Abstract
Previous studies have shown that the selenium (Se) deficiency is an important factor for the etiology of Kashin–Beck disease (KBD). Although KBD is presently controlled in most regions of China, it is still active in the Tibetan Plateau. The present study aimed to assess the nutritional status of selenium in school children by using the Se level in hair as a biomarker in KBD endemic areas of Lhasa in Tibet, China. Hair samples of 155 school children aged 6–15 years were collected in both KBD areas and non-KBD areas of Lhasa in 2013. The Se level in the hair samples was determined by inductive coupled plasma mass spectrometry (ICP-MS). The average concentration of Se in children’s hair was 0.232 μg/g in KBD areas of Lhasa, which was significantly higher than the data reported decades ago. A significant difference in hair Se was observed between the boys (0.255 μg/g) and the girls (0.222 μg/g) in the studied KBD areas (P < 0.01, Mann–Whitney U test), but hair Se did not vary by age or region. School children in KBD endemic areas in Lhasa likely have improved Se status as a result of high Se content staple food substitution with the enforcement of Free Education Policy and Nutrition Improvement Plan in Tibet. Nevertheless, there were still 20.3 % of students with low Se status (hair Se <0.20 μg/g), which showed that Se status of school children was also partly affected by low Se environment in KBD endemic areas of Lhasa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kashin–Beck disease (KBD) is a chronic, endemic, degenerative osteoarthropathy characterized by severe skeletal deformation and dwarfism and usually occurs in adolescence. China has the highest KBD disease incidence, with cases occurring in 303 counties of 15 provinces, autonomous regions, and municipalities of Heilongjiang, Jilin, Liaoning, Inner Mongolia, Hebei, Beijing, Shandong, Shanxi, Henan, Shaanxi, Gansu, Qinghai, Sichuan, Tibet, and Taiwan [1]. With the implementation of KBD prevention and control measures, which included Se supplement with oral sodium selenite tablets, selenized salt, spraying Se onto crops and eating Se-rich food, improving water quality by microbiological filtration, grain substitution, etc., and the improvement of social and economic conditions in endemic areas starting from1980s, the prevalence of KBD has decreased greatly in most regions of China [1, 2]. However, Qinghai, west of Sichuan, and Tibet still have relatively active and serious cases of KBD [3].
Since KBD was first described in 1949 in Russia, various hypotheses about the etiology had been proposed [4–8]. While the etiology remains obscure, there were three dominating hypotheses: Se deficiency in the environment, organic matter in drinking water such as humic acid, and cereal contamination by myco-toxin producing fungi [9–12]. Recent studies have shown that KBD prevalence resulted from interactions of genetic susceptibility and environmental factors including selenium and/or iodine deficiency, low selenium and/or T-2 toxin, etc. [13]. Although there were some problems that need to be resolved, some studies demonstrated that Se deficiency in the environment played an important role in leading the occurrence of KBD. First, KBD most commonly occurs in the areas with an environment of low Se. In China, KBD has been closely related to Se deficiency detected in soil, food grains, animal and human hair, and human blood in disease-affected areas, compared with those in unaffected areas. Second, there has been great success using Se supplementation to prevent and control KBD [9, 14–17]. However, some scientists consider that the environmental low Se was only an external condition but not the initial etiology causing KBD [18, 19].
The two earliest KBD cases were detected in Tibet in 1965 [20]. By the 1970s, there were 13 counties affected with KBD in Tibet, and the combined incidence rate presented in Mozhugongka, Linzhou, and Nimu Counties was 52.0 % [20]. In 1999, an epidemiological survey of KBD areas in Tibet reported 18,700 affected patients, which was double than that of in the 1970s. Young children were considered to be particularly vulnerable to KBD. The incidence rates in children aged 4–12 years, detected by radiography, were 59 children (50.4 %) of 117 children in Sangri [21], 20 children (33.9 %) out of 59 children in Linzhou [21], and 200 out of 331 children in Changdu, in proportion of 60.4 % [22]. Se deficiency was also associated with the KBD prevalence and severity in Tibet, which was observed by previous studies [20, 23, 24].
Since 2001, a series of trials of multiple control measures such as Se supplementation, grain substitution, water quality improvement, and vitamin C supplementation were enforced in eight KBD-affected counties of Tibet, and within a short period, decreased clinical detection and X-ray detection rates of KBD in children were observed [25]. At the same time, the Free Education Policy implemented involving all primary and high boarding schools in farming and pastoral areas in Tibet, with all tuition, food, and lodging expenses free for students [26]. Since 2012, the Nutrition Improvement Plan has directly promoted dietary structure and nutritional conditions for school children in Tibet. However, the Se nutrition impact assessment of these policies in children in KBD areas is lacking.
Se levels in blood, plasma, and serum are usually used to assess the Se status in previous studies [27, 28]. However, collecting the hair sample has the advantage of noninvasive sampling, and easy to transport and store for a long time [29, 30]. Furthermore, Se in people’s hair is correlated with the Se level in the kidney, liver, lung, plasma [31, 32], and blood [33]. Hair Se concentration has been widely used as a criterion of evaluating environmental exposure levels and assess selenium nutritional status [34–36]. Moreover, hair Se concentration is often employed as a bioindicator for long-term daily Se intake [36–38]. The focus and aim of this study were to evaluate and assess Se concentrations in the hair of school children who were living in KBD areas in Lhasa, Tibet, China.
Materials and Methods
Site Description and Subject Selection
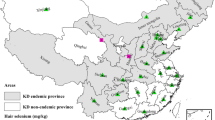
In August 2013, a cross-sectional study was conducted in two counties (Mozhugongka County and LinZhou County) in central of Tibetan Plateau, located in Lhasa Region of Tibet, China. The two counties lie between longitudes 90° 50′–92° 30′ and latitudes 29° 32′–30° 30′, altitude between 3850 and 4200 m. The geographical area of the two counties is 10,029 km2 and has a population of 94,920 at the end of 2010, 97.5 % of whom are Tibetans. Five schools were chosen for the hair sample collection in the two counties. Among them, four schools located in KBD endemic areas, which were Nimajiangre Village, Zhaxigang Village of Mozhugongka County, and Kazi Village, and Alang Village of Linzhou County, and one school situated in non-KBD area was selected as the control, which was Jiangxia Village of Linzhou County (Fig. 1). The five schools had all implemented the Free Education Policy of Tibet, and staple food samples consuming by students in the five schools were also collected.
The study protocol was approved by the Lhasa Health Bureau of Tibet and the agreements of the five schools. Ten percent of total students with informed consent were randomly selected in each school, trying to keep evenly distributed in grades. To guarantee a successful collection of hair samples, children with dyed hair or short hair (length less than 5 mm) were excluded. Under such selection criteria, 123 school children aged 6–15 years old (55 boys and 68 girls) were recruited from KBD areas. A second group consisted of 33 school children aged 6–15 (14 boys and 18 girls) were recruited from non-KBD areas.
Among the selected students from the KBD area, five children were diagnosed to be suspected cases of KBD, which was determined by X-ray photos for the right hand including wrist, and the special doctor analyzed those photos and got the detected results using the diagnostic criteria established by the Ministry of Health of the People’s Republic of China [39].
Sample Collection and Preparation
Approximately 0.5-g hair samples with length of 1–3 cm were collected from the nape of the head as near as possible to the scalp with a pair of stainless steel scissors and stored in polyethylene bags for later sample preparation. The hair samples were washed three times with neutral detergent, rinsed with distilled water, and dried in an oven at a temperature of 60 °C for 6–8 h. Then, the hair samples were cut into small pieces (2–3 mm) for Se determination [24, 40].
Three kinds of staple food consuming in the five investigated schools were collected. Five samples of tsampa as breakfast was made of local higher barley cultivated in Tibet, and five rice samples and five wheat flour samples were imported to Lhasa from other provinces of eastern China. All of the grain samples were oven-dried at 60 °C for 6–8 h to remove all of the moisture. Then, the dried grain samples were crushed in a stainless steel vegetation disintegrator (FW100, Taisite Instrument Co. LTD, Tianjin, China) for a short time and stored for Se determination [41].
Sample Digestion and Analysis
Hair and grain samples (0.1 g each) were placed separately in 50-mL beakers and digested in a mixture of concentrated HNO3 and H2O2 (v:v = 2:1) on a hot plate until the solution became clear. During this process, the temperature was maintained at <150 °C to prevent Se from volatilizing. After cooling, the samples were diluted by deionized water to 10 mL. Blank, parallel, and standard samples for the external calibration procedure were prepared in the same way.
Se concentrations were determined by ICP-MS (ELAN DRC-e, PerkinElmer Instrument Co., Shelton, CT, USA) which has been regarded as an important technique explored for its potential for Se analysis. The detection limit of Se with ICP-MS was 0.01 μg/g. Rapid, quasi-simultaneous, multi-element detection capabilities, high sensitivity, and detection power have made this technique an important tool for the analysis of trace elements in biological samples [42–44]. All of the reagents were of analytical-reagent grade or better, provided by Sino-pharm Chemical Reagent, Beijing, China. The purified water for all dilutions was of 18.3 MΩ cm−1 purity using the Milli-Q (Millipore, Bedford, MA, USA) deionization system.
Accuracy and Precision
For quality control, the accuracy was guaranteed by using of certified reference materials (CRMs). The Se concentration determined in the reference material GBW09101b (human hair, standard reference material, Shanghai Institute of Nuclear Research, Shanghai, China) was 0.580 ± 0.006 mg/kg (n = 8), which was consistent to certified concentration of 0.590 ± 0.040 mg/kg. The measured Se value in reference material GBW10010 (rice, standard reference material, Institute of Geophysical and Geochemical Exploration, Beijing, China) was 0.067 ± 0.006 mg/kg (n = 8), which was comparable to certified value of 0.061 ± 0.015 mg/kg.
Precision was maintained by analyzing parallel samples of replicate assays (10 % of the total samples), obtaining a coefficient of variation of 0.5–5.3 %.
Statistical Analysis
The data was presented as means and standard deviations for continuous variables (mean ± S.D.). The Kolmogorov–Smirnov’s test was carried out to examine if the hair Se followed a Gaussian distribution (P < 0.05). Mann–Whitney U test was used for comparing the difference of hair Se between any two groups. Kruskal–Wallis H test of one-way analysis of variance (ANOVA) was performed to compare Se in the hair of school children among region or age groups. The linear regression analysis was used to evaluate the association between hair Se and children age. Differences were considered significant at level of P < 0.05. The SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used to perform all these statistical analyses. Location map of the study area was made using ArcGIS 10.0 (Esri, Redlands, CA, USA).
Results and Discussion
Kolmogorov–Smirnov’s test result showed that the log transformation Se concentration in the hair samples of all students formed a Gaussian distribution (Fig. 2). As shown in Table 1, the mean hair Se level of children in the KBD areas and non-KBD area were 0.232 ± 0.058 μg/g and 0.236 ± 0.047 μg/g respectively, and no significant difference was observed between two groups (Mann–Whitney U test, P > 0.05). Suspected KBD children had significantly lower hair Se concentrations than the other healthy children in the KBD endemic areas (Mann–Whitney U test, P < 0.01) (Table 2).
The average hair Se levels in boys and girls in KBD areas were 0.255 ± 0.046 and 0.222 ± 0.043 μg/g, respectively (Mann–Whitney U test, P < 0.01). Table 3 summarizes the hair Se levels in all schools as well as in the corresponding gender groups. Although hair Se levels were all higher in boys than those in girls in each of the four KBD endemic villages, the difference was significant only at Nimajiangre, Mozhugongka County, and Zhaxigang, Mozhugongka County (Mann–Whitney U test, P < 0.05). It was found that mean hair Se concentrations of boys (0.268 ± 0.046 μg/g) were also significantly higher than of girls (0.205 ± 0.051 μg/g) (Mann–Whitney U test, P < 0.01) in the non-KBD area. These results were similar to previous findings reported in China. In Gansu Province, the Se status of boys was higher than that of girls in KBD areas such as Longnan and Qingyang Counties. However, in non-KBD areas such as Tianshui and Lanzhou, the levels were not significantly different [45]. A similar phenomenon was also found in the KBD areas of Jilin Province [46]. Moreover, our findings were similar with those based on a national report of biochemical indicators of diet and nutrition in the USA (1999–2002), where the blood Se concentration in boys was higher than that in girls [47]. Consistent observations have been documented in children in New Zealand (5–14 years old) and Slovakia (11–18 years old) [48, 49]. Moreover, in Ganji of Iran, the hair Se levels were significantly lower in women than in men [50]. Potential explanations for the differences between the genders may include a difference in daily food intake and consumption habits (e.g., amount of caloric intake) [51, 52]. Second, different absorption and metabolism characteristics in addition to human characteristics between boys and girls might have an effect on Se levels. Indeed, more attention should be paid to the sex-dependent metabolic differences, and the underlying mechanisms require further research. Moreover, the gender difference should be considered for the prevention and control of KBD in Tibet.
Additionally, Kruskal–Wallis H test of ANOVA was used to obtain a better insight into the difference of hair Se levels among the four KBD-affected villages. No significant differences in hair Se concentrations of boys among the village groups were evident, as well as those of girls (P > 0.05). It can be partly explained that the dietary Se intake is the major factor influencing Se status in human body, the dietary structure of students in the studied schools is broadly consistent with the adoption of the Free Education Policy and Nutrition Improvement Plan in Tibet, and all of the primary schools in Lhasa had adopted unified food supply.
For age comparisons, the participants in KBD areas were divided into an older age group (11–15 years old) and younger age group (6–10 years old). The hair Se concentration in the older age group (11–15 years old) was slightly higher (0.239 ± 0.049 μg/g) than that in the younger age group (6–10 years old) (0.223 ± 0.047 μg/g) (Mann–Whitney U test, P > 0.05). According to the average Se concentration of every age, there was an equation: y = 0.004x + 0.2078 (R 2 = 0.370, P > 0.05). This linear relationship did not include children in age of 6–7 as the number of the two age groups was small. Although there were no significant differences among all the age groups (Kruskal–Wallis H test of ANOVA, P > 0.05), hair Se levels had a rise along with the increase of children age. Increase of food intake may be the factor in the rising of hair Se in older students. This was also observed in children hair samples from North and South Libya and Ankara [34, 37].
On comparison with the recent data of KBD areas in other provinces of China (Table 4), the hair Se concentration of school children in KBD areas of Lhasa was similar to the results reported in Rangtang of Sichuan Province and higher than those in Longnan, Qingyang, Dingxi of Gansu Province, Guide, and Xinghai of Qinghai Province. However, when compared with the non-KBD areas in central of China, the mean hair Se was much lower than the data in Xinxiang (0.53 ± 0.14 μg/g) and Zhengzhou (0.38 ± 0.18 μg/g) of the Henan Province, Nanchong of Sichuan Province (0.44 ± 0.81 μg/g), and Lanzhou of Gansu Province (0.42 ± 0.11 μg/g).
Among the 123 survey school children in KBD area of Lhasa, 36 children (29.3 %, 23 boys and 13 girls) had normal hair Se level, according to the threshold of hair Se value classified by past research efforts related to Se-endemic disease in China [57], quoted in many research papers [51, 58, 59]. The hair Se level of 25 children (20.3 %, 5 boys and 20 girls) was lower than 0.20 μg/g, which was deemed to be in Se deficiency status. The hair Se concentration of 62 children (50.4 %, 27 boys and 35 girls) ranged from 0.20 to 0.25 μg/g and would be indicated on the edge of Se deficiency. These data suggested that Se nutritional status of school children living in KBD areas of Lhasa was still relatively low.
Nevertheless, the mean hair Se level of school children observed in the KBD areas of Lhasa in this study was substantially higher than the data observed decades ago in KBD areas in Tibet, i.e., 0.067 μg/g reported in 1982 [60], as well as 0.142 μg/g reported in 2003 [61], which may benefit from the implementation of the Free Education Policy and Nutrition Improvement Plan in Tibet. Particularly, the staple foods consumed by school children were mainly outsourced foods such as rice and wheat flour from other provinces of China, compared with most of their consumption of locally sourced foods at home, such as tsampa, made of highland barley grains. Results of Se concentration of staple foods collected at schools showed that the average Se concentration of local tsampa was 5.4 ± 1.3 μg/kg (5), which was much lower than that in rice (53.3 ± 9.3 μg/kg (5)) and wheat flour (31.8 ± 5.1 μg/kg (5)) imported from other provinces of China (Mann–Whitney U `test, P < 0.01). Undoubtedly, outsourcing of staple foods provides a major contribution to improvements in the dietary Se intake of school children in KBD endemic area of Lhasa, China.
Whereas students spend 28 weeks of every year (200 days) in school and the rest of the time is spent at home, the dietary pattern at home would also influence the hair Se levels of school children. Therefore, the Se status of students in KBD areas of Lhasa was still partly affected by the low Se environment. This may explain why some of the students in the present study were still Se-deficient.
The present study focuses on assessment of hair Se status of school children in the KBD areas in Lhasa. There were some limitations in this study, including the relatively small sample size, logistic constraints that prevented the collection of every kind of food samples daily consumed by students both at school and at home. The corresponding association of hair Se levels with dietary Se intake at school and at home in KBD areas in Tibet will be further studied.
Conclusion
The current study showed that the nutritional status of Se in children has improved considerably in comparison with the level of decades ago. These changes are primarily caused by the increase in dietary intake of Se for school children, which likely resulted from high Se level grain substitution within the enforcement of the Free Education Policy and Nutrition Improvement Plan for school children in Tibet. Meanwhile, the Se status of school children is partly affected by low Se environment in KBD endemic areas of Lhasa. Se deficiency stays in some students, especially female students. Therefore, it is important to strengthen the supply of Se-rich foods for school children such as seafood, eggs, meats, chicken, dry fruits, and legumes, as others such mushrooms and garlic [30, 62–65]. The dietary Se intake survey of students in their school and family is needed to deeply study the relationship between hair Se levels and daily Se intake, as well as its improvement impact on the prevention and control of KBD in Tibet.
References
Tan J (1989) The Atlas of Endemic Diseases and their Environments in the People’s Republic of China. Science Press, Beijing, In Chinese
Zhao Z, Li Q, Yang P et al (2013) Selenium: a protective factor for Kashin–Beck disease in Qing-Tibet plateau. Biol Trace Elem Res 153:1–4
The Ministry of Health Statistics Information Center. China health development of statistical bulletin in 2006. http://www.moh.gov.cn/newshtml/18903.htm1988.
Fang HC (1988) The relationship of Kashin-Beck and Keshan diseases with hydrogeochemistry. Environ Geo Water Sci 12:23–28
Zamana LV (1995) Possible hydro-geochemical preconditions for Kashin-Beck disease in Transbaikalia. Chinese Geograph Sci 5:185–192
Chasseur C, Suetens C, Nolard N et al (1997) Fungal contamination in barley and Kashin-Beck disease in Tibet. Lancet 350:1074
La GM, Mathieu F, Begaux F et al (2001) Kashin-Beck disease and drinking water in central Tibet. Int Orthop 25:167–169
Chasseur C, Suetens C, Michel V et al (2001) A 4-year study of the mycological aspects of Kashin-Beck disease in Tibet. Int Orthop 25:154–158
Tan J, Wang W, Zhu Z et al (1988) Selenium in environment and Kashin-Beck disease. Chin J Geochem 7:273–280
Yang J (1995) Etiology of Kashin–Beck disease. China J Endemiol 14:201–204 (In Chinese)
Peng A, Wang W, Wang C et al (1999) The role of humic substances in drinking water in Kashin–Beck disease in China. Environ Health Persp 107:293–296
Suetens C, Moreno-Reyes R, Chasseur C et al (2001) Epidemiological support for a multi-factorial etiology of Kashin–Beck disease in Tibet. Int Orthop 25:180–187
Guo X (2008) Progression and prospect of etiology and pathogenesis of Kashin-Beck disease. J Xian Jiaotong Univ (Medical Sciences) 29:481–488 (In Chinese)
Li C (1979) Observation on the efficiency of the cure KBD in 224 cases with selenium and vitamin E, and a discussion on the etiology of KBD. Chin Med J 59:169–171 (In Chinese)
Peng A, Yang C (1991) Examination of the roles of selenium in the Kashin-Beck disease. Biol Trace Elem Res 28:1–9
Xu L, Sen W, Xiong Q et al (1991) Selenium in Kashin-Beck disease areas. Biol Trace Elem Res 31:1–9
Tan J, Zhu W, Wang W et al (2002) Selenium in soil and endemic diseases in China. Sci Total Environ 28:227–235
Moreno-Reyes R, Mathieu F, Boelaert M et al (2003) Selenium and iodine supplementation of rural Tibetan children affected by Kashin–Beck osteoarthropathy. Am J Clin Nutr 78:137–144
Canter PH, Wider B, Ernst E (2007) The antioxidant vitamins A, C, E and selenium in the treatment of arthritis: a systematic review of randomized clinical trials. Rheumatology 46:1223–1233
Wang W, Wang M, Zhu Z et al (1985) Geographic epidemiological research on Keshan Disease, Kashin-Beck Diseases in Tibet. Proceedings of the second chemical geography conference of China. Science Press, Beijing, pp 85–91, In Chinese
Gong H, Zhaxi S, Xirao R et al (2004) Sampling monitoring report on the prevalence condition of Kashin-Beck Disease in Tibet. China J Endemiol 23:91 (In Chinese)
Institute of Kashin-Beck disease in Center for endemic disease control of China (2000). Investigative report on the prevalence condition of Kashin-Beck Disease (KBD) in Tibet China J Endemiol 19:41–43 (In Chinese)
Li S, Li W, Hu X et al (2009) Soil selenium concentration and Kashin-Beck disease prevalence in Tibet. China Front Environ Sci Engin China 3:62–68
Zhang B, Yang L, Wang W et al (2011) Environmental selenium in the Kashin-Beck disease area, Tibetan Plateau, China. Environ Geochem Health 33:495–501
Gong H, Zhaxi D, Xirao R et al (2004) Kashin-Beck disease sampling test report in Tibet. China J Endemiol 23:91 (In Chinese)
Zhang H (2011) Children of farmers, herdsmen to enjoy free education in Tibet. People’s Daily Online. http://english.peopledaily.com.cn/90001/90776/90882/7285747.html
Da Cunha S, Filho FM, Antelo DS et al (2003) Serum sample levels of selenium and copper in healthy volunteers living in Rio deJaneiro city. Sci Total Environ 301:51–54
Li N, Gao Z, Luo D et al (2007) Selenium level in the environment and the population of Zhoukoudian area, Beijing, China. Sci Total Environ 381:105–111
Golub Kina NA, Alffhan GV (1999) The Human Selenium Status in 27 Regions of Russia. J Trace Elements Med Bio 13:15–20
Navarro-Alarcon M, Cabrera-Vique C (2008) Selenium in food and the human body: a review. Sci Total Environ 400:115–141
Piccinini L, Borella P, Bargellini A et al (1996) A case–control study on selenium, zinc, and copper in plasma and hair of subjects affected by breast and lung cancer. Biol Trace Elem Res 51:23–30
Borella P, Bargellini A, Caselgrandi E et al (1997) Observations on the use of plasma, hair and tissue to evaluate trace element status in cancer. J Trace Elem Med Biol 11:162–165
Chen X, Yang G, Chen J et al (1980) Studies on the relations of selenium and keshan disease. Biol Trac Elem Res 2:91–107
Mengüba K, Gökmen CI, Diab N et al (1992) Selenium status of normal Turkish children. Biol Trace Elem Res 133:160
Dong Z, Bank MS, Spengler JD (2015) Assessing metal exposures in a community near a cement plant in the Northeast U.S. Int J Environ Res Public Health 19:952–969
Ashton K, Hooper L, Harvey LJ et al (2009) Methods of assessment of selenium status in humans: a systematic review. Am J Clin Nutr 89:2025S–2039S
Musa-Alzubaidi LI, Kasperek K et al (1982) Hair selenium content during infancy and childhood. Eur J Pediatr 139:295–296
Viju V, Thomas RK, Haswell SJ et al (2013) Maternal hair selenium levels as a possible long-term nutritional indicator of recurrent pregnancy loss. BMC Womens Health 13:40
Ministry of Health of the People’s Republic of China (2010) Diagnosis of Kashin-Beck Disease (WS/T207–2010)
Gao J, Liu Y, Huang Y et al (2011) Daily selenium intake in a moderate selenium deficiency area of Suzhou, China. Food Chem 126:1088–1093
Keshavarzi B, Moore F, Najmeddin A et al (2012) The role of selenium and selected trace elements in the etiology of esophageal cancer in high risk Golestan province of Iran. Sci Total Environ 433:89–97
Forrer R, Gautschi K, Stroh A et al (1999) Direct determination of selenium and other trace elements in serum samples by ICP-MS. J Trace Elem Med Biol 12:240–247
Labat L, Dehon B, Lhermitte M (2003) Rapid and simple determination of selenium in blood serum by inductively coupled plasma-mass spectrometry (ICP-MS). Anal Bioanal Chem 376:270–273
Zaitseva IP, Skalny AA, Tinkov AA et al (2015) The influence of physical activity on hair toxic and essential trace element content in male and female students. Biol Trace Elem Res 163:58–66
Kang F, Li Y, Wei J et al (2013) Analysis of selenium concentration in hair samples of children in Kashin Beck Disease areas in Gansu Province. China J Endemiol 32:556–560 (In Chinese)
Xu S, Zhang X, Lv H et al (2013) Selenium levels inside and outside environment of Jinlin Province Kashin-Beck Disease area. China J Ctrl Endemiol Dis 28:340–342 (In Chinese)
Centers for Disease Control and Prevention (2008) Trace elements: selenium. In: National report on biochemical indicators of diet and nutrition in the US population 1999–2002. pp101–106. http://www.cdc.gov/nutritionreport/99–02/part_4b.html
Brtková A, Magálová T, Béderová A et al (1999) Serum selenium levels in healthy Slovak children and adolescents. Biol Trace Elem Res 67:49–54
Thomson CD, McLachlan SK, Parnell WR et al (2007) Serum selenium concentrations and dietary selenium intake of New Zealand children aged 5–14 years. Br J Nutr 97:357–364
Pan D, Huang H (2013) Hair selenium levels in hepatic steatosis patients. Biol Trace Elem Res 152:305–309
Li S, Bañuelos GS et al (2014) The changing selenium nutritional status of Chinese residents. Nutrients 6:103–1114
Clark NA, Teschke K, Rideout K et al (2007) Trace element levels in adults from the west coast of Canada and associations with age, gender, diet, activities, and levels of other trace elements. Chemosphere 70:155–164
Xu X, Gao Q, Chen Y et al (2012) Study on the selenium nutrition of children in Kashin-Beck disease endemic areas in Rang tang county, Sichuan province. J China West Normal Univ (Natural Sciences) 33:79–82 (In Chinese)
Zhang Q, Wang H, He D et al (2011) Selenium levels inside and outside Qinghai Province Kashin-Beck disease district environment from 2007 to 2009. China J Endemiol 26:119–121 (In Chinese)
Wang D, Yang W, Sun Q et al (1987) Study on the etiology of Kashin Beck disease areas of Henan province and the relationship between environmental and human selenium. China J Endemiol 6(2):108–111 (In Chinese)
Gao G, Zhang J, Si Y et al (2004) Investigation of hair selenium levels of preschool children in Xinxiang districts. J Appl Clin Pediatr 19:609–610 (In Chinese)
Tan J (1990) Chemico-geography of some life elements and endemic diseases with an emphasis on China. Environmental life elements and health. Science Press, Beijing, pp 145–157
Lv Y, Yu T, Zhao W et al (2014) Constraint on selenium bioavailability caused by its geochemical behavior in typical Kashin–Beck disease areas in Aba, Sichuan Province of China. Sci Total Environ 493:737–749
Li S, Xiao T, Zheng B (2012) Medical geology of arsenic, selenium and thallium in China. Sci Total Environ 421–422:31–40
Hou S, Zhu W (1982) Study on the hair selenium concentration and its background value in different natural environment of China. Environ Sci 2:29–35 (In Chinese)
Zhaxi S, Gong H, Gesang D et al (2003) Selenium test results analysis in Tibet Kashin Beck Disease areas. China J Ctrl Endem Dis 18:177 (In Chinese)
Morris YC et al (1970) Selenium contents of foods. J Nutrit 100:1383–1388
Klapec T, Mandic ML, Grgic J et al (2004) Selenium in selected foods grown or purchased in eastern Croatia. Food Chem 85:445–452
Sirichakwal PP, Puwastein P, Polngam J et al (2005) Selenium content of Thai foods. J Food Compos Anal 18:47–59
Pappa EC, Pappas AC, Surai PF (2006) Selenium content in selected foods from the Greek market and estimation of the daily intake. Sci Total Environ 372:100–108
Acknowledgments
We gratefully acknowledge funding from the National 12th Five-Year Plan scientific and technological issues (No. 2013BAC04B03) and the National Natural Science Foundation of China (No. 41171081). We also thank the local government of Tibet Centers for Disease Control and Prevention and Lhasa Centers for Disease Control and Prevention, Tibet Autonomous Region for their help during the field investigations and sampling, which were carried out in August 2013. The editor and two anonymous reviewers are thanked for their helpful comments on the manuscript to improve the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Z., Li, H., Yang, L. et al. Hair Selenium Levels of School Children in Kashin–Beck Disease Endemic Areas in Tibet, China. Biol Trace Elem Res 168, 25–32 (2015). https://doi.org/10.1007/s12011-015-0333-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0333-4