenoprotein genes have been identified in mammals, and a variety of beneficial biological functions of selenoproteins on health have been described [24–26]. Selenoprotein W (SelW), one member of the selenoprotein family, is highly expressed in the muscle of chickens [27] and in proliferating C2C12 myoblasts [28]. However, as SleW decreases during differentiation of C2C12 myoblasts, this shows that SelW is involved in muscle growth and differentiation [28]. It has also been demonstrated that supplemental Se can regulate the expression level of SelW in chicken myoblasts [29]. Regarding the role of SelW in the process of differentiation of C2C12 myoblasts, it is reasonable to hypothesize that SelW may play a role in the process of differentiation in chicken myoblasts treated with Se [30]. As far as the present studies, there was no report that showed the role of Se in the regulation of differentiation in chicken myoblasts and the relationship between SelW and MRFs that play a crucial role in the differentiation of myoblasts. Therefore, the aims of the present study were to determine whether supplemental Se influences the process of differentiation and to establish the possible relationship between SelW and MRFs in chicken myoblasts.
Materials and Methods
Cell Culture of Primary Chicken Embryonic Myoblasts and Se Treatment
The chicken embryonic myoblasts were prepared by us as described by Ruan [29], with some modifications. The myoblasts were isolated from the pectoralis of 12-day-old ISA layer chicken embryos, minced, and digested with 0.1 % collagenase type I (Invitrogen, Carlsbad, CA, USA). To release single cells, the suspension was dispensed by repetitive passage and filtered to remove large debris. The cell suspension was washed twice and subjected to a density gradient centrifugation in three discontinuous layers with 20, 30, and 55 % Percoll (Pharmacia, Uppsala, Sweden), and the cells between the interface of 30 and 55 % Percoll were harvested. The cell mass was washed and resuspended in proliferation medium (PM) that consists of Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL), 10 % horse serum (Hyclone, Logan, UT, USA), 5 % chicken embryonic extract, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Myoblasts were seeded in six-well plates (Jet, China) coated with 0.1 % gelatin (Sigma, St. Louis, MO) at a density of 2 × 105 cells/cm2 with PM at 37 °C in humidified 95 % air–5 % CO2. The differentiation of myoblasts was induced by replacing PM with serum-free differentiation medium (containing DMEM with various levels of Se as sodium selenite containing 10−9 M, 10−8 M, 10−7 M, 10−6 M, 10−5 M, 100 IU/ml penicillin, and 100 μg/ml streptomycin) after 48 h proliferation. The Se contents in the present were chosen based on the previous study work by Ruan [29]. The medium was changed every day. The RNA of myoblasts was harvested at 12, 24, 48, and 72 h after induction of differentiation.
Chicken Embryonic Myoblasts' Morphometric Measurement
The morphology of chicken embryonic myoblasts treated or untreated with Se was visualized under light microscopy (Eclipse-Ti, Nikon, Japan) at ×400 magnification. Under a light microscope, digital images were taken from ten randomly selected fields which contained more than 20 cells.
Determination of the SelW, Myf-5, Myogenin, and MRF4 mRNA Levels by Quantitative Real-Time PCR
Total RNA was extracted from individual cells of myoblasts using TRIzol reagent (Invitrogen, China). The concentration and purity of the total RNA were determined spectrophotometrically at 260/280 nm. First-strand cDNA was synthesized from 5 μg of total RNA using oligo dT primers (TaKaRa, China) and Superscript II reverse transcriptase according to the manufacturer's instructions (Invitrogen, China). Synthesized cDNA was stored at −80 °C before use.
Primer Premier Software 5.0 (PREMIER Biosoft International, USA) was employed to design specific primers for SelW, Myf-5, myogenin, MRF4, and β-actin based on known chicken sequences (Table 1). General PCRs were first performed to confirm the specificity of the primers. The PCR products were electrophoresed on 2 % agarose gels, extracted, cloned into the pMD18-T vector (TaKaRa, China), and sequenced. Quantitative real-time PCR was performed on an ABI PRISM 7500 Detection System (Applied Biosystems, USA). Reactions were performed in a 20-μl reaction mixture containing 10 μl of 2 × SYBR Green II PCR Master Mix (TaKaRa, China), 2 μl of cDNA, 0.4 μl of each primer (10 μM), 0.4 μl of 50 × ROX reference Dye II, and 6.8 μl of PCR-grade water. The PCR procedure consisted of 95 °C for 30 s followed by 40 cycles of 95 °C for 5 s and 60 °C for 34 s. The melting curve analysis showed only one peak for each PCR product. Electrophoresis was performed with the PCR products to verify primer specificity and product purity. A dissociation curve was run for each plate to confirm the production of a single product. The amplification efficiency for each gene was determined using the DART-PCR program [31]. As a housekeeping gene was used as an internal reference, chicken β-actin expression was used. The mRNA relative abundance was calculated according to the method of Pfaffl [32].
Statistical Analysis
Statistical analyses of Se concentration and mRNA levels were performed using SPSS statistical software for Windows (version 13; SPSS Inc., Chicago, IL, USA). When a significant value (P < 0.05) was obtained by one-way ANOVA, further analysis was done. All data showed a normal distribution and passed equal variance testing. Differences between means were assessed by Tukey's honestly significant difference test for post hoc multiple comparisons. Data are expressed as mean ± standard deviation. Bars that do not share a common letter were considered to be different significantly from each other (P < 0.05); bars sharing a common letter are not significantly different (P > 0.05). The correlation was assessed using Pearson's correlation coefficient.
Results
Effects of Se on the Morphology of Chicken Embryonic Myoblasts
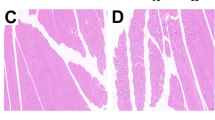
The cells from the 30–55 % Percoll interface were cultured onto gelatin-coated culture dishes. After 48 h proliferation, the medium was changed with a serum-free differentiation medium. The morphology of the cells at different differentiated stages was shown in Fig. 1. As shown in Fig. 1a, after differentiation for 24 h, some cells were aligned in strings with the formation of myotubes. As shown in Fig. 1c, after differentiation for 48 h, the multinucleated myotubes in cells were formed, and myotubes became dense. As shown in Fig. 1b, after differentiation for 72 h, the cells were prominently aligned as strings. However, many of the cells contained vacuoles attributable to aging. Cells that were treated with different levels of Se for 48 h, but not at other time points, are shown in Fig. 1c–h. Whereas Fig. 1d shows that the relatively low concentration of Se (10−9 M) had no effect on the cells' differentiation, Se at the concentration of 10−5 M inhibited their differentiation and significantly decreased cell viability (Fig. 1h). When compared with the control group, the cells cultivated at 10−7 M Se in the medium were increasingly multinucleated and contained myotubes. However, when cultivated at 10−6 M Se, the percentage of multinucleated cells and of myotubes was lower compared to the cells treated with 10−7 M Se, suggesting that at the level of 10−6 M, Se begins to inhibit the differentiation of the cells. Multinucleated cells and myotubes in cells treated with 10−8 M Se were very dense, and it became difficult to distinguish between multinucleated cells and myotubes, which indicated that the optimal Se supplement for differentiation in chicken embryo myoblasts was 10−8 M.
Effect of Se on the morphology of chicken embryonic myoblasts. The chicken embryonic myoblasts were cultured in DMEM with 10−9, 10−8, 10−7, 10−6, and 10−5 M Se for 24, 48, and 72 h. The morphology of treated or untreated myoblasts was visualized under light microscopy (magnification, × 400; bar, 100 μm). Subpanel a represented the cells in the control group at 24 h; b represented the cells in the control group at 72 h; c represented the cells in the control group at 48 h; d represented the cells treated with 10−9 M Se at 48 h; e represented the cells treated with 10−8 M Se at 48 h; f represented the cells treated with 10−7 M Se at 48 h; g represented the cells treated with 10−6 M Se at 48 h; h represented the cells treated with 10−5 M Se at 48 h
Effect of Se on Gene Expression of SelW in Primary Chicken Embryonic Myoblasts during Differentiation
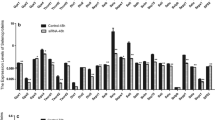
To further demonstrate the effects of Se on the expression of SelW gene in chicken embryonic myoblasts, myoblasts from the chicken pectoral muscle were isolated and cultured in serum-free medium with various Se concentrations, and the level of SelW mRNA expression of muscle cells during the differentiation process was examined (Fig. 2a). The results confirmed that the expression of SelW first increased and then decreased with the increasing Se content in the medium at different time points, except for the group with 10−5 M at 72 h. The greatest increase of SelW mRNA expression was observed in the group of 10−7 M Se content at different time points, which indicated that the optimal content for the expression of SelW in chicken embryonic myoblasts was 10−7 M.
Effect of Se on the expression of SelW, Myf-5, myogenin, and MRF4. The chicken embryonic myoblasts were cultured in DMEM with 10−9, 10−8, 10−7, 10−6, and 10−5 M Se for 12, 24, 48, and 72 h, respectively. SelW, Myf-5, myogenin, and MRF4 mRNA expression levels in chicken embryonic myoblasts were measured by quantitative real-time RT-PCR. Subpanels a, b, c, and d represented the expression of SelW, Myf-5, myogenin, and MRF4, respectively. Bars represent mean ± standard deviation (n = 3/group). Bars with a different small letter are statistically significantly different from 12 h by one-way analysis of variance followed by Tukey's multiple comparison test (P < 0.05); bars sharing a common letter are not significantly different (P > 0.05)
Effect of Se on Gene Expression of MRFs in Primary Chicken Embryonic Myoblasts during Differentiation
To investigate the potential effects of Se on the differentiation of myoblasts, the levels of some MRF transcripts, such as Myf-5, myogenin, and MRF4, were measured by using a quantitative RT-PCR technique. The results in Fig. 2b–d reveal that Myf-5, myogenin, and MRF4 transcripts were increased in the Se-supplemented groups compared to the controls, although a decrease during the differentiation was observed in the 10−5 M Se group.
The Pearson correlation coefficients between Myf-5, MRF4, myogenin, and SelW mRNA levels for different Se supplementations at the same time point are presented in Table 2. Myf-5, MRF4, and myogenin mRNA levels in 24, 48, and 72 h were significantly correlated with the levels of SelW mRNA for the different Se supplementations.
Discussion
Se plays important roles in muscle metabolism. Se deficiency in cattle, humans, and avians causes the degeneration of cardiac and skeletal muscles characterized by the presence of calcium deposits, vascular lesions, and hemorrhages [33, 34]. However, Se also plays a crucial role in the development of cells. In a prior study, FuKun [35] fed mice with different contents of Se for 8 weeks and found that dietary Se levels modulate free thiol levels and specific signaling events during CD4+ T cell activation, which influenced their proliferation and differentiation. In another prior study, Gu [15] indicated that Se was necessary for the normal differentiation of oligodendrocyte lineage cells. He found that the differentiation of O-2A progenitor cells into mature oligodendrocytes, as assessed by the upregulation of myelin-specific genes, required the addition of Se into the culture at an optimal concentration of approximately 30 nM, and he postulated that the effect of Se deprivation on the oligodendrocyte differentiation was caused by the influence of Se on the activity of GSH-Px or other Se-containing enzymes which resulted in an oxidative stress to the cells. These prior studies demonstrate that Se plays important roles in the regulation of the differentiation of mammalian cells. However, it remains unclear whether Se supplement influences the process of differentiation in chicken embryonic myoblasts. In the present study, the morphology assay of chicken embryonic myoblasts showed that the optimal Se supplement (10−8 M) promotes the differentiation of cells; however, excessive Se supplement (10−5 M) inhibited the differentiation of chicken embryo myoblasts and significantly decreased cell viability. These results demonstrated that Se plays a role in the process of differentiation of chicken myoblasts. In contrast, excessive high concentration of Se (10−5 M) presented a toxic function.
In order to further investigate the effect of Se on differentiation of chicken myoblasts, we also examined the mRNA expression of MRFs following the treatment of different contents of Se. It has been known that vertebrate skeletal myogenesis is controlled by the MRF family that is necessary for skeletal muscle determination and terminal differentiation. Gene-targeting experiments have demonstrated that MyoD and Myf-5 are important for myogenic determination and are expressed in proliferating myoblasts, whereas myogenin and MRF4 are important for terminal differentiation and lineage maintenance [36]. Myogenin is crucial for differentiation, and its absence results in a deficiency of muscle fibers despite muscle cell migration and commitment [37]. Similarly, MRF4-deficient mice displayed a range of phenotypes consistent with a late role for MRF4 in the myogenic pathway [38–41]. These results suggest that MRFs play an important role in the process of skeletal muscle development. In the present study, we investigated the expression of Myf-5, MRF4, and myogenin in chicken embryonic myoblasts following the treatment of Se. The data revealed that the mRNA levels of the three MRFs first increased and then tended to decrease after Se was supplemented in the medium, which indicated that an optimal concentration of Se could promote the expression of the three MRFs in chicken embryonic myoblasts, whereas excessive Se inhibited their expression. The higher expression levels of MRFs in optimal-Se-treated groups indicated that optimal Se can promote the differentiation of chicken myoblasts, and the results were consistent with the results of the morphology assay.
SelW is expressed ubiquitously in various tissues, but it is specifically high in the skeletal muscle and brain of mammals [28, 42, 43]. Due to the high expression of SelW in skeletal muscles of the chicken [27], SelW may play a crucial role in the biological function of this tissue. It has been reported that SelW played a role in the process of differentiation in C2C12 myoblasts [28] and had a relationship with MyoD in C2C12 myoblasts [30]. Accordingly, SelW may also play a role in the process of differentiation in chicken embryonic myoblasts. In the present study, we examined the possible relationship between SelW and MRFs in chicken myoblasts treated with Se. The data indicated that the production of SelW is highly favored both in proliferating and differentiated myoblasts, consistent with the observation by Noh [30] with rat myoblasts and different, however, with those of Loflin [28] and Whanger [44]. This contradiction may result from different levels of Se in the medium and diverse kinds of animals or cell lines. The high expression of SelW in differentiated chicken myoblasts may be related to the role of SelW in the regulation of differentiation of chicken myoblasts, but this needs to be further studied. In the present study, the mRNA level of the SelW also first increased and then tended to decrease after Se was supplemented in the medium. It is interesting that the expression levels of SelW and MRFs have the same trend following the treatment of Se in this study. Further correlation analysis showed that MRF mRNA levels in 24, 48, and 72 h (differentiation time points) were significantly correlated with SelW mRNA content for the different Se. We thus hypothesize that SelW may have a possible relationship with MRFs in chicken myoblasts, indicating that SelW may play a role in the process of differentiation of chicken embryonic myoblasts.
In summary, Se at optimal levels promotes the differentiation of chicken embryonic myoblasts, and the expression of SelW and the MRF levels in chicken embryonic myoblasts treated with Se are highly correlated. This demonstrates that SelW might have a possible relationship with MRFs in the process of differentiation in chicken myoblasts.
References
Combs GF Jr, Clark LC, Turnbull BW (2001) An analysis of cancer prevention by selenium. Biofactors 14:153–159
Li JL, Gao R, Li S, Wang JT, Tang ZX, Xu SW (2010) Testicular toxicity induced by dietary cadmium in cocks and ameliorative effect by selenium. Biometals 23:695–705
Schweizer U, Schomburg L, Savaskan NE (2004) The neurobiology of selenium: lessons from transgenic mice. J Nutr 134:707–710
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241
Hoffmann PR, Berry MJ (2008) The influence of selenium on immune responses. Mol Nutr Food Res 52:1273–1280
Kaur P, Bansal MP (2005) Effect of selenium-induced oxidative stress on the cell kinetics in testis and reproductive ability of male mice. Nutrition 21:351–357
Martin-Romero FJ, Kryukov GV, Lobanov AV, Carlson BA, Lee BJ, Gladyshev VN, Hatfield DL (2001) Selenium metabolism in Drosophila: selenoproteins, selenoprotein mRNA expression, fertility, and mortality. J Biol Chem 276:29798–29804
Jackson-Rosario SE, Self WT (2010) Targeting selenium metabolism and selenoproteins: novel avenues for drug discovery. Metallomics 2:112–116
Chariot P, Bignani O (2003) Skeletal muscle disorders associated with selenium deficiency in humans. Muscle Nerve 27:662–668
Mahmoud KZ, Edens FW (2005) Influence of organic selenium on hsp70 response of heat-stressed and enteropathogenic Escherichia coli-challenged broiler chickens (Gallus gallus). Comp Biochem Physiol C Toxicol Pharmacol 141:69–75
Schubert JR, Muth OH, Oldfield JE, Remmert LF (1961) Experimental results with selenium in white muscle disease of lambs and calves. Fed Proc 20:689–694
Van Vleet JF, Ferrans VJ (1976) Ultrastructural changes in skeletal muscle of selenium-vitamin E-deficient chicks. Am J Vet Res 37:1081–1089
Bartholomew A, Latshaw D, Swayne DE (1998) Changes in blood chemistry, hematology, and histology caused by a selenium/vitamin E deficiency and recovery in chicks. Biol Trace Elem Res 62:7–16
Ou BR, Jiang MJ, Lin CH, Liang YC, Lee KJ, Yeh JY (2011) Characterization and expression of chicken selenoprotein W. Biometals 24:323–333
Gu J, Royland JE, Wiggins RC, Konat GW (1997) Selenium is required for normal upregulation of myelin genes in differentiating oligodendrocytes. J Neurosci Res 47:626–635
Nishina A, Sekiguchi A, Fukumoto RH, Koketsu M, Furukawa S (2007) Selenazoles (selenium compounds) facilitate survival of cultured rat pheochromocytoma PC12 cells after serum-deprivation and stimulate their neuronal differentiation via activation of Akt and mitogen-activated protein kinase, respectively. Biochem Biophys Res Commun 352:360–365
Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA (2002) Myogenic specification of side population cells in skeletal muscle. J Cell Biol 159:123–134
Muntoni F, Brown S, Sewry C, Patel K (2002) Muscle development genes: their relevance in neuromuscular disorders. Neuromuscul Disord 12:438–446
Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238
Tajbakhsh S, Buckingham M (2000) The birth of muscle progenitor cells in the mouse: spatiotemporal considerations. Curr Top Dev Biol 48:225–268
Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S et al (1991) The myoD gene family: nodal point during specification of the muscle cell lineage. Science 251:761–766
Gayraud-Morel B, Chretien F, Flamant P, Gomes D, Zammit PS, Tajbakhsh S (2007) A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev Biol 312:13–28
Behne D, Kyriakopoulos A (2001) Mammalian selenium-containing proteins. Annu Rev Nutr 21:453–473
Kryukov GV, Gladyshev VN (2004) The prokaryotic selenoproteome. EMBO Rep 5:538–543
Zhang Y, Fomenko DE, Gladyshev VN (2005) The microbial selenoproteome of the Sargasso Sea. Genome Biol 6:R37
Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9:775–806
Li JL, Ruan HF, Li HX, Li S, Xu SW, Tang ZX (2011) Molecular cloning, characterization and mRNA expression analysis of a novel selenoprotein: avian selenoprotein W from chicken. Mol Biol Rep 38:4015–4022
Loflin J, Lopez N, Whanger PD, Kioussi C (2006) Selenoprotein W during development and oxidative stress. J Inorg Biochem 100:1679–1684
Ruan H, Zhang Z, Wu Q, Yao H, Li J, Li S, Xu S (2012) Selenium regulates gene expression of selenoprotein W in chicken skeletal muscle system. Biol Trace Elem Res 145:59–65
Noh OJ, Park YH, Chung YW, Kim IY (2010) Transcriptional regulation of selenoprotein W by MyoD during early skeletal muscle differentiation. J Biol Chem 285:40496–40507
Peirson SN, Butler JN, Foster RG (2003) Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res 31:e73
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Avanzo JL, de Mendonca CX Jr, Pugine SM, de Cerqueira Cesar M (2001) Effect of vitamin E and selenium on resistance to oxidative stress in chicken superficial pectoralis muscle. Comp Biochem Physiol C Toxicol Pharmacol 129:163–173
Hassan S, Hakkarainen J, Jonsson L, Tyopponen J (1990) Histopathological and biochemical changes associated with selenium and vitamin E deficiency in chicks. Zentralbl Veterinarmed A 37:708–720
Hoffmann FW, Hashimoto AC, Shafer LA, Dow S, Berry MJ, Hoffmann PR (2010) Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J Nutr 140:1155–1161
Perry RL, Rudnick MA (2000) Molecular mechanisms regulating myogenic determination and differentiation. Front Biosci 5:D750–767
Salmon M, Owens GK, Zehner ZE (2009) Over-expression of the transcription factor, ZBP-89, leads to enhancement of the C2C12 myogenic program. Biochim Biophys Acta 1793:1144–1155
Patapoutian A, Yoon JK, Miner JH, Wang S, Stark K, Wold B (1995) Disruption of the mouse MRF4 gene identifies multiple waves of myogenesis in the myotome. Development 121:3347–3358
Rawls A, Morris JH, Rudnicki M, Braun T, Arnold HH, Klein WH, Olson EN (1995) Myogenin's functions do not overlap with those of MyoD or Myf-5 during mouse embryogenesis. Dev Biol 172:37–50
Zhang W, Behringer RR, Olson EN (1995) Inactivation of the myogenic bHLH gene MRF4 results in up-regulation of myogenin and rib anomalies. Genes Dev 9:1388–1399
Yoon JK, Olson EN, Arnold HH, Wold BJ (1997) Different MRF4 knockout alleles differentially disrupt Myf-5 expression: cis-regulatory interactions at the MRF4/Myf-5 locus. Dev Biol 188:349–362
Yeh JY, Beilstein MA, Andrews JS, Whanger PD (1995) Tissue distribution and influence of selenium status on levels of selenoprotein W. FASEB J 9:392–396
Jeong DW, Kim EH, Kim TS, Chung YW, Kim H, Kim IY (2004) Different distributions of selenoprotein W and thioredoxin during postnatal brain development and embryogenesis. Mol Cells 17:156–159
Whanger PD (2000) Selenoprotein W: a review. Cell Mol Life Sci 57:1846–1852
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30871902), the Science Foundation of the Education Department of Heilongjiang Province (11551030), the Postdoctoral Science Foundation (grant no. LRB06-262), the Postdoctoral Science Foundation of Heilongjiang Province (grant no. LBH-Z07250), and the Study Abroad Foundation of Heilongjiang Province (LC201031). The authors thank the members in the veterinary internal medicine laboratory in the College of Veterinary Medicine, Northeast Agricultural University, for help in analyzing the data.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, Q., Yao, HD., Zhang, ZW. et al. Possible Correlation between Selenoprotein W and Myogenic Regulatory Factors in Chicken Embryonic Myoblasts. Biol Trace Elem Res 150, 166–172 (2012). https://doi.org/10.1007/s12011-012-9520-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9520-8