Abstract
The objective of this study was to evaluate the toxicity of individual and mixtures of di(n-butyl) phthalates (DBP) and their active metabolite monobutyl phthalate (MBP) and arsenic (As) on spatial cognition associated with hippocampal apoptosis in mice. Mice were exposed, individually or in combination, to DBP (50 mg/kg body weight, intragastrically), MBP (50 mg/kg body weight, intragastrically), and As (10 mg/L, per os) for 8 weeks. The Morris water maze test showed that mice exposed to DBP/MBP combined with As exhibited longer escape latencies and the lower average number of crossing the platform. The As content in the hippocampus after As exposure increased as compared to those without As exposure. In mice exposed to DBP/MBP combined with As, pathological alterations and oxidative damage to the hippocampus were found. Expression of apoptosis-related protein: Bax and caspase-3 were significantly increased in the hippocampus, while there was no significant change in expression of Bcl-2. The results suggested that DBP and MBP combined with As can induce spatial cognitive deficits through altering the expression of apoptosis-related protein and As played a critical role in cognition impairments. And the joint exposure has antagonistic effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditionally, research on toxicity of environmental pollutants has been focused on individual toxicants. However, organisms are actually exposed to a cocktail of various pollutants at low concentrations in the ecosystem. Interactions of chemicals in a mixture could be synergistic, antagonistic, potentiated, or inhibitory depending on the species of chemicals involved. Arsenic (As) is a well-known toxicant, which has been exposed to millions of people worldwide through food and water [1]. As is reported to cause a wide array of adverse health effects, including respiratory, gastrointestinal, hematologic, hepatic, renal, dermal, immunologic, and neurological effects [2, 3]. Epidemiological studies revealed an association between As in drinking water and the risk of cognitive impairment, including disturbed visual perception and visuomotor integration, psychomotor speed, attention, speech, and memory [2, 4]. Also, chronic exposure to inorganic As via drinking water results in a dose-dependent reduction in the intellectual functions of children [5, 6]. Early-life exposure to As-contaminated water has been linked to increased mortality and morbidity in infants and cognitive deficits in school-aged children [7]. Furthermore, evidence from animal studies showed that As can be transported through the placenta from adult mice to their fetuses, resulting in learning and behavioral deficits [8, 9].
Phthalic acid esters (PAEs) are a group of synthetic compounds that have been used in a wide variety of industrial and consumer applications for more than 50 years. Phthalates are not bound covalently to plastics and hence, are easily released into the environment [10]. Children likely have multiple sources and routes of exposure, as current research indicates that almost all children’s urine samples contain measurable concentrations of phthalates metabolites [11]. Di (n-butyl) phthalates (DBP) is deemed as an endocrine disrupting chemical (EDC) because it can interfere with the generation and action of many hormones, including testosterone, estradiol, luteinizing hormones, and others [12, 13]. Accumulating evidence has shown that perinatal exposure to DBP has adverse effects on the neurodevelopment of neonatal and immature brains of rat offspring [14]. Regarding the adverse effects of DBP, the most concern is with its estrogenic and anti-androgenic activities in vitro [15]. Besides its adverse effects on reproduction, recent evidence highlights its influence on neurological functions in children, adult and aging brains, as endocrine disruptors [16]. Mono-n-butylphthalate (MBP) is considered the main and active hydrolysate of DBP by enzyme esterase [17], which can suppress steroidogenesis by fetal-type Leydig cells in primates and rodents [18].
Because of their widespread and constant presence in the environment, humans are exposed either concurrently or sequentially, through various routes of exposure, to a large number of chemicals from a variety of sources over varying periods of time [19]. The magnitude of the problem is immense because daily exposures to mixture of chemicals have become a routine. As exposure to both As and phthalates of environmental origin is likely, it was a logical step forward to investigate their combined effect in addition to the traditional focus on the effect of a single agent. Therefore, the current study was designed to determine the neurological effect of exposure to As, DBP, and MBP individually or mixtures in mice, especially, at low concentrations which are thought to be individually safe. In fact, only a limited number of studies are available on the combined effects of these compounds. Exposure to DBP and Benzoapyrene (BaP), individually or as mixtures, was found to affect the reproductive system of male rats, adversely, via oxidative stress [20]. Studies have revealed that DBP and its metabolite MBP can inhibit the enzymatic activity of superoxide dismutase (SOD) and thus impair the free radical scavenging mechanism [21]. Xu et al. [22] further reported the potential neurotoxic effect exhibited by DBP and Diethyl Phthalate (DEP) on zebrafish embryos. DBP and MBP inhibited testosterone production in MLTC-1 cells. This inhibition of testosterone production occurred in the absence of any change in Leydig cell viability and was dose-dependent [23].
Apoptosis, or programmed cell death, is a normal feature in the development of the central nervous system and can be experimentally induced by a variety of conditions and compounds. It is regulated by a number of anti- and pro-apoptotic genes expressing homologous proteins and by enzymatic cascades. One of the gene families closely related to these regulatory pathway is the Bcl-2 family, which comprises several Bcl-2 related genes that promote (e.g., Bax) or inhibit apoptosis (e.g., Bcl-2). Caspases are cysteine proteases that are thought to be critical in the execution of apoptotic cell deaths. Caspase-3 is considered to be a prominent effector caspase. Activation of this proteolytic enzyme has been demonstrated in many acute and chronic neurodegenerative disorders, such as Alzheimer’s disease [24] and Parkinson’s disease [25]. The purpose of present study was to investigate neurotoxicity of DBP/MBP combined with As at a low concentration. The expression of Bax, Bcl-2, and caspase-3 in the hippocampus were studied. In addition, sensitive biomarkers for neurotoxicants, acetylcholinesterase (AChE), and nitric oxide synthase (NOS) activity were also measured. Our findings provide basic experimental evidence for better understanding of co-exposure induced neurotoxicity.
Materials and Methods
Chemicals and Reagents
Analytical grade (>99 %) sodium arsenite was purchased from Sinopharm Chemical Reagent Co. Ltd. As standard was purchased from the National Center of Analysis and Testing for Nonferrous Metals and Electronic Materials (NCATN) in China. DBP (99 % purity) was purchased from Sigma-Aldrich (St. Louis, MO, USA), while MBP (>99.4 %) was purchased from TCI, Japan. Corn oil was obtained from local commercial sources.
Animals
Eighty (80) 3-week-old SPF male (10 ± 2 g) ICR mice were purchased from the Comparative Medicine Center of the Jiangsu University (the license number SYXK (SU) 2013–0036). Mice were allowed to acclimatize to their new environment for a period of 3 days before commencement of experiment. During the experiments, mice were fed under controlled environmental conditions, which included a temperature of 22 ± 2 °C and humidity of 58 ± 2 %. They were kept in plastic cages padded with wood shavings at a 12-h light/dark cycle. Mice were fed with basal diet and given free access to drinking water. All experimental procedures conformed to The Code of Ethics of the World Medical Association for experiments involving humans (EC Directive 86/609/EEC for animal experiments). They were approved by the Jiangsu University Committee on Animal Care and Use.
Experimental Design and Exposure
The mice were randomly divided into eight groups (ten mice per group). Sodium arsenite was dissolved in the drinking water, 10 mg/L [26]. DBP and MBP were given to the mice by oral gavage once daily, after dissolution in corn oil. Test solutions were administered continuously for 8 weeks to mice. The daily doses of DBP and MBP, given alone, was designed at 50 mg/kg, which was based on the LOAEL of a previous study [27]. Detailed doses can be seen in Table 1.
At the end of the experimental period, every mice were weighed and anesthetized with sodium pentobarbital. The brain, liver, and kidneys of each mice were collected, rinsed with cold saline, weighed, and used for biochemical and elemental analyses. Also, the hippocampus of five mice from each group were excised, fixed in 10 % formalin, and stained with hematoxylin and eosin (H&E) for histological examination, respectively. Another five hippocampus of the mice from each group was carefully separated, transferred into liquid nitrogen, and stored at −196 °C for western blot analysis.
Morris Water Maze Test
The Morris water maze test was used as previously described in the literature [28] to evaluate the learning and memory abilities of mice following as, DBP and MBP exposure. The test was performed in a 1.2-m diameter pool with a 9.5-cm diameter platform placed in the SE quadrant of the pool, located in a room with a temperature of 25 °C. A pickup camera recorded the tracks of the mice in the pool. The procedure consisted of 4 days of the hidden platform acquisition test and a probe trial test. In the hidden platform acquisition test, the training began 6 h after the end of each day’s exposure. The mice were released into the water from the NE, NW, and SW quadrants, respectively. The time required for the mice to find the escape platform was recorded. Each mouse was allowed 60 s to search for the platform. If the mice could not find the platform within that time, the escape latency was recorded as 60 s, and the mice were led to the platform and placed on it for 60 s. Each mouse was trained three times a day to find the escape platform, and this continued for 4 days. After 1 days of no training, the platform was removed, and each mouse was subjected to the probe trial test to determine their memory ability. The mice were also put into the pool from the NE, NW, and SW quadrants, and the camera recorded their tracks for 60 s.
Mice behavior, which included escape latency and the number of passes through the area where the platform had been, were recorded by automated video tracking (Shandong Academy of Medical Sciences, Jinan, China). The biomarkers for the MWM were “escape latency” for learning and “number of crossing the platform” for memory.
Histopathological Analyses
Sections of the brain and the hippocampus of each group were excised, fixed in 10 % formalin and dehydrated in ascending grades of ethanol, cleared in xylene, and embedded in paraffin. The cut sections were stained with H&E and viewed under a light microscopy (Axioskop 40, Zeiss, Germany) by an experienced and board-certified veterinary pathologist who was blind to the animals assigned to each experimental group.
As Content Assay
Briefly, 0.1 g samples of the brain, liver, and kidney were digested with HNO3 and H2O2 in microwave digestion system (Anton Paar 3000, Australia). Before measurement, added 10 mL 5 % ascorbic acid and 5 % sulfocarbamide to the digested samples, then diluted to 10 mL with 5 % hydrochloric acid, As concentration was measured using Vista-MPX Simultaneous ICP-OES (Varian Inc.,USA) [29]. As determination was done in triplicates.
Biochemical Assay
Malondialdehyde (MDA) and SOD in the brain, liver, and kidney, NOS and AChE in the brain were assayed by commercial kits (Institute of Biological Engineering of Nanjing Jianchen, Nanjing, China).
Western Blotting
Western blot analyses were performed to detect the protein expression of Bax, Bcl-2, and caspase-3. β-actin was used as a control. Briefly, the hippocampus was homogenized with a Dounce homogenizer (1:10, w/v) in a RIPA Lysis Buffer (Beyotime, China) solution containing protease inhibitors. The total protein concentration in the lysates was determined using a BCA protein assay kit (Biyuntian, China). The samples employed for Western blotting contained 40 μg of protein from the hippocampus in each lane. The proteins were mixed with an equal volume (20 μl) of SDS-PAGE loading buffer, separated via SDS-PAGE using 12 % SDS-PAGE gels and transferred to a polyvinylidene difluoride (PVDF) membrane. The membranes were blocked with blocking buffer containing defatted milk powder for 2 h and incubated overnight at 4 °C with anti-rabbit Bax (1:1000), Bcl-2 (1:1000), caspase-3 (1:1000), and β-actin antibodies (1:500) (Cell Signaling Technology. USA). The membrane was washed three times with Tris-buffered saline containing 0.05 % Tween-20 (TBST) for 15 min and then incubated at room temperature for 2 h with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2000) (Cell Signaling Technology, USA). After that, the membrane was detected using enhanced chemiluminescence (ECL). Images of resulting gels were captured by image master VDS. The images was analysis by Quantity One 4.6.9.
Statistical Analysis
Statistical analysis was performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Numerical data were expressed as means ± standard deviation (SD). Statistical significance of the differences between the experimental and control groups was tested using one-way analysis of variance (ANOVA) followed by LSD’s test. The level for statistical significance and interactive effect was set at p < 0.05.
Results
Morris Water Maze Test
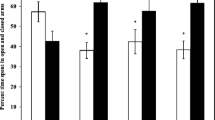
In the Morris water maze test, mice were able to find the platform within 60 s. As + DBP and As + DBP + MBP groups significantly exhibited longer escape latencies to the hidden platform compared with the control (Fig. 1a). In hidden platform trial, the average number of mice crossing the platform site was significantly different in As + DBP, As + MBP, and As + DBP + MBP compared to mice in the control group (Fig. 1b). Also, As + DBP + MBP group exhibited longer escape latencies and less average number of crossing platform compared with DBP + MBP. Using analysis of variance of factorial design, it was observed that the combination exposure to As, DBP, and MBP has antagonistic effect on escape latency and average number of mice crossing the platform site. The combination of As, DBP, and MBP exposure resulted in memory impairment and decreased learning in mice to some extent.
Effects on spatial learning and memory following exposure to As, DBP, and MBP individually or in combination in the Morris water maze. a Escape latency in the hidden platform acquisition test. b Number of crossing platform in the probe trail test. The results are means ± SD (n = 10). a p < 0.05, compared with control; e p < 0.05, As + DBP + MBP compared with DBP + MBP; *p < 0.05, interaction of joint exposure
As Levels in Soft Tissues
As levels in the tissue samples (brain, liver and kidney) (Table 2) were determined in this study. There were no significant differences in the levels of As in the tissues between the groups without As exposure and the control group; however, significant increases were observed in As, As + DBP, As + MBP and As + DBP + MBP as compared to the control group. Also, the level of As in As + DBP + MBP was significant increases as compared to DBP + MBP. In addition, the As level in soft tissues increased with DBP and MBP concentration in combination, especially in the brain. A possible explanation of this phenomenon is stimulation of As accumulation by DBP/MBP in the brain of mice.
Histological Examination of the Hippocampus
After 8 weeks of exposure, the hippocampus of the mice was examined and analyzed. It was found that pyramidal cells in the CA1 region and granule cells in the dentate gyrus (DG) region of the mice in the control group were neatly arranged, with structures intact and with clear edges (Fig. 2). As + DBP, As + MBP and As + DBP + MBP exposed groups appeared to show pathological alterations, as the arrangement of cells was seen to be loose and disordered, with swelling deformations in cell shape as compared to the control. All morphological changes indicated that, combined exposure affected the histology of the hippocampus in mice.
Effects on Lipid Peroxidation and Superoxide Dismutase
Sensitive biochemical variables changed significantly after expose to As, DBP and MBP, especially in combination. The activity of SOD and the level of MDA in the tissues are shown in Table 3. A significant decrease in the activity of SOD was observed not only in the liver and kidney of mice, but also in the brain when exposed to As + DBP, As + MBP and As + DBP + MBP as compared to the control. Also, As + DBP + MBP showed a significant decrease in the activity of SOD and increase in levels of MDA compared with DBP + MBP. The levels of MDA was significantly increased in the tissues after exposure to As + DBP, As + MBP and As + DBP + MBP as compared to the control. There were no significant differences in the activity of SOD or MDA between the group expose to DBP + MBP and the control. Using analysis of variance of factorial design, it was observed that the combination exposure to As, DBP and MBP has antagonistic effect on the MDA and SOD in the tissues.
Effects on NOS and the Activities of AChE
The activities of NOS and AChE in the brain of mice in the 16 groups are showed in Fig. 3 and Fig. 4. iNOS and TNOS activity were markedly increased in the brains of As + DBP, As + MBP, and As + DBP + MBP groups, as compared to that of the control. There were, however, no significant differences between the groups exposed to As, DBP and MBP individually and the control. As can be seen in Fig. 4, AChE activity was also significantly inhibited in brain when expose to As + DBP, As + MBP, and As + DBP + MBP. The iNOS and TNOS, AChE activity were markedly increased in As + DBP + MBP group compared with DBP + MBP. Using analysis of variance of factorial design, it was observed that the combination exposure to As, DBP, and MBP has antagonistic effect on the iNOS and TNOS, AChE activities in the brain of mice.
Effect on NOS activity in the brain of mice following exposure to As, DBP, and MBP individually or in combination. a TNOS expression in the brain, b iNOS expression in the brain. The results are means ± SD of triplicate samples. a p < 0.05, compared with control; e p < 0.05, As + DBP + MBP compared with DBP + MBP; *p < 0.05, interaction of joint exposure
Western Blotting
The expression levels of Bax, Bcl-2 and caspase-3 in the hippocampus of mice are shown in Fig. 5. Bax and caspase-3 protein expression levels in the hippocampus were increased in groups following exposure to DBP/MBP combined with As compared to the control, whereas Bcl-2 was changed inversely. In particular, no significant change was observed in mice exposed to As, DBP, and MBP individually.
Discussion
Morris water maze is a powerful tool used in the study of spatial learning and memory in a variety of species [30]. It is an ideal system to evaluate the cognitive effects of environmental pollutants on rodents in behavioral neuroscience. In the present study, the longer escape latency and less number of crossing platform in As + DBP, As + MBP, and As + DBP + MBP showed the spatial acquisition deficit and memory impairment. However, no significant differences were observed in As, DBP, MBP, and DBP + MBP groups. These results provided novel evidence that DBP/MBP combined with As can cause learning and memory deficits, especially, As may play an important role in combination. To determine possible underlying mechanisms for the injured behavioral development in the young mice, the present study focused on what happened in the hippocampus, a brain area related to navigation [31].
The hippocampus participates in diverse biological functions, including motivation, spatial navigation, adaptive timing, and declarative (notably, episodic) memory [32]. Extensive evidence indicates that hippocampal neuronal injury can result in functional deficits in learning and memory [33]. It has been proved that many neurological disorders are related to cell death or apoptosis that may be involved in neuron loss [34]. Nakazawa et. al reported that DG and CA1 regions could reflect the capability of behavioral performance [35]. In this study, the results showed that the combined exposure of As, DBP, and MBP (10 mg/L, 50 mg/kg, 50 mg/kg) greatly decreased the number of neurons and altered the normal brain structure in hippocampal regions, whereas there were no significant differences between As, DBP, MBP individually, or DBP + MBP and the control. The result may be a reasonable explanation for the phenomenon, which was described in the Morris water maze.
An immediate question raised was how combined exposure impacted spatial acquisition in association with neuronal apoptosis in the hippocampus. To address such a question, the caspase cascades (one of the characteristic biomarkers for apoptosis that plays an essential role in both initiation and execution of cell death) were investigated. Therefore, the expression of three apoptosis-related protein, Bax/Bcl-2 and caspase-3, were determined in the hippocampus. The caspase and Bcl-2 families are important regulators of programmed cell death in experimental models of brain injury [36]. Li et al. [37] reported that increased caspase-3 activity in the hippocampus was correlated to impaired spatial learning and memory. Bcl-2 can protect cells from apoptosis by acting at a point downstream from release of mitochondrial cytochrome c, thereby preventing a caspase-3-dependent proteolytic cascade [38]. In the current study, over-expression of apoptosis-related protein: Bax/Bcl-2 ratio and caspase-3 were observed in the impaired brains of As + DBP, As + MBP, and As + DBP + MBP exposed mice. The data suggests that the neuronal apoptosis observed in the combined exposure to the hippocampus was correlated to increased caspase-3 activity, and Bax/Bcl-2 was also involved in that change. Several reasons may account for these observations. First, Lossi et al. [39] demonstrated that the physiological process of apoptosis in an immature brain is relatively active, and thus, the immature brain is considered to be more vulnerable to diverse types of endogenous and exogenous stimuli, which may trigger the over-activation of pathologic apoptosis . Our data indicated that DBP/MBP exposure at a dose of 50 mg/kg individually was not likely to cause much neuronal apoptosis in early weanling mice; however, it changed when combined with As, which may alter the cognitive abilities of the mice at a relatively low dose as well. Hence, the results showed that As may be crucial to the combined exposure. Meanwhile, As as well as its metabolites generates ROS, including nitricoxide (NO−) and superoxide anion (O2−), which results in tissue damage [40, 41]. Increased ROS generation may lead to the mitochondrial membrane disruption [42] causing the release of cytochrome c and finally apoptosis of the cells [43], contributing to As-induced cytotoxicity.
DBP is reported to have adverse effects on ROS scavenging pathway either by increasing the concentration of ROS or by decreasing the activity of anti-oxidant enzymes [44]. So, oxidative stress injuries leading to neuronal apoptosis were a possible mechanism for the combined neurotoxicity. Therefore, lipid peroxidation and SOD activities in soft tissues in addition to NOS and AChE in the brain of mice were investigated in this study.
Also, the presence of the blood-CNS barrier may limit the transportation of As, and DBP/MBP from the periphery to the CNS and thus limit the effects of their combined exposure on the CNS. Studies using experimental animals have shown that As can cross the blood brain barrier and accumulate in different regions of the brain, which can destroy functions of the peripheral and central nervous system, and suggests that As may play an essential role in causing neurological diseases [2, 45]. Meanwhile, growing evidence suggests that oxidative stress has a central role in the neuropathology of neurodegenerative diseases. It has been suggested that the loss of cell function results from the increased oxidative damage to proteins and DNA [46]. Lu et al. [47] reported that oxidative stress-induced JNK/ERK pathway activation-regulated apoptosis played a crucial role in As-induced neuronal cell deaths. Anti-oxidant enzymes are considered to be the first line of cellular defense against oxidative damage by suppressing the formation of ROS, or opposing their actions [48]. As exposure has been found to cause oxidative damage to the biological system by enhancing generation of free radical species, which in turn may be responsible for increased lipid peroxidation, decreased SOD and glutathione (GSH) levels [49]. In this study, lipid peroxidation and SOD activities were changed more markedly in the liver and kidneys than in the brain. This suggests that the blood-CNS barrier may limit the effect of the exposure. However, the result of this study also showed that the combined exposure induced oxidative damage not only in the liver and kidneys but also in the brain. In addition, the As content of brain in mice exposed to As individually or mixtures was significantly higher than those without As exposure and suggests that As can cross the blood brain barrier and accumulate in the brain.
In recent years, much attention has been given to understand As exposure impact on cholinergic mechanisms. AChE, an enzyme involved in the metabolism of acetylcholine and a neuromodulator at the cholinergic synapses, also plays a major role in synaptic plasticity, specifically in learning and memory [50], and its activity can be used as a biomarker of As neurotoxicity [51]. Decreased activity of AChE in the whole brain has been reported following exposure to As through drinking water in rats [52]. Exposure to As and gallium arsenite has been found to decrease the activity of AChE associated with impairment in learning and memory in rats [53]. All the results demonstrated that the AChE activity in the brain was significantly inhibited and suggested that brain AChE might be a major target after exposure to As. To date, AChE inhibition by phthalates in animal species has not be reported, except in aquatic animals. In our present study, no obvious decrease in AChE activity following DBP/MBP or As individual exposure, while AChE inhibition, was observed when DBP/MBP combined with As in the hippocampus. Also, the joint exposure has antagonistic effect. As the hippocampus has a crucial role to modulate learning and memory [54], damage to the cholinergic system in the hippocampus may be associated with learning and memory deficits as observed in the present study.
The role of free radicals and nitric oxide (NO) in the neurotoxicity of environmental chemicals and in the pathogenesis of neurodegenerative diseases is well accepted [53]. NO is an unstable molecule that plays key roles in morphogenesis and synaptic plasticity and acts as a messenger molecule or neurotransmitter in the brain [55]. However, involvement of neuronal nitric oxide synthase (nNOS) and NO levels has also been shown in As neurotoxicity [56]. It has been shown that As generates free radical species including hydroxyl radicals, superoxide anions, NO, and others and thus impairs the anti-oxidant system in the brain and other biological tissues [57]. Chattopadhyay et al. [58] in an interesting study on human fetal brain explants demonstrated that As exposure increased generation of NO associated with enhanced ROS and apoptosis. NO readily combines with DA and produces peroxynitrite anions and semiquinones, reactive species implicated to damage biological membranes [59]. Our results showed that elevated NOS levels occurred in brain due to the exposure to As, DBP, and MBP in combination as compared to the control group, indicating that neurotoxicity of combined exposure may involve changes in NO production. In this respect, the connection between learning and memory deficits, pathological alterations, over-expression of apoptosis-related protein, and oxidative stress injuries were investigated throughout this series of experiments.
Conclusions
In summary, although it is difficult to comment on the exact mechanism of neurotoxicity of DBP/MBP combined with As, our findings not only indicated that DBP/MBP combined with As at low doses (50 mg/kg, 50 mg/kg, 10 mg/L) impaired learning and memory capabilities in mice but also showed that this deficit in spatial cognition might be correlated to oxidative stress injuries causing neuronal apoptosis in the hippocampus; also, the joint exposure has antagonistic effect, and As may play an essential role in this deficit although the detailed mechanism needs more investigation. The results of the present study exhibited that DBP/MBP can stimulate the accumulation of As in the brain of mice.
References
Abernathy CO, Liu YP, Longfellow D, Aposhian HV, Beck B, Fowler B, Goyer R, Menzer R, Rossman T, Thompson C (1999) As: health effects, mechanisms of actions, and research issues. Environ Health Perspect 107:593–597
Rodriguez VM, Jimenez-Capdeville ME, Giordano M (2003) The effects of arsenic exposure on the nervous system. Toxicol Lett 145:1–18
Duker AA, Carranza EJM, Hale M (2005) Arsenic geochemistry and health. Environ Int 31:631–641
Tsai SY, Chou HY, The HW, Chen CM, Chen CJ (2003) The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology 24:747–753
Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A, Slavkovich V, Lolacono NJ, Cheng Z, Hussain I (2004) Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect 112:1329–1333
Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC (2006) Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. NeuroToxicology 27:210–216
Hamadani JD, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, Arifeen SE, Huda SN, Vahter M (2011) Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. Int J Epidemiol 40:1593–1604
Jin Y, Xi S, Li X, Lu C, Li G, Xu Y, Qu C, Niu Y, Sun G (2006) Arsenic speciation transported through the placenta from mother mice to their newborn pups. Environ Res 101:349–355
Xi S, Sun W, Wang F, Jin Y, Sun G (2009) Transplacental and early life exposure to inorganic arsenic affected development and behavior in offspring rats. Arch Toxicol 83:549–556
Mikula P, Svobodova Z, Smutna M (2005) Phthalates: toxicology and food safety—a review. Czech J Food Sci 23:217–223
Koch HM, Drexler H, Angerer J (2003) An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int J Hyg Environ Health 206:77–83
O’Connor JC, Frame SR, Ladics GS (2002) Evaluation of a 15-day screening assay using intact male rats for identifying antiandrogens. Toxicol Sci 69:92–108
Shultz VD, Phillips S, Sar M, Foster PMD, Gaido KW (2001) Altered gene profiles in fetal rat testes after in utero exposure to di(n-butyl) phthalate. Toxicol Sci 64:233–242
Li X, Jiang L, Cheng L, Chen H (2014) Dibutyl phthalate-induced neurotoxicity in the brain of immature and mature rat offspring. Brain and Development 36:653–660
Shen O, Du G, Sun H, Wu W, Jiang Y, Song L, Wang X (2009) Comparison of in vitro hormone activities of selected phthalates using reporter gene assays. Toxicol Lett 191:9–14
Weiss B (2011) Endocrine disruptors as a threat to neurological function. J Neurol Sci 305:11–21
Tanaka A, Matsumoto A, Yamaha T (1978) Biochemical studies on phthalic esters. III. Metabolism of dibutyl phthalate (DBP) in animals. Toxicology 9:109–123
Hallmark N, Walker M, McKinnell C, Mahood IK, Scott H, Bayne R, Coutts S, Anderson RA, Greig I, Morris K, Sharpe RM (2007) Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environ Health Perspect 115:390–396
Suk WA, Olden K, Yang RSH (2002) Chemical mixtures research: significance and future perspectives. Environ Health Perspect 110:891–892
Chen XM, An H, Ao L, Sun L, Liu WB, Zhou ZY, Wang YX, Cao J (2011) The combined toxicity of dibutyl phthalate and benzo(a)pyrene on the reproductive system of male Sprague Dawley rats in vivo. J Hazard Mater 186:835–841
Prasanth GK, Divya LM, Sadasivan C (2009) Effects of mono and di(n-butyl) phthalate on superoxide dismutase. Toxicology 262:38–42
Xu H, Shao X, Zhang Z, Zou Y, Chen Y, Han S, Wang S, Wu X, Yang L, Chen Z (2013) Effects of Di-n-butyl phthalate and diethyl phthalate on acetylcholinesterase activity and neurotoxicity related gene expression in embryonic zebrafish. Bull Environ Contam Toxicol 91:635–639
Chen X, Zhou QH, Leng L, Chen X, Sun ZR, Tang NJ (2013) Effects of di(n-butyl) and monobutyl phthalate on steroidogenesis pathways in the murine Leydig tumor cell line MLTC-1. Environ Toxicol Pharmacol 36:332–338
Roth KA (2001) Caspases, apoptosis, and Alzheimer disease: causation, correlation, and confusion. J Neuropathol Exp Neurol 60:829–838
Andersen JK (2001) Does neuronal loss in Parkinson’s disease involve programmed cell death? Bioessays 23:640–646
Lu K, Abo RP, Schlieper KA, Graffam ME, Levine S, Wishnok JS, Swenberg JA, Tannenbaum SR, Fox JG (2014) Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: an integrated metagenomics and metabolomics analysis. Environ Health Perspect 122:284–291
Mylchreest E, Wallace DG, Cattley RC, Foster PMD (2000) Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to di(n-butyl) phthalate during late gestation. Toxicol Sci 55:143–151
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in rat. J Neurosci Methods 11:47–60
Habibi E, Ghanemi K, Fallah-Mehrjardi M, Dadolahi-Sohrab A (2013) A novel digestion method based on a choline chloride-oxalic acid deep eutectic solvent for determining Cu, Fe, and Zn in fish samples. Anal Chim Acta 762:61–67
D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Rev 36:60–90
Lever C, Wills T, Cacucci F, Burgess N, O’Keefe J (2002) Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature 416:90–94
Tracy AL, Jarrard LE, Davidson TL (2001) The hippocampus and motivation revisited: appetite and activity. Behav Brain Res 127:13–23
Leib SL, Heimgartner C, Bifrare YD, Loeffler JM, Tauber MG (2003) Dexamethasone aggravates hippocampal apoptosis and learning deficiency in pneumococcal meningitis in infant rats. Pediatr Res 54:353–357
Magliozzi R, Howell OW, Reeves C, Roncaroli F, Nicholas R, Serafini B, Aloisi F, Reynolds R (2010) A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 68:477–493
Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S (2002) Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297:211–218
Clark RS, Kochanek PM, Chen M, Watkins SC, Marion DW, Chen J, Hamilton RL, Loeffert JE, Graham SH (1999) Increases in Bcl-2 and cleavage of caspase-1 and caspase-3 in human brain after head injury. FASEB J 13:813–821
Li ZG, Zhang WX, Grunberger G, Sima AAF (2002) Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res 946:221–231
Swanton E, Savory P, Cosulich S, Clarke P, Woodman P (1999) Bcl-2 regulates a caspase-3/caspase-2 apoptotic cascade in cytosolic extracts. Oncogene 18:1781–1787
Lossi L, Cantile C, Tamagno I, Merighi A (2005) Apoptosis in the mammalian CNS: lessons from animal models. Vet J 170:52–66
Del Razo LM, Quintanilla-Vega B, Brambila-Colombres E, Calderon-Aranda ES, Manno M, Albores A (2001) Stress proteins induced by arsenic. Toxicol Appl Pharmacol 177:132–148
Luna AL, Acosta-Saavedra LC, Lopez-Carrillo L, Conde P, Vera E, De Vizcaya-Ruiz A, Bastida M, Cebrian ME, Calderon-Aranda ES (2010) Arsenic alters monocyte superoxide anion and nitric oxide production in environmentally exposed children. Toxicol Appl Pharmacol 245:244–251
Larochette N, Decaudin D, Jacotot E, Brenner C, Marzo I, Susin SA, Zamzami N, Xie Z, Reed J, Kroemer G (1999) Arsenite induces apoptosis via a direct effect on the mitochondrial permeability transition pore. Exp Cell Res 249:413–421
Banerjee N, Banerjee M, Ganguly S, Bandyopadhyay S, Das JK, Bandyopadhay A, Chatterjee M, Giri AK (2008) Arsenic-induced mitochondrial instability leading to programmed cell death in the exposed individuals. Toxicology 246:101–111
Wang Y, Song L, Chen J, He J, Liu R, Zhu Z, Wang X (2004) Effects of di-butyl phthalate on sperm motility and oxidative stress in rats. Natl J Androl 10:253–256
Nagaraja T, Desiraju T (1994) Effects on operant learning and brain acetylcholine esterase activity in rats following chronic inorganic arsenic intake. Human Exp Toxicol 13:353–356
Sarvestani NN, Khodagholi F, Ansari N, Farimani MM (2013) Involvement of p-CREB and phase II detoxifying enzyme system in neuroprotection mediated by the flavonoid calycopterin isolated from Dracocephalum kotschyi. Phytomedicine 20:939–946
Lu TH, Tseng TJ, Su CC, Tang FC, Yen CC, Liu YY, Yang CY, Wu CC, Chen KL, Hung DZ, Chen YW (2014) Arsenic induces reactive oxygen species-caused neuronal cell apoptosis through JNK/ERK-mediated mitochondria-dependent and GRP 78/CHOP-regulated pathways. Toxicol Lett 224:130–140
Xu Z, Wang Z, Li JJ, Chen C, Zhang PC, Dong L, Chen JH, Chen Q, Zhang XT, Wang ZL (2013) Protective effects of selenium on oxidative damage and oxidative stress related gene expression in rat liver under chronic poisoning of arsenic. Food Chem Toxicol 58:1–7
Flora SJS, Bhadauria S, Pant SC, Dhaked RK (2005) Arsenic induced blood and brain oxidative stress and its response to some thiol chelators in rats. Life Sci 77:2324–2337
Lane RM, Kivipelto M, Greig NH (2004) Acetylcholinesterase and its inhibition in Alzheimer disease. Clin Neuropharmacol 27:141–149
Yousef MI, El-Demerdash FM, Radwan FME (2008) Sodium arsenite induced biochemical perturbations in rats: ameliorating effect of curcumin. Food Chem Toxicol 46:3506–3511
Kannan GM, Tripathi N, Dube SN, Gupta M, Flora SJS (2001) Toxic effects of arsenic (III) on some hematopoietic and central nervous system variables in rats and guinea pigs. J Toxicol Clin Toxicol 39:675–682
Flora SJS, Bhatt K, Mehta A (2009) Arsenic moiety in gallium arsenide is responsible for neuronal apoptosis and behavioral alterations in rats. Toxicol Appl Pharmacol 240:236–244
Butterweck V, Böckers T, Korte B, Wittkowski W, Winterhoff H (2002) Long-term effects of St. John’s wort and hypericin on monoamine levels in rat hypothalamus and hippocampus. Brain Res 930:21–29
Chen SM, Swilley S, Bell R, Rajanna S, Reddy SLN, Rajanna B (2000) Lead induced alterations in nitrite and nitrate levels in different regions of the rat brain. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 125:315–323
Rios R, Zarazúa S, Santoyo M, Sepúlveda-Saavedra J, Romero-Díaz V, Jiménez V, Pérez-Severiano F, Vidal-Cantú G, Delgado J, Jiménez-Capdeville M (2009) Decreased nitric oxide markers and morphological changes in the brain of arsenic-exposed rats. Toxicology 261:68–75
Barchowsky A, Dudek EJ, Treadwell MD, Wetterhahn KE (1996) Arsenic induces oxidant stress and NF-KB activation in cultured aortic endothelial cells. Free Radic Biol Med 21:783–790
Chattopadhyay S, Bhaumik S, Nag Chaudhury A, Das Gupta S (2002) Arsenic induced changes in growth development and apoptosis in neonatal and adult brain cells in vivo and in tissue culture. Toxicol Lett 128:73–84
Antunes F, Nunes C, Laranjinha J, Cadenas E (2005) Redox interactions of nitric oxide with dopamine and its derivatives. Toxicology 208:207–212
Acknowledgements
This work was supported financially by the Priority Academic Program Development of Jiangsu Higher Education Institutions, Collaborative Innovation Center of Technology and Material of Water Treatment, Specialized Research Fund for the Doctoral Program of Chinese Universities from the Ministry of Education (20113227110020).
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Guanghua Mao and Zhaoxiang Zhou co-first authors.
Guanghua Mao and Zhaoxiang Zhou contributed equally to this work.
Rights and permissions
About this article
Cite this article
Mao, G., Zhou, Z., Chen, Y. et al. Neurological Toxicity of Individual and Mixtures of Low Dose Arsenic, Mono and Di (n-butyl) Phthalates on Sub-Chronic Exposure to Mice. Biol Trace Elem Res 170, 183–193 (2016). https://doi.org/10.1007/s12011-015-0457-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0457-6