Abstract
People in Bangladesh are often exposed to low to high levels of multiple metals due to contaminated groundwater with various heavy metals such as arsenic (As), lead (Pb), and manganese (Mn). However, the effects of concomitant exposure of these three metals on neurobehavioral changes are yet to be studied. Therefore, this study was intended to assess the neurotoxic effect of As, Pb, and Mn in a mouse model. Elevated plus maze (EPM) and Morris water maze (MWM) tests were conducted to evaluate anxiety, learning, and spatial memory impairment, respectively. The mice exposed to a combination of metals spent least time exploring the open arms and had longer latencies to find the hidden platform than the control and individual metal exposure groups in EPM and MWM tests. Moreover, concomitant multi-metal exposure remarkably decreased the activities of cholinergic and antioxidant enzymes, brain-derived neurotropic factor (BDNF), and nuclear factor erythroid 2–related factor 2 (Nrf2) levels and significantly increased interleukin-6 (IL-6) level in the brain tissue compared to the control and individual metal-exposed mice. Among the mice treated with a single metal, the As-treated mice showed the highest toxic effects than Pb- or Mn-treated mice. Taken together, the present study demonstrated that exposure to a mixture of As, Pb, and Mn, even at lower doses than individual metals, significantly augmented anxiety-like behavior and impaired learning and spatial memory compared to exposure to individual metals, which was associated with the changes of BDNF, Nrf2, IL-6 levels, and related enzyme activities in the brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Groundwater is the primary source of drinking water for people in rural areas of Bangladesh and people use tube-well water as their key source of drinking water [1]. Unfortunately, this water is contaminated with harmful elements, especially arsenic (As) in many parts of Bangladesh [2]. Drinking of As-contaminated water caused human suffering and also posed a socio-economic challenge for the affected countries like Bangladesh. For a long time, high As-contaminated drinking water intake has been linked with many kinds of cancers and non-cancerous illnesses, such as peripheral neuropathy, dermatological conditions, cardiovascular disease, and diabetes [3, 4]. Moreover, chronic As exposure leads to As deposition in a number of susceptible organs and thereby impairing the specific activities of those organs [5, 6]. For instance, in As exposure experimental mice, As crosses the blood–brain barrier and deposits in the brain, causing reactive oxygen species (ROS) generation, which in turn leads to anxiety-like disorders, shortages in normal locomotion, and spatial memory development [7]. Furthermore, higher concentration and prolonged duration of As exposure affect neurological and cognitive impairment both in humans and rodents [4]. Children’s intellectual function is obstructed by continuous As exposure at moderate to high dosages, and there is an age-dependent link between As exposure and cognition [8].
Aside from arsenic, high amounts of manganese (Mn), lead (Pb), nickel (Ni), chromium (Cr), and other inorganic compounds have been identified in groundwater in many parts of Bangladesh [7, 9]. Previous research found that water from tube-wells in various parts of Bangladesh contained higher amounts of Mn and Pb above the permissible limit [7, 9, 10]. Mn is a necessary mineral that the body uses for a variety of purposes; however, long-term exposure to excessive Mn is linked to cognitive decline because it damages the central nervous system [11]. For example, chronic Mn exposure can impair locomotor activity in experimental animals by exacerbating neurodegenerative damage [9, 12]. Human exposure to higher Mn concentration impairs attention and memory functioning [13]. According to a number of studies, excessive Mn accumulation in the brain results in damage to the hippocampal region followed by cognitive decline. Reactive nitrogen species, dopaminergic neurodegeneration, and the production of oxygen and reactive nitrogen free radicals are all connected to Mn-induced neurotoxicity [11, 14]. Furthermore, Pb is also a highly toxic metal and can accumulate in air, soil and groundwater [7, 15]. Excessive exposure to Pb leads to oxidative stress through lipid peroxidation, resulting in the generation of reactive oxygen species [16]. In addition, chronic exposure to Pb is associated with the deposition in the soft organs of the animal body, which in turn exerts toxic effects on cardiovascular, hepatic, and nervous systems [15, 17]. Results from epidemiological and experimental studies stated that Pb exposure has been linked to neurodevelopmental disorders, neurodegenerative diseases, and cognitive impairment [18]. For instance, in both humans and laboratory animals, Pb can deposit in the brain and induces neurobehavioral and biochemical alterations leading to Alzheimer’s disease, Parkinson’s disease, and other neurological illnesses like behavioral issues, mental retardation, nerve damage, etc. [18, 19].
People are frequently exposed to a variety of heavy metals, including As, Mn, Pb, etc. Prolonged exposure to these metals raises the risk of developing serious illnesses and disorders [20]. For example, chronic co-exposure of As and cadmium (Cd) enhances nephrotoxicity more vigorously than individual metal exposure in experimental animals [21]. Another study reported that concomitant exposure to As, Pb, Cd, and mercury (Hg) is associated with deteriorated renal parameters in adolescents [22]. Additionally, As, Mn, and Pb exposures can induce neurotoxicity by reducing the expression of tyrosine hydroxylase and vesicular monoamine transporter in the striatum of mice [20]. On the other hand, in laboratory animals, concurrent exposure to As and Pb had antagonistic effects on neurobehavioral activities and blood indices linked to liver and brain functioning [7]. Compared to the effects of the two metals alone, exposure to both As and Pb reduced the effects related hepatic and neurological toxicity [7, 23]. Also, our recent study demonstrated that co-exposure to As plus Mn and As plus Pb can attenuate the effects of As-induced neurobehavioral and biochemical changes in mice [1, 7]. Thus, the effects of a mixture of As, Pb, and Mn exposures on animal models are inconsistence as evident by the limited number of research. Furthermore, to the best of our knowledge, the effects of exposure to As, Mn, and Pb at relatively lower doses on neurobehavioral changes have not been investigated yet in animal models. Therefore, this study is undertaken to evaluate the combined impact of As, Mn, and Pb on anxiety-like behavior, learning, and memory impairment using a mouse model.
Materials and Methods
Experimental Design and Animal Maintenance
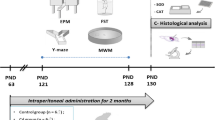
Swiss Albino male mice were collected from the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b). Animals were acclimatized for seven days and divided into five groups: (a) control, (b) As-treated, (c) Pb-treated, (d) Mn-treated, and (e) As + Mn + Pb–treated. Each metal-exposed and control group was composed of eight mice. Sodium arsenite, lead acetate, and manganese chloride (10 mg/kg body weight) were provided to the stomach of the mice through an oral gavage tube, once a day for 30 days. The co-exposed (As + Mn + Pb) group received the three metals as total of 10 mg/kg body weight (equal concentration), whereas the control group received only distilled water as a vehicle. The doses of the metals for individual and multi-metal exposure groups were selected from the articles published previously [1, 7, 24]. All experimental mice were housed in plastic cages in the animal house with unlimited access to icddr,b formulated rodent food pellet and distilled water throughout the experimental period as described earlier [9]. The animal experimental design and timeline are shown in Fig. S1. Behavioral assays were carried out during the light cycle between 9:00 am and 4.00 pm, and experimental mice were moved to the behavior testing room 30 min before to start experiment as mentioned previously [1]. All animal experiments were carried out in accordance with the ethical requirements for animal experimentation guidelines of the Institute of Biological Sciences, University of Rajshahi, Bangladesh (No.: 110(16)/320/IAMEBBC/IBSc).
Assessing Anxiety-Like Behavior in EPM
The elevated plus maze (EPM) test is a method to measure rodent’s anxiety response. The EPM is a lab instrument with two open arms (50 × 10 cm), two closed arms (50 × 10 × 40 cm), and a plus sign of 50 cm above the floor [12]. In this experiment, each mouse was positioned on the center of the platform facing its closed arm, and it was given 5 min to explore the maze. Spent time in both arms of each mouse of every group during the given time was recorded [7]. When all four paws were on closed arm, the mouse was said to have entered a closed arm [9]. After every test, the maze was cleaned with 70% ethanol to prevent results from being influenced by smell cues. Anxiety is assessed as experimental animals spend more time in the closed arm than in the open arm of EPM [1].
The MWM Test for Learning and Memory Evaluation
The Morris water maze (MWM) is a widely used method for learning and spatial memory test of rodents. On the 31st day following the start of the experiment, a MWM test was conducted to evaluate the toxic effect of co-exposure to three metals on learning and spatial memory impairment in mice. The water maze consists of a large black circular pool of 120 cm in diameter, filled with water to a depth of 30 cm at a temperature of 25 ± 2 °C. A black platform with a diameter of 8 cm is fixed in the center of the pool’s northeast (NE) quadrant, 1 cm below the water’s surface. Mice from respective groups were put into the maze to assess their ability to learn and remember objects in space. A visual indication posted on the inside of the pool wall, and every trail had a separate starting position. On the first 2 days, every mouse in every experimental group was trained to locate the platform. There was a 60-s time limit (cutoff time) to locate the secret platform and 20 s to stay on it. An experimenter placed mice on the underwater platform and gave them 20 s to stay there if they could not locate it in the allotted time. The experimenter was blinded to the experimental dose and groups. The latency of each mouse to find the platform was recorded using stopwatch. Every day, three trials were conducted daily following a 30-min break as previously mentioned [1]. Mice were towel-dried after each maze test and kept in a heated cage for a minimum of 5 min before being put back in their home cage. Each trial’s escape latency to discover the platform was noted, and the mean latency time of the experimental animals per day was calculated manually by averaging the three trials [1]. Mice that did not find the platform within the cutoff time at least twice out of three trials were excluded from the consecutive day of testing [7]. Two mice from each group failed to meet the criteria and were excluded from data analysis. A considerable decrease in escape latency as compared from the initial session was revealed as actual learning. Following a week of training, a prob test was conducted without the platform. Out of the 60 s, the amount of time spent in the preferred quadrant was noted; more time spent in the target quadrant was associated with improved performance on probe test.
Biochemical Examination of Experimental Mice Brain Tissue Homogenates
The whole mouse brain of each mouse was taken from all experimental groups after being anesthetized with diethyl ether. Brains were then rinsed with phosphate-buffered saline (PBS) and weighed with an electronic balance and homogenized using a blender on ice, resuspended with PBS (0.1 M, pH 7) containing 0.5% Triton X100 (Sigma-Aldrich, Germany). Subsequently, the homogenates underwent a 30-min centrifugation at 2660 g and 4 °C, and the supernatant was extracted for biochemical parameter analysis as described earlier [25]. Concentration of total protein, activities of acetylcholinesterase (AChE), butyrylcholinesterase (BChE), superoxide dismutase (SOD), and reduced glutathione reductase (rGR) in experimental mice brain tissue homogenates were analyzed as the methods outlined in Islam et al. [26]. Brain-derived neurotrophic factor (BDNF—catalog no. BDNF- E-El-M0203, lot no. 1PUVMHWDL4), nuclear factor-erythroid factor 2–related factor 2 (Nrf2—catalog no. E-EL-M2607, lot no. KL14POF60144), and interleukin-6 (IL-6—catalog no. E-EL-M0044, lot no. XF34HL047424) proteins were measured using ELISA kits (Elabscience, USA), respectively, according to the manufacturer’s protocol.
Statistical Analysis
The standard error of the mean (mean ± SEM) is used to present all data. Tukey’s multiple comparisons, and repeated measures ANOVA tests were used to determine the statistical significance between the experimental groups. P values less than 0.05 were considered statistically significant. GraphPad Prism 7.05 was used to create the graphs and analyze the data.
Results
Multi-metal Exposure Induces Synergistic Anxiety-Like Behavior in Mice
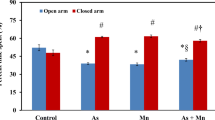
The result of the EPM test of metal-exposed and control mice is shown in Fig. 1. The percentages of time in open arms for control mice and mice treated with As, Mn, Pb, and As + Mn + Pb groups were 50.72 ± 1.09, 35.79 ± 1.71, 42.78 ± 1.318, 39.78 ± 1.731, and 28.61 ± 1.40, respectively. On the other hand, the percentages of time spent in closed arms of the four groups were 49.28 ± 1.09, 64.21 ± 1.71, 57.22 ± 1.318, 60.22 ± 1.731, and 71.39 ± 1.40, respectively. The findings demonstrated that, in comparison to control, individual exposure to As, Pb, and Mn groups considerably reduced the time spent in open arms and increased the time spent in closed arms. Most importantly, multi-metal-exposed mice spent significantly less time in open arms compared to individual metal-exposed mice as well as the control mice [F (4,25) = 31.13; p < 0.0001], although the concentrations of As, Pb, and Mn in combined exposure were two-thirds lower than those of individual metals.
Percentage of time spent in open of multi-metal-exposed mice in EPM. The time spent in open arms of control (C), arsenic (As), manganese (Mn), lead (Pb), and As + Mn + Pb–exposed mice groups is presented in dot plots. The data were expressed as mean ± SEM (where n = 6). Using Tukey’s multiple comparison test, groups were compared (****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05) and ordinary one-way ANOVA (p < 0.0001)
Multi-metal Exposure Induces Robust Learning and Memory Impairment in the MWM Test
The Morris water maze (MWM) is a neuropsychological test used to evaluate spatial memory and learning ability in experimental animals. The mean latency time for control animals to find the platform on day 1 was 34.194 ± 2.430 s, which decreased rapidly over the next 7 days, and the time on day 7 was 11.833 ± 0.749 s (Fig. 2A). On the other hand, the mean latency times of As-, Mn-, Pb-, and As + Pb + Mn–exposed groups were 37.556 ± 2.252, 32.694 ± 2.252, 36.750 ± 2.383, and 43.500 ± 2.487 s, respectively, on day 1 [F (4,25) = 3.091, p = 0.0338] and those were 25.722 ± 0.969, 16.583 ± 0.871, 20.500 ± 1.190 and 30.917 ± 1.625 s, respectively, on day 7 [F (4,25) = 44.41 p < 0.0001] (Fig. 2A). These results showed that after 7 days of learning, the mean latency times of the As-, Pb-, and Mn-exposed groups were slightly reduced and required more time than the control group. Statistically significant (p < 0.05) changes were found in the mean latency times at day 7 of the As-, Pb-, Mn-, and As + Mn + Pb–exposed groups compared to the control group. However, mice of multi-metal-exposed group had longer mean latency times all over the 7-day course compared to the control. Also, the latency time of As + Mn + Pb–exposed group was significantly (p < 0.05) longer than the individual metalexposed groups from days 3 to 7.
Effect of multi-metal exposure on learning and memory impairment of mice in MWM. A Latency time of control (C), arsenic (As), manganese (Mn), lead (Pb), and As + Mn + Pb–exposed mice were expressed as mean ± SEM, where n = 6 for each group of mice. Mice were trained three times a day. *Significantly distinct from control group at p < 0.05 in repeated measures ANOVA test. #Significantly distinct from control group at p < 0.05 in ordinary one-way ANOVA. B Morris’s water maze probe trial for experimental mice. Mean ± SEM was used to express the time spent in the NE quadrant of the control (C), arsenic (As), manganese (Mn), lead (Pb), and As + Mn + Pb groups. The dot plots correspond to Fig. 2. Tukey’s multiple comparison test (*p < 0.05, ***p < 0.001, ****p < 0.0001) and ordinary one-way ANOVA (p < 0.0001) were used to compare groups
On the probe day, following the 7-day trial, the platform was removed from the pool and a probe test was performed to assess mouse learning ability. The time spent in the preferred quadrant of all the experimental mice groups was measured and presented in Fig. 2B. The time spent in the desired quadrant by control, As-, Mn-, Pb-, and As + Mn + Pb–exposed mice were 45.17 ± 1.046, 34.83 ± 0.8333, 41.5 ± 1.088, 38.00 ± 0.9309, and 30.83 ± 0.8724 s, respectively. The findings demonstrated that, in comparison to the control mice, the As-, Pb-, Mn-, and As + Mn + Pb–exposed animals spent comparatively less time in the desired quadrant [F (4,25) = 33.96, p < 0.0001]. Moreover, the combined exposure mice spent less time in the preferred quadrant than the individual metal-exposed groups, indicating that spatial memory and learning impairments were more obvious in the multi-metal treated mice than in the individual metal-treated group (p < 0.05).
Multi-metal Exposure Reduced the Cognitive Marker—BDNF Expression in the Brain
BDNF is one of the most crucial elements in the development of memory and neural plasticity. BDNF levels in the brain tissue of control, As-, Mn-, Pb-, and As + Mn + Pb–treated groups were 538.50 ± 29.19, 310.83 ± 12.19, 454.13 ± 16.77, 335.50 ± 24.45, and 246.63 ± 6.473 pg/mg, respectively (Fig. 3). A markedly decreased amount of BDNF was found in the brain tissue of all three metal-exposed mice groups. Among the individual metal-exposed groups, a low level of BDNF was found in the As-exposed mice brain. Moreover, multi-metal exposure significantly reduced BDNF levels in the brain tissue compared to Mn- and Pb-exposed mice (p < 0.05).
BDNF levels in the brain tissue of experimental mice. The mice in the control (C), arsenic (As), manganese (Mn), lead (Pb), and As + Mn + Pb groups were expressed as mean ± SEM, with n = 6. A one-way ANOVA (p < 0.0001) and Tukey’s multiple comparison test (*p < 0.05, ****p < 0.0001) revealed a significant difference among means
Reduction of Cholinesterase Activity in the Brain of Multi-metal-Exposed Mice
In general, cholinesterase activity is used as a biomarker of cognitive impairment. The impact of multi-metal exposure on the activity of cholinesterase (AChE and BChE) in the brain of experimental mice is shown in Fig. 4. The result showed that AChE activity was 150.914 ± 4.09, 112.953 ± 2.03, 131.790 ± 2.36, 121.580 ± 2.90, and 105.284 ± 2.12 mU/mg in the brain tissue of control, As-, Mn-, Pb-, and As + Mn + Pb–treated groups mice, respectively. A considerably lower AChE activity was found in the brain of the multi-metal-exposed mice compared to control mice and all individual metal-exposed mice groups [F (4,25) = 40.21, p < 0.0001]. Similarly, a remarkable decreased BChE activity was noted in multi-metal-exposed mice brains compared to control and individual metal-exposed mice groups [F (4,25) = 31.73, p < 0.0001]. In addition, multi-metal exposure significantly reduced AChE and BChE activity in the brain tissue compared to Mn- and Pb-exposed mice (p < 0.01).
Cholinesterase activity in brain tissue in experimental mice. A AChE and B BChE. The mean ± standard error of mean (SEM) was expressed for the control (C), arsenic (As), manganese (Mn), lead (Pb), and As + Mn + Pb groups, with n = 6 for each. Tukey’s multiple comparison test (****p < 0.0001, ***p < 0.001, **p < 0.01) and ordinary one-way ANOVA (p < 0.0001) revealed significantly different means
Exposure to Multi-metals Decreased the Amount of Nrf2 in the Mouse Brain
Nrf2 is a crucial component of antioxidant defense as it can trigger the transcription of antioxidant enzymes. The effects of heavy metal exposure on Nrf2 level in the brain tissue of experimental mice are shown in Fig. 5. Nrf2 levels of control, As-, Mn-, Pb-, and As + Mn + Pb–treated groups were 10.585 ± 0.881, 3.944 ± 0.311, 7.274 ± 0.503, 5.401 ± 0.342, and 2.661 ± 0.256 ng/mg, respectively. Significantly reduced Nrf2 levels were detected in the brain tissue of metal-exposed mice compared to control mice [F (4,25) = 36.67, p < 0.0001]. Among the individual metal exposure groups, the lowest level of Nrf2 was found in the brain of As-exposed mice. Moreover, mice co-exposed to multi-metals showed a significant reduction of Nrf2 protein in the brain tissue compared to individual metal (Pb and Mn)-exposed mice (p < 0.01), indicating severe destruction of the antioxidant system in the brain.
Brain tissue Nrf2 levels in experimental mice. The mean ± standard error of mean (SEM) for each group of mice — control (C), arsenic (As), manganese (Mn), lead (Pb), and combined group (As + Mn + Pb) was determined, where n = 6 for each group. Tukey’s multiple comparison test (****p < 0.0001, ***p < 0.001, **p < 0.01) and ordinary one-way ANOVA (p < 0.0001) revealed significant differences between means
Multi-metal Exposure Disrupted the Oxidative System in the Brain
Multi-metals induced significant reductions of both SOD and rGR activity in the brain tissue compared to the control and the individual As-, Mn-, and Pb-exposed mice brains (p < 0.05) (Fig. 6). The brain tissue of mice exposed to multi-metals showed a substantial decrease in SOD activity compared to control animals [F (4,25) = 27.15, p < 0.0001]. Similarly, rGR activity was significantly decreased in the brain tissue of multi-metal-exposed mice compared to control mice [F (4,25) = 39.40, p < 0.0001]. It was noted that the exposure of As + Mn + Pb significantly decreased both enzyme activities in the brain tissue when compared with Mn and Pb exposures (p < 0.01).
Activity of antioxidant enzymes A SOD and B rGR in the brain tissue of experimental mice. Control (C), arsenic (As), manganese (Mn), lead (Pb), and As + Mn + Pb-exposed mice were stated as mean ± SEM, where n = 6 for each group of mice. Significantly different among means were conducted by Tukey’s multiple comparison test (****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05) and ordinary one-way ANOVA (p < 0.0001)
Multi-metal Exposure Enhances Inflammation in the Brain
IL-6, a well-established inflammatory marker, is associated with pathophysiological processes of tissue damage. A significant increment of IL-6 levels was noted in the brain tissue of As-, Mn-, Pb-, and As + Mn + Pb–exposed groups of mice (Fig. 7). Results showed that IL-6 level was higher in the As-exposed mice brain among the individual metal exposure groups; however, the highest level was detected in the multi-metal-exposed mice. It is noted that a significantly increased IL-6 level was detected in the brain tissue of multi-metal-exposed mice compared to Mn- and Pb-exposed mice (p < 0.05). The elevation of IL-6 level indicates severe inflammatory response in multi-metal-exposed mice.
IL-6 levels in brain tissue of experimental mice. Control (C), arsenic (As), manganese (Mn), lead (Pb), and As + Mn + Pb mice were expressed as mean ± SEM, where n = 6 for each group of mice. Significantly different means were conducted by Tukey’s multiple comparison test (****p < 0.0001, ***p < 0.001, *p < 0.05) and ordinary one-way ANOVA (p < 0.0001)
Discussion
Concomitant exposure to several heavy metals through water, air, or food poses a serious health burden by affecting various organs. Consequently, immune system, gastrointestinal and kidney dysfunction, nervous system disorders, vascular damage, skin lesions, cancer, and birth defects are examples of complications associated with heavy metal mediated toxicity [27]. Numerous studies have documented the detrimental effects of individual As, Pb, and Mn on changes in neurobehavior [1, 7, 9]. In this study, we examined the combined effects of three heavy metals (As, Pb, and Mn) at low dosages on neurotoxicity in mice that are present in groundwater in several locations of Bangladesh. Exposure to heavy metals might be an important cause of neurological disorders in individuals, and MWM and EPM tools are used to assess neurological disorder associated behavioral parameters (e.g., anxiety-like behavior, learning, and spatial memory) in rodents [7, 18]. Anxiety is a feeling of distress, fear, and disquiet, and it is noted that As, Pb, and Mn exposures develop anxiety and stress in mice [1, 18]. Similarly, in the current study, we have noted that the exposure of individuals to As, Pb, and Mn induces anxiety in mice. Most importantly, we found that multi-metal exposure developed severe anxiety-like behavior at lower doses of multi-metal than the individual metals at higher doses. In addition, multi-metal-exposed mice showed significantly higher impairment of learning and memory development compared to individual metal-exposed mice in the MWM test. Previous reports showed that mice exposed to mixtures of As and Pb or As and Mn metals had less anxiety-like behavior and less memory impairment than As-exposed mice, as well as As induces severe disturbance in mice among the tested metals [1, 7]. Interestingly, it has been reported that Pb decreased the concentration of As in the brain of adult mice co-exposed to As with Pb when compared with the same dose of As in concomitant exposure [28]. However, the findings of the current study showed that exposure to a mixture of three metals (As, Pb, and Mn) intensified the neurotoxic effects of the individual metal on anxiety-like behavior, learning, and memory impairment even though they were at lower concentrations in the mixture.
Learning and memory are the key sub-components of cognitive functions, and BDNF is a key molecule associated with neuronal plasticity changes and plays a crucial role in learning and memory development [29]. In comparison to control mice, the brain tissue of each metal-exposed mouse in this study showed a lower amount of BDNF, which is linked to learning and memory deficits. Moreover, BDNF level was remarkably diminished in the brain tissue of the multi-metal-exposed mice compared to all other experimental groups. Accordingly, these mice had the poorest learning capacity and highest memory impairment among the experimental mice groups. Reduction of BDNF level causes aggregation of amyloid-β, induces apoptosis of nerve cells and inflammation in the brain, and ultimately impairs memory consolation in the brains of experimental animals [26]. Also, patients with cognitive impairments such as Parkinson’s, Alzheimer’s, and Huntington’s diseases have shown decreased BDNF levels [30]. Therefore, the findings of our study imply that multi-metal exposure inhibits BDNF expression and thereby diminishes BDNF-mediated memory functions.
A reduced cholinesterase activity in the brain tissue is associated with increased inflammation and oxidative stress, which in turn impairs memory development and capacity for learning [26]. For instance, reduced levels of AChE and BChE activity have been associated with heavy metals–induced neurotoxicity in human and experimental animals [7, 9]. Furthermore, it has been shown that As and Pb suppress both monoaminergic and cholinergic function in the hippocampus of lab animals [31]. Similarly, in this study, we found remarkably decreased AChE and BChE activity in brain tissue at lower levels of As + Mn + Pb–exposed mice. Cholinergic dysfunction is characterized in Alzheimer’s disease, and decreased BChE activity is associated with Aβ pathology [32] and impaired learning and memory formation in laboratory animals [33, 34].
Endogenous antioxidant enzyme systems scavenge increased free radicals and maintains redox homeostasis in living cells. As exposure causes oxidative stress by disrupting the pro/antioxidant balance and has a significant impact on disease presentations, particularly nervous system illnesses [35]. Furthermore, oxidative stress induced by As, Pb, or Mn exhibits a vital role in the pathogenesis of neurodegenerative processes and also the development of anxiety and impairment of cognitive functions in experimental animals [36, 37]. ROS levels above a certain threshold damage memory formation by altering the hippocampus and signaling molecules involved in synaptic plasticity [38]. Here, we have found significantly reduced SOD and rGR activity in brain tissues of the mice exposed to individual and mixture of three metals. Importantly, the tested antioxidant enzyme activities were significantly decreased in multi-metal-exposed mice, indicating higher levels of ROS generation in the brains of these mice. Transcription factor Nrf2 controls the expression of proteins involved in the antioxidant system, thus providing protection against ROS-induced injury and inflammation [39]. Cellular oxidative stress causes Nrf2 translocation and binding to the antioxidant response element (ARE), which stimulates the production of antioxidant-related genes [40]. In our study, we have observed a noteworthy reduction of Nrf2 level in the brain of multi-metal-exposed mice compared to individual metal (Mn and Pb) and control mice. The lowest amount of Nrf2 in the brain of multi-metal-exposed mice suggests the highest damage to brain tissue in these mice through the severest ROS production. Aforementioned studies have confirmed that the reduction of Nrf2 expression in brain tissue inactivates the Nrf2-signaling pathway, resulting in impaired cognitive functions and depression-like behavior manifestation [41]. In the current study, we have noted a higher level of IL-6 with a lower Nrf2 level in the brain of multi-metals exposed mice in comparison to that of Mn- and Pb-exposed as well as control mice. An increased risk of neuropsychiatric conditions including depression and Alzheimer’s disease is linked to elevated IL-6 levels [42, 43]. Also, Nrf2 inactivation promotes the upregulation of IL-6 expression [44]. Thus, multi-metal exposure could disrupt the antioxidant system and increase the inflammation that induces cell damage and organ dysfunction, via perturbing IL-6 and Nrf2 expressions. Therefore, the enhancement of severe anxiety-like behavior and memory impairment in multi-metal-exposed mice is consistent with previous reports on the roles of BDNF, cholinesterase, ROS, Nrf2, and IL-6.
The BDNF protein is primarily active at the synaptic junctions, mediates cellular communication and signaling, and influences synaptic plasticity directly. Concomitant exposure to As, Pb, and Mn downregulated BDNF and cholinergic enzyme system in the brain compared to individual metal exposure. Furthermore, compared to single metal exposure, multi-metal exposure significantly weakened the antioxidant system by downregulating Nrf2 in brain regions. This may be due to the accumulation of high amounts of toxic metals in the organs, which in turn induced inflammation through upregulation of inflammatory cytokine IL-6 expression. Therefore, upregulation of IL-6 followed by reduction of BDNF and Nfr2 along with reduced antioxidant enzymes activity in the brain implied severe neurotoxicity in multi-metal-exposed animals.
The concentrations of As, Mn, and Pb used in this study were apparently higher than those of environmental doses, although the concentrations of the metals in drinking water or other environmental sources varies based on the geographical, anthropogenic activities, and many other reasons. In our population-based study, the highest concentration of arsenic observed in one tube well water was approximately 1800 µg/L which was much higher than maximum permissive limit of arsenic in drinking water (10 µg/L) set by WHO [45, 46]. The analysis of the effects at low concentration of any environmental chemicals is the best approach; however, in the case of short-term exposure, low concentration may not show effects, and in some endemic areas, as we mentioned above, the people are exposed to very high concentration of environmental chemicals. Therefore, even we used apparently high doses of metals, but the doses may not be completely non-relevant to environmental dose. Future research is required to address the two important issues with this study. First, it is plausible to measure the accumulation of metals in brain, particularly to correlate the concentrations of metals in brain with neurobehavioral toxicity. Second, a dose-dependent investigation is required to identify the exact dose relevant to the human exposure to environmental As, Pb, and Mn. Humans are typically exposed to a variety of metals simultaneously at different concentrations. However, it is rather difficult to assess the combined effects or to distinguish the effects of one metal from other. Therefore, despite the aforementioned limitations, the results of this study provide some important insights into the neurobehavioral toxicity caused by multi-metal exposure.
Conclusion
The current study found that individual exposure to As, Pb, and Mn resulted in altered biochemical indices linked to oxidative stress, inflammation, and neurotoxicity, as well as anxiety-like behavior and reduced spatial memory and learning. The effects of arsenic (As) were more noticeable than those of Pb and Mn. However, a mixture of these toxic metals at lower doses than individual metals increased the severity of toxicity for developing anxiety and impairing learning and memory, which were associated with greater changes in biochemical indices, such as BDNF, Nrf2, cholinesterase, ROS, and IL-6.
Data Availability
No datasets were generated or analysed during the current study.
References
Biswas S, Anjum A, Banna HU, Rahman M, Siddique AE, Karim Y, Nikkon F, Haque A, Hossain K, Saud ZA (2019) Manganese attenuates the effects of arsenic on neurobehavioral and biochemical changes in mice co-exposed to arsenic and manganese. Environ Sci Pollut Res 26:29257–29266
Ahmad SA, Khan MH, Haque M (2018) Arsenic contamination in groundwater in Bangladesh: implications and challenges for healthcare policy. Risk Manag Healthc Policy 11:251–261
Parvez F, Chen Y, Argos M, Hussain AZ, Momotaj H, Dhar R, van Geen A, Graziano JH, Ahsan H (2006) Prevalence of arsenic exposure from drinking water and awareness of its health risks in a Bangladeshi population; results from a large population-based study. Environ Health Perspect 114:355–359
Tyler CR, Allan AM (2014) The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep 1:132–147
Lewchalermvong K, Rangkadilok N, Nookabkaew S, Suriyo T, Satayavivad J (2018) Arsenic speciation and accumulation in selected organs after oral administration of rice extracts in Wistar rats. J Agric Food Chem 66:3199–3209
Rahman MM, Mandal BK, Chowdhury TR, Sengupta MK, Chowdhury UK, Lodh D, Chanda CR, Basu GK, Mukherjee SC, Saha KC, Chakraborti D (2003) Arsenic groundwater contamination and sufferings of people in North 24-Parganas, one of the nine arsenic affected districts of West Bengal, India. J Environ Sci Health A Tox Hazard Subst Environ Eng 38:25–59
Aktar S, Jahan M, Alam S, Mohanto NC, Arefin A, Rahman A, Haque A, Himeno S, Hossain K, Saud ZA (2017) Individual and combined effects of arsenic and lead on behavioral and biochemical changes in mice. Biol Trace Elem Res 177:288–296
Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A, Slavkovich V, Lolacono NJ, Cheng Z, Hussain I, Momotaj H (2004) Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect 112:1329–1333
Anjum A, Biswas S, Rahman M, Rahman A, Siddique AE, Karim Y, Aktar S, Nikkon F, Haque A, Himeno S, Hossain K, Saud ZA (2019) Butyrylcholinesterase-a potential plasma biomarker in manganese-induced neurobehavioral changes. Environ Sci Pollut Res 26:6378–6387
Bhuiyan MA, Islam MA, Dampare SB, Parvez L, Suzuki S (2010) Evaluation of hazardous metal pollution in irrigation and drinking water systems in the vicinity of a coal mine area of northwestern Bangladesh. J Hazard Mater 179:1065–1077
Liang G, Qin H, Ma S, Huang X, Lv Y, Qing L, Li Q, Huang Y, Chen K, Huang Y, Shen Y (2015) Effects of chronic manganese exposure on the learning and memory of rats by observing the changes in the hippocampal cAMP signaling pathway. Food Chem Toxicol 83:261–267
Bardullas U, Limón-Pacheco JH, Giordano M, Carrizales L, Mendoza-Trejo MS, Rodríguez VM (2009) Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol Appl Pharmacol 239:169–177
Bouabid S, Tinakoua A, Lakhdar-Ghazal N, Benazzouz A (2016) Manganese neurotoxicity: behavioral disorders associated with dysfunctions in the basal ganglia and neurochemical transmission. J Neurochem 136:677–691
Zhang Q, Liu J, Duan H, Li R, Peng W, Wu C (2021) Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J Adv Res 34:43–63
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5:47–58
Sharma P, Chambial S, Shukla KK (2015) Lead and neurotoxicity. Ind J Clin Biochem 30:1–2
Xia D, Yu X, Liao S, Shao Q, Mou H, Ma W (2010) Protective effect of Smilax glabra extract against lead-induced oxidative stress in rats. J Ethnopharmacol 130:414–420
Banna HU, Anjum A, Biswas S, Mondal V, Siddique AE, Roy AK, Nikkon F, Haque A, Himeno S, Salam KA, Hossain K, Saud ZA (2022) Parental lead exposure promotes neurobehavioral disorders and hepatic dysfunction in mouse offspring. Biol Trace Elem Res 1:1
Sanders T, Liu Y, Buchner V, Tchounwou PB (2009) Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health 24:15–46
Lee D, Kim H, Sung K, Kim Y, Kim K (2021) Mixed exposure to As, Mn, and Pb and dopamine neurotransmission in the striatum. J Adv Pharm Educ Res 11:115–118
Liu J, Liu Y, Habeebu SM, Waalkes MP, Klaassen CD (2000) Chronic combined exposure to cadmium and arsenic exacerbates nephrotoxicity, particularly in metallothionein-I/II null mice. Toxicology 147:157–166
Sanders AP, Mazzella MJ, Malin AJ, Hair GM, Busgang SA, Saland JM, Curtin P (2019) Combined exposure to lead, cadmium, mercury, and arsenic and kidney health in adolescents age 12–19 in NHANES 2009–2014. Environ Int 131:104993
Saritha S, Davuljigari CB, Kumar KP, Reddy GR (2019) Effects of combined arsenic and lead exposure on the brain monoaminergic system and behavioral functions in rats: reversal effect of MiADMSA. Toxicol Ind Health 35:89–108
Gupta A, Kumar A, Naqvi S, Flora SJ (2021) Chronic exposure to multi-metals on testicular toxicity in rats. Toxicol Mech Methods 31(1):53–66
Reza ASM, Hossain MS, Akhter S, Rahman MR, Nasrin MS, Uddin MJ, Sadik G, KhurshidAlam AHM (2018) In vitro antioxidant and cholinesterase inhibitory activities of Elatostema papillosum leaves and correlation with their phytochemical profiles: a study relevant to the treatment of Alzheimer’s disease. BMC Complement Altern Med 18:1–8
Islam J, Shila TT, Islam Z, Kabir E, Haque N, Khatun M, Khan S, Jubayar AM, Islam F, Nikkon F, Hossain K, Saud ZA (2023) Clerodendrum viscosum leaves attenuate lead-induced neurotoxicity through upregulation of BDNF-Akt-Nrf2 pathway in mice. J Ethnopharmacol 304:116024
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M (2021) Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol 12:643972
Pohl HR, Roney N, Abadin HG (2011) Metal ions affecting the neurological system. Met Ions Life Sci 8:62
Miranda M, Morici JF, Zanoni MB, Bekinschtein P (2019) Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci 2019:363
Bathina S, Das UN (2015) Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci 11:1164–1178
Reddy GR, Devi BC, Chetty CS (2007) Developmental lead neurotoxicity: alterations in brain cholinergic system. Neurotoxicology 28:402–407
DeBay DR, Reid GA, Macdonald IR, Mawko G, Burrell S, Martin E, Bowen CV, Darvesh S (2017) Butyrylcholinesterase-knockout reduces fibrillar β-amyloid and conserves 18FDG retention in 5XFAD mouse model of Alzheimer’s disease. Brain Res 1671:102–110
Sankhwar ML, Yadav RS, Shukla RK, Pant AB, Singh D, Parmar D, Khanna VK (2012) Impaired cholinergic mechanisms following exposure to monocrotophos in young rats. Hum Exp Toxicol 31:606–616
Yadav RS, Chandravanshi LP, Shukla RK, Sankhwar ML, Ansari RW, Shukla PK, Pant AB, Khanna VK (2011) Neuroprotective efficacy of curcumin in arsenic induced cholinergic dysfunctions in rats. Neurotoxicology 32:760–768
Flora SJ (2011) Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med 51:257–281
Chtourou Y, Trabelsi K, Fetoui H, Mkanne G, Kallel H, Zeghal N (2011) Manganese induces oxidative stress, redox state unbalance and disrupts membrane bound ATPases on murine neuroblastoma cells in vitro: protective role of silymarin. Neurochem Res 36:1546–1557
Kasperczyk S, Słowińska-Łożyńska L, Kasperczyk A, Wielkoszyński T, Birkner E (2015) The effect of occupational lead exposure on lipid peroxidation, protein carbonylation, and plasma viscosity. Toxicol Ind Health 31:1165–1171
Massaad CA, Klann E (2011) Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal 14:2013–2054
Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT (2012) Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. J Med 367:1098–1107
Chen B, Lu Y, Chen Y, Cheng J (2015) The role of Nrf2 in oxidative stress-induced endothelial injuries. J Endocrinol 225:83–99
Yao W, Zhang JC, Ishima T, Dong C, Yang C, Ren Q, Ma M, Han M, Wu J, Suganuma H, Ushida Y (2016) Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci Rep 6:30659
Baune BT, Konrad C, Grotegerd D, Suslow T, Birosova E, Ohrmann P, Bauer J, Arolt V, Heindel W, Domschke K, Schöning S (2012) Interleukin-6 gene (IL-6): a possible role in brain morphology in the healthy adult brain. J Neuroinflammation 9:125
Labenz C, Toenges G, Huber Y, Nagel M, Marquardt JU, Schattenberg JM, Galle PR, Labenz J, Wörns MA (2019) Raised serum interleukin-6 identifies patients with liver cirrhosis at high risk for overt hepatic encephalopathy. Aliment Pharmacol Ther 50:1112–1119
Kobayashi EH, Suzuki T, Funayama R, Nagashim T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M (2016) Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 7:11624
Khatun M, Haque N, Siddique AE, Wahed AS, Islam MS, Khan S, Jubayar AM, Sadi J, Kabir E, Shila TT, Islam Z, Sarker MK, Banna HU, Hossain S, Sumi D, Saud ZA, Barchowsky A, Himeno S, Hossain K (2024) Arsenic exposure-related hypertension in bangladesh and reduced circulating nitric oxide bioavailability. Environ Health Perspect 132(4):47003
Siddique AE, Rahman M, Hossain MI, Karim Y, Hasibuzzaman MM, Biswas S, Islam MS, Rahman A, Hossen F, Mondal V, Banna HU, Huda N, Hossain M, Sultana P, Nikkon F, Saud ZA, Haque A, Nohara K, Xin L, Himeno S, Hossain K (2020) Association between chronic arsenic exposure and the characteristic features of asthma. Chemosphere 246:125790
Acknowledgements
We also thank Dr. Megumi Yamamoto, National Institute for Minamata Disease, Japan.
Funding
This research work was supported by the University of Rajshahi (166/5/52/RABI/BINGAN-30/2022–23).
Author information
Authors and Affiliations
Contributions
Ehsanul Kabir: Investigation, Methodology, Data curation, Formal analysis, Writing – original draft. Tasnim Tabassum Shila: Investigation, Methodology, Data curation, Formal analysis Jahidul Islam: Investigation, Methodology, Validation. Sharmin Akter Beauty: Investigation. Shakhawoat Hossain: Data curation, Formal analysis. Farhadul Islam: Data curation, Formal analysis, Review & editing, Farjana Nikkon: Resources, Funding acquisition, Seiichiro Himeno: Review & editing, Khaled Hossain: Funding acquisition, Review & editing, Zahangir Alam Saud: Conceptualization, Funding acquisition, Project administration, Supervision.
Corresponding author
Ethics declarations
Ethical Approval
The Institute of Biological Sciences, University of Rajshahi, Bangladesh, provided institutional ethical approval for the animal experiment (No.: 110(16)/320/IAMEBBC/IBSc.).
Consent to Participate
Not applicable.
Consent for Publication
All authors of the manuscript have read the manuscript and have agreed to submit it in its current form for consideration for publication in Biological Trace Element Research.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ehsanul Kabir and Tasnim Tabassum Shila have equal contribution to this work.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplementary file1
Fig. S1. Timeline of animal experiment: All experimental groups received normal food pellets and drinking water. Additionally, the control group received distilled water and the other groups received respective metal-containing solutions through oral gavage respectively. The Elevated plus maze and Morris water maze tests, which spanned from day 31 to day 40. Following the sacrifice of each experimental mouse, a homogenate of the brain was prepared for biochemical analysis. (PNG 377 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kabir, E., Shila, T.T., Islam, J. et al. Concomitant Exposure to Lower Doses of Arsenic, Lead, and Manganese Induces Greater Synergistic Neurotoxicity Than Individual Metals in Mice. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04260-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04260-y