Abstract
The present study was designed to evaluate the impact of gamma radiation (60Co) on freshwater prawn Macrobrachium rosenbergii by using electron microscopic (SEM, TEM) studies. One set of prawns (experimental group) was irradiated (3, 30, 300, and 3000 mGy) by Theratron Phoenix TeleCobalt Unit [P-33], while other set of prawns (control group) was maintained (non-irradiated) separately. Scanning electron microscopic observations of gills and hepatopancreas showed fused and swollen lamella, abnormal gill tips, wrinkled lamellar epithelium, and necrotic epithelium surface in irradiated groups, while no such abnormalities were obvious in the control group. Transmission electron microscopic studies showed damaged nucleus, granulated mitochondria, vacuoles with crystalline granular inclusions, destructed membrane, vacuoles filled with granules, rough endoplasmic reticulum with residual bodies, shrunken mitochondria, dilated rough endoplasmic reticulum, and dilated cisternae of the Golgi body in irradiated groups. The structural abnormalities of vital organs could affect physiological functions such as respiration, osmo-ionic regulation and storage, secretion of the gills, and hepatopancreas, which in turn could adversely affect the growth and survivability of M. rosenbergii.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radiation is one of the most widespread sources of environmental stress in living environment and their exposure causes oxidative stress and metabolic changes in the living organisms (Mohamed 2011). The International Commission on Radiological Protection (ICRP) emphasized the need to protect non-human biota from the potential effects of ionizing radiation (ICRP 2007). The severity of a toxicant can be measured at the level of the molecular, cellular, tissue, organ, individual, or population (Moore 1985). The detection of responses to toxicants at the cellular or tissue level is of great value and where histopathological studies reflected the impact of toxicants on metabolic processes at the cellular level (Triebskorn and Kohler 1996; Mathur and Gupta 2008).
Scanning electron microscope (SEM) helps to study the organ’s morphological characteristics and abnormalities if any in treated samples. Transmission electron microscope (TEM) studies reveal the cell organelle features and their pathological conditions in treated samples. Ultrastructural alterations were studied by several authors for assessing the effects of organic chemicals and metals (Segner and Braunbeck 1998). In the last few decades, many studies focused on the influences of exogenous factors on crustaceans. Bhavan and Geraldine (2000) reported that always a close correlation exists between different kinds of stresses and ultrastructure of tissues.
The gill is used as a model organ for assessing the environmental radiation impact on testing the species and performs multifunctions like gas exchange (McMahon and Wilkens 1983), osmo-ionic homeostasis (Mantel and Farmer 1983), and transbranchial NaCl absorption and water excretion (Kirschner 2004). A Crustacean hepatopancreas acts as a primary organ responsible for absorption and storage of ingested materials and plays a major role in accumulating, neutralizing, and eliminating harmful chemicals (Abdelmeguid et al. 2009). The present study was designed to evaluate the impact of gamma radiation (60Co, 3, 30, 300, and 3000 mGy) on freshwater prawn Macrobrachium rosenbergii by using electron microscopic (SEM, TEM) studies.
Materials and methods
Experimental design and Gamma irradiation
Macrobrachium rosenbergii was collected from the river Cauvery (11° 29′ N; 79° 50′ E), Tiruchirappalli, Tamil Nadu, India, acclimatized in Environmental Research Laboratory, Jamal Mohamed College, Tiruchirappalli, and fed with boiled chopped goat liver ad libitum (Stalin et al. 2013). Prawns (n = 10) were irradiated (3, 30, 300, and 3000 mGy) along with Thermo Luminescence Disks (TLD, BARC, India) for dose measurement (Sadiq Bukhari et al. 2012) by using Theratron phoenix (P-33) Tele cobalt unit (60Co radionuclide source)in GVN Cancer Cure Research Centre and Hospital, Tiruchirappalli (Tamil Nadu, India). Control (non-irradiated) and irradiated groups were maintained in the laboratory for the next 96 h and were sacrificed.
Scanning electron microscopic studies
The animals were anesthetized on ice for 3 min, and gills and hepatopancreas were dissected out following Poljaroena et al. (2010). Gills and hepatopancreas were fixed in 2.5% Glutaraldehyde in 0.1 M PBS, pH 7.4, at 4 °C for 24 h and were post-fixed in 1% Osmium Tetraoxide (OsO4) in 0.1 M PBS, pH 7.4 for 1 h and dehydrated through increasing concentrations of ethanol. Tissues were coated with platinum and palladium in a Hitachi ion-sputtering apparatus (E2500). The processed tissues were examined in a Hitachi S-2500 scanning electron microscope (SEM) operating at 15 KV in the Centre for an Advanced Study in Botany, in the University of Madras, Chennai (Tamil Nadu, India) following Cheng et al. (2010).
Transmission electron microscopic studies
The gills and the hepatopancreas tissues were kept in vivo condition prior placing it into the fixative medium (fixed in 3% Glutaraldehyde and post-fixed by 1% OsO4). Tissues were dehydrated in ascending graded alcohol and cleared by propylene oxide. Tissues were molded with epoxy resin, kept in an incubator at 60 °C for 48 h, cooled down (Manush et al. 2007), sectioned (1 μm) through ultra microtome (Leica ultracut UCT) with a glass knife, and stained by toludine blue. The sections were transmitted in Tecnai TR spirit (Netherland) and photographed in Histopathological Unit, Christian Medical College (CMC), Vellore (Tamil Nadu, India).
Histological gradations
Semi-thin sections were cut at 1-m thickness and stained with methylene blue. The histological changes if any were examined and ranked semiquantitatively (Mishra and Mohanty 2008; Stalin et al. 2013): none (0%), mild changes (< 10%), moderate changes (10 to 50%), severe changes (50 to 70%), and extended severe changes (> 70%) in prawn tissues. Data was represented as the mean of slides (25 slides/organ) for each prawn, i.e., 250 slides per group.
Results
Scanning electron microscopic studies
Gill
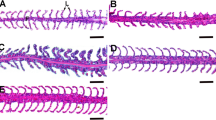
Scanning electron micrographs of gills of M. rosenbergii in the control prawn show the filament (F), lamella (L), interlamellar region (IL), and pillar cells (Fig. 1 (A1 and A2)). Gills of M. rosenbergii, treated with 3 mGy and 30 mGy Cobalt-60 gamma, showed fused lamellae (FLU), abnormal gill tip (AG), and wrinkling of the lamellar epithelium (WLE) (Fig. 1 (A3 and A4; A5 and A6)). The 300-mGy Cobalt-60 gamma–treated to M. rosenbergii showed swollen lamellae (SL) besides fused lamellae (FLU) and abnormal gill tip (AG) (Fig. 1 (A7 and A8)). Epithelial necrosis with and without blood emerging (asterisk shaped) besides fused lamellae (FLU), abnormal gill tip (AG), and swollen lamellae (SL) was observed in the gills of the animal treated with 3000-mGy Cobalt-60 gamma radiation (Fig. 1 (A9 and A10)).
Scanning electron microscopic images of control and 60Co gamma irradiated freshwater prawn M. rosenbergii. (A) Gill: Control (A1–A2), 3 mGy (A3–A4), 30 mGy (A5–A6), 300 mGy (A7–A8), and 3000 mGy (A9–A10) groups. F filament, L lamella, IL interlamellar region, FLU fusion of lamellae, AG abnormal gill tip, WLE wrinkling of the lamellar epithelium, SL swollen lamellae, * epithelial necrosis
Hepatopancreas
Scanning electron micrographs of hepatopancreas of M. rosenbergii in the control prawn were found with B cells, S cells, L-lipid droplets, and epithelial in normal structure (Fig. 2 (B1 and B2)). However, the hepatopancreas of M. rosenbergii treated with 3-mGy Cobalt-60 gamma showed with large lipid droplets inside the B cell, extrusion of large and small lipid (L) droplets, few completely mineralized lamellae body (LB), and irregular bulbous surface with necrotic crystalline fragments (N) (Fig. 2 (B3 and B4)). About 30-mGy Cobalt-60 gamma–irradiated prawn showed small and rare lipid droplets inside a B cell (decaying surface) (B), large lipid droplets inside the B cell (L), extrusion of large and small lipid droplets, lamellae body (LB), vacuoles formation(V), and necrosis (N) (Fig. 2 (B5 and B6)). About 300-mGy Cobalt-60 gamma–treated prawn was observed with extrusion of large and small lipid droplets, lamellae body (LB), vacuoles formation (V), and small and rare lipid droplets inside a B cell (decaying surface) (B) (Fig. 2 (B7 and B8)). Scanning electron micrographs of M. rosenbergii treated with 3000-mGy Cobalt-60 gamma showed small and rare lipid (L) droplets inside a B cell disintegrated apical part of a cell and decaying surface, necrotic epithelium surface, formation of vacuoles (V), and lamellae body (LB) (Fig. 2 (B9 and B10)).
Scanning electron microscopic images of control and 60Co gamma irradiated freshwater prawn M. rosenbergii. (B) Hepatopancreas: Control (B1–B2), 3 mGy (B3–B4), 30 mGy (B5–B6), 300 mGy (B7–B8), and 3000 mGy (A9–A10) groups. B B cells, S S cells, L lipid droplets; N necrosis; L (circle) large lipid droplets inside the B-cell; LB extrusion of large and small lipid droplets, lamellae body; V vacuoles formation, B small and rare lipid droplets inside a B cell (decaying surface)
Transmission electron microscopic studies
Gill
Transmission electron microscopic observation of control prawn gill showed pillar cell (PC) and hemocoelic space (HS). The lamellar region of the gills showed an epithelial cell with cuticle (C), nucleus (N), and mitochondria (M) associated with infolded cell membranes (IC) (Fig. 3 (A1 and A2)). Cobalt-60 gamma–irradiated (3 mGy) prawn gills showed HS, the E cell with centrally placed nucleus (N), few mitochondrial swelling with degenerated cristae, and vacuolated area with degenerated mitochondrae (MV). The lamellar region characterized by elevated cuticle (EC), vacuoles with crystalline granular inclusion (VC), and granular cells (Fig. 3 (A3 and A4)). About 30-mGy-irradiated prawn was observed with necrosis of mitochondria (M), damaged nucleus (N), lamellar region characterized by elevated cuticle (EC), and vacuoles with crystalline granular inclusion (VC) (Fig. 3 (A5 and A6)). About 300-mGy gamma–irradiated prawn was observed with necrosis of mitochondria (M), damaged nucleus (N), elevated cuticle (EC), vacuoles with crystalline granular inclusion (VC), formation of vacuoles(V), and granular cells(GC) (Fig. 3 (A7 and A8)). About 3000-mGy-irradiated prawn gills showed damaged nucleus (N), mitochondria with granular cell (Gc) formations, VC found at the basal regions of the gills, mitochondrial degeneration and devoid of cristae, and MV in adajcent intralamellar septal cell(s) (Fig. 3 (A9 and A10)).
Transmission electron microscopic images of control and 60Co gamma irradiated freshwater prawn M. rosenbergii. (A) Gill: Control (A1–A2), 3 mGy (A3–A4), 30 mGy (A5–A6), 300 mGy (A7–A8), and 3000 mGy (A9–A10) groups. PC pillar cell, HS hemocoelic space, C epithelial cell with cuticle, N nucleus, M mitochondria, IC infolding of cell membrane, MV vacuolated mitochondria, EC elevated cuticle in lamellar region, VC vacuoles with crystalline granular inclusion
Hepatopancreas
The hepatopancreas of control prawns showed E cell with centrally placed nucleus (N), few mitochondria (M), rough endoplasmic reticulum (RER), and rich in glycogen granules (G) and without microvilli (Fig. 4 (B1 and B2)). Hepatopancreas of 3 mGy irradiated group of prawns showed absorptive (R) cell with centrally placed nucleus (N), large lipid vacuoles (LV), hepatocyte vacuoles filled with granular inclusions (VG), cytoplasm with many electron dense granules (EG), shrunken mitochondria (SM), dilated rough endoplasmic reticulum (DRER), and dilated cisternae of the Golgi body (DGB) in the tubule epithelium (Fig. 4 (B3 and B4)). About 30-mGy treated prawn was observed with vacuolated cell with granular inclusions (VG), nucleus (N), dilated cisternae of the Golgi body (DGB), dilated Golgi body complex (GC), lipid vacuoles (LV), residual bodies in rough endoplasmic reticulum (RB), shrunken mitochondria (SM), and dilated rough endoplasmic reticulum (DRER) (Fig. 4 (B5 and B6)). About 300-mGy treated prawn was observed with necrotic nucleus (N), glycogen granules (G), microvilli (MV), dilated cisternae of the Golgi body(DGB), dilated Golgi body complex (GC), lipid vacuoles (LV), residual bodies in rough endoplasmic reticulum (RB), shrunken mitochondria (SM), and dilated rough endoplasmic reticulum (DRER) (Fig. 4 (B7 and B8)). Lipid vacuoles LV, vacuolated cell with granular inclusions (VG), shrunken mitochondria (SM), dilated rough endoplasmic reticulum (DRER), dilated cisternae of the Golgi body (DGB), and residual bodies (RB) formation in RER were observed in the hepatopancreatic tubule epithelium of 3000 mGy irradiated prawns (Fig. 4 (B9 and B10)).
Transmission electron microscopic images of control and 60Co gamma irradiated freshwater prawn M. rosenbergii. (B) Hepatopancreas: Control (B1–B2), 3 mGy (B3–B4), 30 mGy (B5–B6), 300 mGy (B7–B8), and 3000 mGy (A9–A10) groups. E embryonic cell, N nucleus, M mitochondria, RER rough endoplasmic reticulum, G glycogen granules, R absorptive cell, LV lipid vacuoles, VG vacuolated cell with granular inclusions, EG electron dense granules, SM shrunken mitochondria, DRER dilated rough endoplasmic reticulum, DGB dilated cisternae of the Golgi body, RB residual bodies in rough endoplasmic reticulum
Histological gradations
In irradiated groups, the histological anomalies increase as dose increases. Maximum histological changes were found at 3000 mGy followed by lower level. Semiquantitative scoring of gill and hepatopancreas abnormalities observed by scanning electron microscopic studies was done (Table 1). Similar scoring was done for the gill and hepatopancreas abnormalities observed by transmission electron microscopic studies (Table 2).
Discussion
The impact of radiation on biota has been identified as priority area for future research within both scientific and regulatory communities especially in crustaceans in aquatic ecosystem (Fuller et al. 2015). In this study, the effects of 60Co gamma radiation at dose level of 3, 30, 300, and 3000 mGy on the gill and hepatopancreas of freshwater crustacean M. rosenbergii were investigated by electron microscopic studies.
Scanning electron microscope
Gill
The SEM study showed fused to swollen lamellae (FLU-SL), abnormal gill tip (AG), and wrinkling of the lamellar epithelium (WLE) which may reduce the surface area and in turn likely to lead to decreased ionic permeability as suggested by McNamara and Lima (1997). Segner (1987) reported that gill epithelial cells in decapods crustaceans undergo structural reorganization in response to low dose radiation.
Hepatopancreas
Hepatopancreas of irradiated group of prawns showed various levels of abnormalities in various organelles. Icely and Nott (1980) reported several types of granulate inclusion, responsible for regulation of the concentrations of metals in the blood, in cells. Hence, gamma radiation exposure may disturb the regulations of the concentrations of metals. They may act as a “barrier” to the diffusion of potentially harmful amounts of essential and non-essential metals into the blood. Hence, it is likely that the gamma radiation exposure may disturb the regulations of the concentrations of metals.
Transmission electron microscopic
Gill
TEM study revealed mitochondrial swelling with degenerated cristae, vacuolated area with degenerated mitochondrae, vacuoles with crystalline granular inclusion and granular cells, necrosis of mitochondria, damaged nucleus (N), lamellar region characterized by elevated cuticle, and formation of vacuoles (V) and granular cells. Manush et al. (2007), in the gills of M. rosenbergii exposed to 35 °C, observed severe degeneration of mitochondria with no cristae, irregular nuclear membrane and marginated chromatin. Gills of M. olfersii showed flattened hemilamella, narrow, hemolymph-filled space, and layers of pillar cells (Freire and McNamara 1995).
Hepatopancreas
The electron microscopic studies in controlled hepatopancreas of M. rosenbergii showed a normal arrangement with intact nuclear membrane, less number of nuclear pores and vacuoles, eccentric position of nucleolus, and more number of microvilli (Ramalingam and Ramarani 2007). Tissue destruction and bacterial accumulation found in the hepatopancreatic tubular lumen of Penaeus vannamei infected with Vibrio anguillarum (Esteve and Herrera 2000). The hepatocytes possessed distinct boundaries and apical membranes showed long microvilli which represented the brush border during TEM analysis (Abdelmeguid et al. 2009).
The folded basal lamina in the tubules evidenced in P. argentinus and P. monodon exposed to pesticides (Vogt 1987). TEM study on P. serratus exposed to 100 ng/μL petroleum compound revealed cellular disorganization and increased thickness in basal lamina, degeneration of the hepatopancreatic epithelium. No attempt has however been made to assess the impact of (mGy) of 60Co gamma radiation at low level (Sadiq Bukhari et al. 2012). Hence, the present work is a novel attempt to study the 60Co gamma (3, 30, 300, and 3000 mGy) radiation-induced ultrastructural studies in adult freshwater crustaceans (prawn) Macrobrachium rosenbergii.
Conclusion
The impact of 60Co gamma radiation was studied on Macrobrachium rosenbergii. Ultrastructural damages were found in gills and hepatopancreas of M. rosenbergii exposed even at 3-mGy level. However, no lethality was observed in the select dose levels. Hence, it is recommended that radiation output may be kept at below 3-mGy level in order to protect the biota from radiation effect.
Data availability
Not applicable.
Abbreviations
- 60Co:
-
Cobalt-60
- SEM:
-
Scanning electron microscopic
- TEM:
-
transmission electron microscopic
- ICRP:
-
International Commission on Radiological Protection
- TLD:
-
Thermo luminescence disks
- BARC:
-
Bhabha Atomic Research Centre
- GVN:
-
G.Viswanathan
- PBS:
-
Phosphate-buffered saline
- OsO4 :
-
Osmium tetroxide
- mGy:
-
milligray
References
Abdelmeguid NE, Awad HE, Ibrahim AM, Yousef NA (2009) Ultrastructural changes in Hepatopancreas of Palaemon serratus, following treatment with petroleum carcinogenic compounds. Pak J Nutr 8(6):770–778
Bhavan PS, Geraldine P (2000) Histopathology of the hepatopancreas and gills of the prawn Macrobrachium malcolmsonii exposure to endosulfan. Aquat Toxicol 50(4):331–339
Cheng Z, Liu X, Han M, Ma W (2010) Adsorption kinetic character of copper ions onto a modified chitosan transparent thin membrane from aqueous solution. J Hazard Mater 182:408–415
Esteve M, Herrera F (2000) Hepatopancreatic Alterations in Litopenaeus vannamei (Boone, 1939) (Crustacea: Decapoda: Penaeidae) Experimentally Infected with a Vibrio alginolyticus. Journal of Invertebrate Pathology 76:1–5
Freire CA, McNamara JC (1995) Fine structure of the gills of the fresh-water shrimp Macrobrachium olfersii (Decapoda), effect of acclimation to high salinity medium and evidence for involvement of the lamellar septum in ion uptake. J Crustac Biol 15:103–116
Fuller N, Lerebours A, Smith JT, Ford AT (2015) The biological effects of Ionising radiation on Crustaceans: A review. Aquat Toxicol 167:55–67
Icely JD, Nott JA (1980) Accumulation of copper within the “Hepatopancreatic” Caeca of Corophium volutator (Crustacea: Amphipoda). Mar Biol 57:193–199
ICRP (2007) Recommendations of the International Commission on Radiological Protection. Ann ICRP 37:2–3 Publication 103
Kirschner LB (2004) The mechanism of sodium chloride uptake in hyper regulating aquatic animals. J Exp Biol 207:1439–1452
Mantel LH, Farmer LL (1983) Osmotic and ionic regulation. In: Mantel LH (ed) The biology of Crustacea. Internal Anatomy and Physiological Regulation, vol 5. Academic Press, New York, pp 53–161
Manush SM, Pal AK, Das T, Chatterjee N, Sarma K, Mukherjee SC (2007) Ultrastructural alteration in the gill of Macrobrachium rosenbergii acclimated to three temperatures. Asian J Cell Biol 2(1):1–10
Mathur S, Gupta AK (2008) Histoenzymological study on the toxicity of copper sulphate in the digestive glands of Lymnaea luteola. J Environ Biol 29:201–204
McMahon BR, Wilkens JL (1983) Ventilation, perfusion and oxygen uptake. In: Mantel L, Bliss D (eds) Biology of Crustacea, vol 6. Academic Press, New York, pp 289–372
McNamara JC, Lima AG (1997) The route of ion and water movements across the gill epithelium of the freshwater shrimp M. olfersii (Decapoda, Palaemonidae), evidence from ultrastructural changes induced by acclimation to saline media. Biol Bull 192:321–331
Mishra AK, Mohanty B (2008) Acute toxicity impacts of hexavalent chromium on behavior and histopathology of gill, kidney and liver of the freshwater fish, Channa punctatus (Bloch). Environ Toxicol Pharmacol 26(2):136–141
Mohamed NE (2011) Effect of chitosan on oxidative stress and metabolic disorders induced in rats exposed to radiation. J Am Sci 7(6):406–417
Moore MN (1985) Cellular responses to pollutants. Mar Pollut Bull 16:134–139
Poljaroena J, Vanichviriyakita R, Tinikula Y, Phoungpetcharaa I, Linthonga V, Weerachatyanukula W, Sobhona P (2010) Spermatogenesis and distinctive mature sperm in the giant freshwater prawn, Macrobrachium rosenbergii (De Man, 1879). Zool Anz 249:81–94
Ramalingam K, Ramarani S (2007) Effect of Pseudomonas aeruginosa on the giant freshwater prawn, Macrobrachium rosenbergii-histopathological and electron microscopic study. J Environ Biol 28(3):627–635
Sadiq Bukhari A, Syed Mohamed HE, Broos KV, Stalin A, Singhal RK, Venubabu P (2012) Histological variations in liver of freshwater fish Oreochromis mossambicus exposed to 60Co gamma irradiation. J Environ Radioact 113:57–62
Segner H (1987) Response of fed and starved roach, Rutius rutilus to sublethal copper contamination. J Fish Biol 30:423–437
Segner H, Braunbeck T (1998) Cellular response profile to chemical stress. In: Schüürmann G, Markert B (eds) Ecotoxicology, J. Wiley and Spektrum Akad, Verlag, Heidelberg, pp 521–569
Stalin A, Broos KV, Sadiq Bukhari A, Syed Mohamed HE, Singhal RK, Venu-babu P (2013) Effects of Co60 gamma irradiation on behavior and gill histoarchitecture of giant freshwater prawn Macrobrachium rosenbergii (DE MAN). Ecotoxicol Environ Saf 92:155–160
Triebskorn R, Kohler HR (1996) The impact of heavy metals on the grey garden slug, Deroceras reticulatum (Muller), metal storage cellular effects and semiquantitative evaluation of metal toxicity. Environ Pollut 93:327–343
Vogt G (1987) Monitoring of environmental pollutants such as pesticides in prawn aquaculture by histopathological diagnosis. Aquaculture 67:157–164
Acknowledgements
Authors sincerely thank the Management of GVN Cancer Cure Research Centre and Hospital, Tiruchirappalli and Miss Reeta, Welcome Research Unit, CMC, Vellore, for their technical support.
Author information
Authors and Affiliations
Contributions
Stalin Arumugam contributed to the study conception, study design, study methods, data collection, analysis, and first draft preparation. Suganthi Palai and Mathivani Subramanian contributed to the improvement of study design and study methods. Gokula Varadharajan contributed to the data analysis, data presentation and improving the first draft. Stalin Arumugam, Suganthi Palani, Mathivani Subramaniyan, and Gokula Varadharajan commented on earlier versions of the manuscript, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Responsible Editor: Georg Steinhauser
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arumugam, S., Palani, S., Subramanian, M. et al. Ultrastructural alteration in Gill and Hepatopancrease of freshwater prawn Macrobrachium rosenbergii exposed to 60Co gamma radiation. Environ Sci Pollut Res 28, 11348–11356 (2021). https://doi.org/10.1007/s11356-020-11394-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11394-8