Abstract
The causes of Kashin–Beck disease (KBD) in children are multifactorial, and particular consideration has been given to childhood selenium (Se) deficiency. In this study, dietary intake of Se and mercury (Hg) was determined at KBD areas to investigate the Se status and risks. Therefore, total Hg and Se levels were investigated in scalp hair samples and in daily intake food samples of 150 schoolchildren in Yongshou County of Shaanxi, China. The results showed that the average concentration of Se in children’s hair has risen to 302 ng g−1 and significantly increased compared to the data reported decades ago. Children at KBD endemic areas likely have improved Se status due to the Se supplementation in food at recent decades. However, all the children in the study areas still showed lower Se status compared to those in other non-KBD areas of China. The probable daily intake of Se in the study areas was still lower after stopping Se supplementation in food at KBD areas, which is 17.96 μg day−1. Food produced locally cannot satisfy the lowest demand for Se nutrition for local residents. If the interactions of Se–Hg detoxification are considered, Hg intake from food exacerbates Se deficiency at the KBD areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential trace element in the diet because it is necessary to make the selenocysteine and selenomethionine found in some selenoproteins. Several physiological functions are restricted by Se, such as antioxidant selenoenzyme synthesis, heavy metal detoxification, reproductive function potentiation, anticancer DNA repair and immune system enhancement (Mehdi et al. 2013; Ognjanovic et al. 2012). Previous studies suggested that Se is the fundamental for the redox-mediated prevention and specially repairs brain’s and neuroendocrine tissue’s oxidative damage (Whanger 2001; Zhang et al. 2014; Rocha et al. 2014). For example, the antioxidant selenoenzyme is an important component of several antioxidant systems (e.g., glutathione peroxidase, thioredoxin reductases and selenoproteins) that actively protect human tissues against the damage produced by free radicals and reactive oxygen species (Li and Shah 2004).
Childhood Se deficiency is the most common phenomenon in underdeveloped countries or regions, which can result in physiological and pathological impairments. Epidemiological studies show that Se deficiency can attribute to the occurrence of muscular dystrophy, reducing reproductive capacity, night blindness, necrosis of internal organs and cancer (Taylor et al. 2009; Afridi et al. 2015). Additionally, another well-known chronic, endemic, degenerative osteoarthropathy to China caused by Se deficiency is Kashin–Beck disease (KBD), which is associated with more than 90% mortality and usually occurs in adolescence in low-Se areas. China has the highest KBD incidence, cases of which were found at 303 counties in 15 provinces. The daily intake of Se in food is < 10 μg day−1 at KBD areas (Whanger 2001); therefore, it is important to uptake adequate Se for maintaining the normal physiological function and activity of essential selenoproteins (Zhang et al. 2014).
The mechanisms of intestinal Se absorption are not fully clear, which differ with the chemical form of the element. Previous study suggests that selenite is absorbed by simple diffusion; selenate is cotransported by sodium selenate and exchange selenate/OH−; selenoamino acid (selenomethionine, selenocysteine) follows the mechanisms of amino acid absorption (Mehdi et al. 2013). The ingested selenoamino acid is absorbed in the small intestine by an active mechanism similar to that used for methionine, which is via the transport system of neutral amino acids sodium (Vendeland et al. 1994; Mehdi et al. 2013). After absorption, Se is carried to the plasma and then is delivered to all tissues. Se concentrations in the whole blood, plasma and urine are used as biological markers of Se nutritional status of recent intake and the activity of GSH-Px in red blood cells can reflect Se status over a 3-month period indirectly (Lorenzo Alonso et al. 2005). Additionally, there is a significant correlation between the dietary intake of Se and its concentration in whole blood. Hair has been shown to be a major medium for excretion of nutrient elements and toxic metals, and the concentration of element in hair is up to tenfold higher than the levels found in blood or urine samples. Moreover, Se concentrations in hair are suggested to provide an assessment reflecting a longer interval of Se status in the human body (Thomas et al. 2013).
Mercury (Hg) and its compounds [e.g., methylmercury (MeHg)] are nonessential and deleterious to humans and animals (Zhou et al. 2013), which can cross the blood–brain and placental barriers, affecting the central nervous system, kidneys and liver and causing attention deficit and mental retardation during childhood (Du et al. 2016; Zhou et al. 2016). Hg can exert its neurotoxicity to increase free-radical production, resulting in inactivates antioxidant defenses; binding to Se forming selenomercury (HgSe) complexes, reducing Se bioavailability for glutathione peroxidase activity (Sakamoto et al. 2013). The Se antagonizes against Hg toxicity by forming less toxic HgSe precipitates in animal tissues which has been recognized for nearly several decades (Ganther et al.1972; Khan and Wang 2009; Zhang et al. 2014). However, the physiological detoxification of HgSe formation may result in Se deficiency, reducing corresponding selenoprotein activity and synthesis, which cause series of consequent adverse effects (Taylor et al. 2009; Zhang et al. 2014). Toward a molecular understanding of the Se–Hg antagonism, the antidotal effect of antagonism between Hg and Se occurs by ensuring that general selenoprotein activity and function are maintained (Khan and Wang 2009). In other words, not only is Se great for Hg detoxification, but Hg can also have an adverse effect on Se bioavailability.
No studies have focused on the effect of Hg on Se bioavailability at KBD areas. It is noteworthy to find whether Hg intake exacerbates Se deficiency in low-Se areas. We conducted an environmental and epidemiologic study in a KBD community in central China, aiming to investigate Se status and determine the dietary intake of Se and Hg and their concentrations in human hair. Study methods were chosen because human Se and Hg concentrations in hair samples widely used as a criterion of indicator for exposure to Hg and assessment of nutritional status for Se through dietary intake (Rocha et al. 2014; Fang et al. 2012).
Materials and methods
Study area
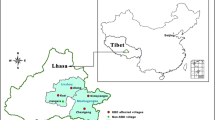
Our study was conducted in Yongshou County of Shaanxi Province in central China, which is located about 80 km northeast of the center of Xianyang City, at an elevation from 600 to 1476 m (Fig. 1). The climate mainly belongs to temperate continental monsoon climate with annual temperature of 10.8 °C and annual rainfall of 302.7 mm. Previous study have showed that the mean soil Se concentration in Kashin–Beck disease areas of Yongshou was 86.5 μg kg−1, which defined as Se-deficient soils (Qian 2016). The local economy is undeveloped, as the per capita gross domestic product (22,240 RMB, 3400 USD) was about half of the national average in China in 2014. The population in 2014 was 200,000, with the rural population constituting over 80% of the total. Our study was conducted in five villages: Qianjin, Yingli, Xinhua, Nansong and Shangqiu. Among them, one site located in non-KBD endemic area was selected as the control, which was Qianjin village. The other four sites were situated at KBD endemic areas.
Sample collection and preparation
Sampling was conducted in July 2013. In total, hairs of 150 children (30 for each village) in the Yongshou were sampled and male and female was equally divided. To guarantee an effective collection of hair samples, children with dyed or short hair were not considered as the participators. The ages of the children ranged between 5 and 14 years of both genders, who lived in the corresponding village more than 3 years. The parents of sampled subjects were interviewed and asked to complete a questionnaire in order to collect details concerning physical data, ethnic origin, health, medical reports, body weight (bw) and dietary habits. Approximately 0.5 g hair samples with length of 1–3 cm were cut with stainless steel scissors from the occipital region of the scalp, bundled together by stapler, placed and sealed in polythene bags, properly identified and brought to the laboratory. Hair samples were washed with nonionic detergent, acetone, distilled water and then dried in an oven at 60 °C overnight prior to analysis.
Numerous studies have showed that food intake was the major exposure route of nutrients and metals (Zhang et al. 2014; Du et al. 2016). Therefore, six types of diet samples were investigated in the five villages of the county based on the dietary habits of local residents. These comprised staple food (raw wheat); vegetables (vigna sinensis, cabbage, pepper, potato and tomato); meat (pork); poultry (chicken and duck); fish (grass carp, yellow-headed catfish and sliver carp). Raw wheat as staple food samples from each children’s home was collected; homegrown vegetables were sampled in the local field; meat, poultry and fish were purchased from the nearest markets; tap water was randomly collected from children’s home (n = 8 for each village). The fresh samples were freeze-dried and weight before and after drying. Biological samples were completely ground to a fine powder in a pre-cleaned food blender and then poured through a 150-µm sieve to analyze Se and Hg concentrations. Egg samples were broken and homogenized directly to analyze Se and Hg concentrations. Latex gloves were used throughout the whole preparing campaign.
All children and their guardian gave their informed consent for inclusion before they participated in the study. Besides, the study protocol was approved by the Yongshou Health Bureau of Shaanxi and the agreements of the five villages and was conducted in accordance with the Declaration of Helsinki, and the protocol obtained ethics approval from the Institute of Geochemistry, Chinese Academy of Sciences.
Analytical methods
For Se analysis, all the biological samples were placed separately in 250-mL beakers, digested in a mixed acids of concentrated HNO3 and HClO4 (V:V = 9:1) and stayed overnight. Then the solution was placed on a hot plate until the solution became clear, subsequently cooled down, adding 5 mL HCl, placed on a hot plate to the solution became clear once again. During the operation, the temperature was controlled less than 150 °C to prevent Se from volatilizing. The analysis of Se was carried out by means of atomic fluorescence spectrophotometer (AFS) (Beijing Haiguang Instrument, Co., Ltd).
Hair Hg levels were measured in a sample of 20 children from each site (10 males and 10 females), so 100 hair samples were measured from the five villages. All the biological samples were measured in triplicate following US EPA Method 7473 using a Milestone™ DMA-80 Direct Hg Analyzer without acid digestion, after method optimization and validation (USEPA 2007). Quality assurance and quality control are shown in the Supporting Information (Text S1 and Table S1).
Se/Hg intake assessment
Based on the present understanding of Se–Hg antagonism by forming a selenomercury compounds in tissue, a new criterion for assessing probable daily intake (PDI) of Se and Hg exposure was proposed and detailed by Zhang et al. (2014), as shown below:
Briefly, BRV represents the benefit–risk value, which indicates either health benefits (if 0 < BRV < ∇Se) or health risks (if BRV < 0 or BRV > ∇Se); ΔSe represents the minimal Se amount required for normal biological function when Hg exposure is zero, which is 11 nmol kg−1 bw day−1 (equivalent to 0.83 μg kg−1 bw day−1 or 26 μg d−1 recommended by the Chinese Nutrient Society if bw is averaged to be 32 kg for children in the study area (CNS 1990) or 40 μg d−1 for 9–13 years recommended by US FNB (2000). ∇Se represents a threshold value for Se poisoning which considered the protective effects from Hg exposure, and the ∇Se is 170 nmol kg−1 day−1 (equivalent to 430 μg day−1, or 13.3 and 11.4 μg kg bw−1 day−1, respectively, for Chinese residents and US residents); the PDI of Se (PDISe) and Hg (PDIHg) was assessed using a food frequency questionnaire. C refers the Hg concentrations of the exposed medium; and IR refers to the daily intake rate (the rate of ingestion or inhalation); and i is the intake of a potentially Hg- or Se-contained substance such as water, wheat, fish, vegetable, meat and egg. Therefore, Ci and IRi represent the concentrations and intake mass of Hg- or Se-contained substance. The molar concentrations were used during the above calculations; for example, PDI is measured in nmol kg bw−1 day−1.
Result and discussion
Se and Hg concentrations in hair
As shown in Fig. 2, the mean hair Se levels of children in the KBD areas were ranged from 283 ± 14 to 320 ± 77 ng g−1, and that in non-KBD area was 287 ± 35 ng g−1. It is interesting that Se concentrations in hair did not differed significantly between the KBD and non-KBD areas. The reason may be that the difference of Se concentrations in the foods was not significantly different among the five villages, which resulted similar dietary intake of Se at KBD areas and non-KBD areas. Additionally, no significant differences in hair Se concentrations of boys among the village groups were evident, as well as those of girls between the five groups (Table 1, T test, p > 0.05 for all). Over 94% of the hair, Se concentrations in the study sites were between 250 and 350 ng g−1.
Based on our study in the KBD area of Yongshou, 97% of the participants (146 children) had normal hair Se level, according to the threshold of hair Se value classified by past research efforts related to Se-endemic disease in China (Tan 1990). Furthermore, it is noteworthy that the mean hair Se level of children observed in the KBD areas in our study was much higher than the data observed decades ago at KBD areas in central area of Shaanxi Province, which was 59 ± 21 ng g−1 in 1980s (Li et al. 1982). Compared with the study of KBD areas in other sites of China, the hair Se concentrations of children at KBD areas of our study area were slight higher (Chen et al. 2015; Wang et al. 2016), which may benefit from Se supplementation in food and dietary intake at KBD areas. Particularly, the Se concentrations of staple foods consumed by children were obviously increased in the study area compared to those of decades ago, which will be discussed in the below section. However, in comparison with the data reported in non-KBD areas, the mean hair Se was much lower than the data in central China (Fang et al. 2012; Gao et al. 2011; Jin et al. 2006).
Hair Hg for all 100 children ranged from 22.6 to 252.2 ng g−1, with an average concentration of 83.0 ± 43.1 ng g−1. Approximately 68% of the children were in the range of 50–150 ng g−1, and all the hair Hg concentrations did not exceed the reference value of 1000 ng g−1 as suggested by the United States Environmental Protection Agency (USEPA) (1997) and the WHO’s recommended threshold value of 2000 ng g−1 (Fang et al. 2012). The average hair Hg from our study area was lower than that reported for fish-eating children populations around the world, such as children in Amazonia (2870 ± 2130 to 16,550 ± 11,440 ng g−1) (Barbosa et al. 2001), Negro river basin of Brazil (18,520 ng g−1) (Barbosa, et al. 2001), Barreiras of Brazil (5640 ± 5550 ng g−1) (Dorea and Barbosa 2005) and Island of Madeira of Portugal (3820 ng g−1) (Rocha et al. 2014). Compared to some urban areas and background areas in Qingdao City (260 ng g−1, Zhang 2009) and Anhui Province (480 ng g−1, Niu et al. 2016) of China, the hair Hg concentrations also showed much lower concentrations in our study area, which illustrated that there is no immediate risk of Hg exposure at the KBD areas of Yongshou.
There is a negative significant relation between nanomolar Hg and Se for all hair samples (p < 0.01, Fig. 3), indicating the evidence for Se–Hg antagonism. Se is the essential trace element to make the selenoproteins that present an important role in many body functions, such as antioxidant defense, the formation of thyroid hormones and cancer prevention (Mehdi et al. 2013). Additionally, Se induces antibody formation in the immune system. In contrast, Hg may cause a variety of adverse effects, with damage to the central nervous system and kidneys being the most prominent; MeHg, the more toxic form, can pose a threat to the sensory, visual, and auditory functions, cerebellum and the cardiovascular system (Du et al. 2016). In living organisms, the physiological/biochemical interaction of antagonism between Se and Hg depends on their mole concentrations (Khan and Wang 2009), while the microcosmic mechanism likely involves the compound of insoluble and biologically unavailable selenomercury (HgSe) precipitates (Pinheiro et al. 2005). Approximately 1:1 or greater molar ratios of Se/Hg often interpreted to indicate detoxification or formation of metabolically inert HgSe precipitates, although less than 1:1 molar ratio often suggested the possibility of Hg toxicity (Khan and Wang 2009). The constant of Hg and Se binding affinity (1045) is extraordinarily intimate, which is about 106-fold higher than that of Hg and sulfur (1039). Thus, an interaction between Se and Hg can easily form the metabolically inert HgSe precipitates. Previous study has showed that the Hg and Se in blood bind to plasma protein to generate high molecular weight chelate, which was described as (Hg–Se) n -selenoprotein P (or (Hg–Se) n -SelP) (Yang et al. 2008), which was suggested to be the precursor of the HgSe(s) (Khan and Wang 2009). Recently, X-ray absorption near edge structure has proved the existence of inert HgSe(s) granules in vivo. Additionally, the more toxic species of MeHg can disrupt the glutathione (GSH) system maturation reducing the GSH-Px in the developing brain of children, but this toxic effect can be protected by Se because of Se decreasing the overall oxidative stress induced by MeHg (Zhang et al. 2014). The significantly negative correlation between Hg and Se may imply that acute Hg toxicity might be partially alleviated by Se. Biologically, Se is incorporated in multiple forms of glutathione peroxidases and thioredoxin reductases, which can interact with Hg to form nontoxic metallothionine. Therefore, the reaction of Se and Hg to inert HgSe granules in vivo results in the relation of the two elements in children’s hair.
Previous studies showed that gender could be an important factor on hair Se levels in populations (Chen et al. 2015; Thomson et al. 2007; Brtková et al. 1999). For example, in the KBD areas of Tibet, the Se status of boys was higher than that of girls (Chen et al. 2015). Similar phenomenon was also found in the KBD areas of Jilin Province (Xu et al. 2013) and Gansu Province (Xu et al. 2013) in China. Moreover, in some non-KBD areas as in the USA, the blood Se concentration in boys was also higher than that in girls, and the same phenomenon was documented in the New Zealand and Slovakia (Thomson et al. 2007; Brtková et al. 1999). In addition to hair Se concentrations, Díez et al. (2008, 2011) suggested that Hg also showed significant higher concentrations in males than that in females. Potential explanations for the differences of both Se and Hg between the genders may include a difference in daily food intake of energy, consumption habits, iron status or hormonal influences, which can influence the bioavailability of trace elements (Clark et al. 2007; Kazi et al. 2008; Li et al. 2014). However, in our study area in Yongshou, the status of Se and Hg in children’s hair did not show the trend of higher concentrations in male than that in female and had no difference between the two genders. The reason may be that the daily food intake and consumption habits for boys and girls were similar in our study area based on our questionnaire.
Se and Hg concentrations in staple food
Wheat is one of the staple foods of humans. Indeed, staple food consumption has been demonstrated to be accounted for 70% of total dietary Se intake in rural populations in inland China (CNS 1990). The Se concentrations averaged 20.91 ng g−1 in wheat collected from each child’ home in the KDB areas and did not differ significantly among the four villages (p > 0.05). Additionally, no significantly different Se concentrations were also observed in the control village, which is 20.5 ng g−1. Nevertheless, the mean wheat Se level observed in the KBD areas of Yongshou in the study was obviously higher than the data observed decades ago at KBD areas in Shaanxi Province, i.e., 7 ± 3 ng g−1 sampled in 1982 (Li et al. 1982), as well as 16.5 ng g−1 reported in 1995 (Zhu et al. 1995). The Se levels in food are be closely related to the Se levels in the soils where the plants are grown (Wang et al. 2016). In a recent study, the average soil Se levels in the KBD area of Yongshou was 86.5 ng g−1 (Qian 2016), which were reduced compared to the topsoil concentrations in Guanzhong Plain of Shaanxi (100–117 ng g−1) and China as a whole (240 ng g−1) (Chen et al. 1984). Therefore, the low Se content in the local soils produced low Se level in local agricultural products such as wheat, vegetables, meat, fish and poultry (Table 2). However, compared to soil Se level decades ago, the Se content was obviously elevated due to the implementation of Se fertilizer to soil in the process of agricultural production. Although the wheat Se concentration had more than doubled since 1980s, the wheat Se level was still in the range of Se deficiency according to the threshold of Se in environment and health (Combs et al. 1987).
Hg concentrations in wheat are shown in Table 1. Hg concentrations in wheat collected from the children’s homes ranged from 0.2 to 1.6 ng g−1, with a mean of 0.7 ng g−1, and the mean concentration of all the samples was 30-fold lower than the tolerance limit of Hg in human food (20 ng g−1) as recommended by the Chinese National Standard Agency (CNSA 1994). The averages of wheat Hg concentrations at each site ranged from 0.6 to 0.8 ng g−1, and no significant differences were observed. The Hg levels in food are mainly controlled by the Hg levels in the soils and atmosphere. According to our survey, our study areas are relatively isolated from direct anthropogenic Hg emissions sources (e.g., chlor-alkali plants, cement production, non-ferrous metal smelters and coal combustion), so that the primary sources of Hg are limited (Qian 2016).
Probable daily intake (PDI) of Se and Hg
The PDI assessment of Se and Hg presents many difficulties due to the absence of specific Chinese food composition tables. Analysis of a person’s diet in detail is impossible, so we have analyzed the Se and Hg content in the main foods consumed in KBD and control communities, including wheat, vegetable, meat, poultry, fish and water (Table 2). According to the results, wheat (34%), vegetables (31%) and meat (26%) were the main routes of Se intake for residents in Yongshou, whereas a combination of fish, poultry and water constituted only 9% of the total PDISe (Table 2). In the 1980s, Environmental Groups of Scientific Expedition had investigated Se intake by grain and hair Se concentrations and significant correlation was observed, indicating the main source of Se intake was from local staple food. However, with the economy development, people’s diets are developing from simplification to diversification; the PDISe of wheat by estimation from the present illustrative assessment was reduced and accounted for about 34%, and there was no significant correlation between PDISe of wheat and hair Se concentrations (p > 0.05).
In general, all the children from KBD and non-BD in Yongshou showed PDISe lower than the RDA (20 μg d−1 for 1–3 years; 30 μg d−1 for 4–8 years; 40 μg d−1 for 9–13 years) by US FNB and the lowest safe intake of Se for a human is (0.83 μg kg−1 bw day−1 or 26 μg d−1 if bw is averaged to be 32 kg for children in the study area) recommended by CNS. Our results suggested that locally produced staple and non-staple food could not satisfy the lowest demand for Se nutrition for local residents.
To reduce the risks of disease resulted from lower Se intake, the government of Shaanxi Province decided to provide the Se salt to alleviate Se deficiency since the late 1990s, which finally got effective control of KBD. However, unfortunately, salt with Se was stopped serving in the province in June 1, 2012. Short-term clinical symptoms may show no signs of malnutrition causing by the lack of specific trace nutrients, as it is not completely related to economic situation and deficient caloric intake. Deficiency of trace nutrients is associated with invisible health impairments; however, it can induce long-term damages, including the risks for developing severe disease and the serious injury to a healthy life (Rocha et al. 2014). Therefore, stopping supply of salt with Se may increase a higher risk of developing severe KBD in these areas.
Bade on our calculated data, the average PDISe at KBD areas was 18.3 μg day−1 and was not significantly different with the control site of Qianjin (17.7 μg day−1). As hair Se levels can be a good indicator for nutritional status for Se through dietary intake, the concentrations of Se in hair samples were similar among the five study sites as we stated above. The PDISe in our study areas may be comparable to that in regions with high rates of Se deficiency such as KBD area in Jilin Province and 6 other counties in Shaanxi Province in China, where similar Se concentrations in staple food were found (Zhu et al. 2010; Yang et al. 2010). However, the average PDISe in Yongshou was 4–26 times less than that in regions with adequate Se intake levels (e.g., the US range of 71–152 μg day−1 and 85–478 μg day−1 in Wanshan, China) (ATSDR 1996; FNB 2000; Zhang et al. 2014) and approximately 2 times less than in areas with moderate rates of Se deficiency (e.g., 34 μg day−1 in the UK and 44 μg day−1 in Suzhou, China) (Gao et al. 2011). According to the results, wheat (32%), vegetables (32%) and meat (27%) were the main routes of Se intake for residents in Yongshou, whereas a combination of fish, poultry and water constituted only 9% of the total PDISe (Table 2).
According to our estimates from the present illustrative assessment, all of the sites in the Yongshou area exhibited PDIHg values (mean 3.380 μg day−1, 0.06–0.17 μg kg bw−1 day−1) below the provisional tolerable weekly intake (PTWI) of 4 μg kg bw−1 week−1 (equivalent to 0.57 μg kg bw−1 day−1) by the Joint Food and Agriculture Organization (FAO)/WHO Expert Committee on Food Additives (JECFA 2010) due to much lower food Hg concentrations in our study areas.
Assessment of Se–Hg interactions
Studying the physiological role of Se has lately been rapidly developing field in biomedical research (Gao et al. 2011), and many researchers have also been concerned about Se protection against Hg toxicity (e.g., Kuklinski et al. 1992; Zhang et al. 2012, 2014; Afridi et al. 2015). However, the Hg binds to Se forming HgSe precipitates, reducing Se availability for essential Se-dependent proteins and enzyme synthesis activity (Kobal et al. 2004). It reasonably assumed that not only does Se have an effect on Hg’s bioavailability, but conversely, Hg can also have an effect on Se physiological functions (Afridi et al. 2015). The basic propensity of Hg, in combination with Se sequestration in vivo, may prohibit biosynthesis of essential selenoproteins. And the “protective effect” of Se on the detoxification of Hg can explain that sufficient free Se must be applied to maintain normal Se-dependent selenoproteins synthesis and activity (Afridi et al. 2015; Hill et al. 2003). Therefore, an assessment that considers Se–Hg interactions is appropriate to evaluate the risk from exposure to Hg and deficiency to Se suggested by Zhang et al. (2014). If the detoxification of 1:1 molar ratios of Se/Hg was considered (Khan and Wang 2009), the Hg intake would reduce approximately 10% of the PDISe in Yongshou KBD areas. Additionally, based on calculation of Eq. (1), we found that all the sites showed BRV < 0, indicating health risks exacerbated when assessed using criteria that considered the protective interactions between Se–Hg based on their molar concentrations. These results also indicate that previous study considering only the Hg in the environment and foods at KBD areas may have underestimated the level of risk for the local children.
Conclusion
The results of the present study show that children at KBD areas tend to exhibit lower hair Se concentrations, and these are associated with lower daily intake of Se. The nutritional status of Se has improved considerably in comparison with the level of decades ago because of the supplemental treatments to increase Se in food and dietary intake. However, the PDI of Se was much lower than the recommended dietary allowance suggested by FND and WHO. Our results of Se nutritional status of children living at KBD areas of Yongshou suggested that these children face a threatening nutritional risk from deficiency of Se. Significantly negative relation between nanomolar Hg and Se concentrations in hair samples indicated the evidence for Se–Hg antagonism, forming less toxic HgSe precipitates. If Hg-protective Se–Hg interactions are considered to evaluate the risk of Se deficiency, health risks will exacerbate in the KBD areas.
References
Afridi, H. I., Kazi, T. G., Talpur, F. N., et al. (2015). Assessment of selenium and mercury in biological samples of normal and night blindness children of age groups (3–7) and (8–12) years. Environmental Monitoring and Assessment, 187(3), 1–11.
ATSDR, Agency for Toxic Substances and Disease Registry. (1996). Toxicological profile for selenium (update). Atlanta, GA: Public Health Service, Department of Health and Human Services.
Barbosa, A. C., Jardim, W., Dórea, J. G., et al. (2001). Hair mercury speciation as a function of gender, age, and body mass index in inhabitants of the Negro River Basin, Amazon, Brazil. Archives of Environmental Contamination and Toxicology, 40(3), 439–444.
Brtková, A., Magálová, T., Béderová, A., et al. (1999). Serum selenium levels in healthy slovak children and adolescents. Biological Trace Element Research, 67(1), 49–54.
Chen, Z., Li, H., Yang, L., et al. (2015). Hair selenium levels of school children in Kashin–Beck disease endemic areas in Tibet, China. Biological Trace Element Research, 168(1), 1–8.
Chen, D., Ren, S., & Li, J. (1984). Selenium in soils of Shaanxi province. Acta Pedologica Sinica, 102(2), 588–589.
Chinese National Standard Agency (CNSA). (1994). Tolerance limit of mercury in foods (pp. 171–173). Beijing, China: CNSA. (in Chinese).
Clark, N. A., Teschke, K., Rideout, K., et al. (2007). Trace element levels in adults from the west coast of Canada and associations with age, gender, diet, activities, and levels of other trace elements. Chemosphere, 70, 155–164.
CNS. (1990). Chinese nutrition society recommended daily dietary nutrient supply. Acta Nutrimenta Sinica 12(1), 1–9.
Combs, G. F., Spajjholz, J. E., Levander, O. A., & Oldfield, J. E., eds. (1987). Selenium in biology and medicine. In Proceedings of the third international symposium on selenium in biology and medicine (pp. 859–876). Beijing, New York: Van Nostrand Reinhold Company.
Díez, S., Esbrí, J. M., Tobias, A., et al. (2011). Determinants of exposure to mercury in hair from inhabitants of the largest mercury mine in the world. Chemosphere, 84(5), 571–577.
Díez, S., Montuori, P., Pagano, A., et al. (2008). Hair mercury levels in an urban population from southern Italy: Fish consumption as a determinant of exposure. Environment International, 34(2), 162–167.
Dorea, J. G., & Barbosa, A. C. (2005). Fish consumption and blood mercury: Proven health benefits or probable neurotoxic risk? Regulatory Toxicology and Pharmacology, 42(2), 249–250.
Du, B., Li, P., Feng, X., et al. (2016). Mercury exposure in children of the Wanshan mercury mining area, Guizhou, China. International Journal of Environmental Research and Public Health, 13(11), 1107. doi:10.3390/ijerph13111107.
Fang, T., Aronson, K. J., & Campbell, L. M. (2012). Freshwater fish–consumption relations with total hair mercury and selenium among women in eastern China. Archives of Environmental Contamination and Toxicology, 62(2), 323–332.
FNB, Food and Nutrition Board USA Institute of Medicine. (2000). Dietary references intakes for vitamin C, vitamin E, selenium and carotenoids. Washington: National Academy Press.
Ganther, H. E., Goudie, C., Sunde, M. L., et al. (1972). Selenium: Relation to decreased toxicity of methylmercury added to diets containing tuna. Science, 175, 1122–1124.
Gao, J., Liu, Y., Huang, Y., et al. (2011). Daily selenium intake in a moderate selenium deficiency area of Suzhou, China. Food Chemistry, 126(3), 1088–1093.
Hill, K. E., Zhou, J., McMahan, W. J., et al. (2003). Deletion of selenoprotein P alters distribution of selenium in the mouse. Journal of Biological Chemistry, 278(16), 13640–13646.
JECFA, (Joint FAO/WHO Expert Committee on Food Additives). (2010). Joint FAO/WHO food standards programme, committee of the codex alimentarius commission, 33rd session. Geneva, Switzerland, July 5–9.
Jin, L., Liang, L., Jiang, G., & Xu, Y. (2006). Methylmercury, total mercury and total selenium in four common freshwater fish species from Ya-Er Lake, China. Environmental Geochemistry and Health, 28, 401–407.
Kazi, T. G., Afridi, H. I., Kazi, N., et al. (2008). Copper, chromium, manganese, iron, nickel and zinc levels in biological samples of diabetes mellitus patients. Biological Trace Element and Research, 122(1), 1–18.
Khan, M. A. K., & Wang, F. (2009). Mercury–selenium compounds and their toxicological significance: Toward a molecular understanding of the mercury–selenium antagonism. Environmental Toxicology and Chemistry, 28, 1567–1577.
Kobal, A. B., Horvat, M., Prezelj, M., et al. (2004). The impact of long-term past exposure to elemental mercury on antioxidative capacity and lipid peroxidation in mercury miners. Journal of Trace Elements in Medicine and Biology, 17, 261–274.
Kuklinski, B., Buchner, M., Muller, T., & Schweder, R. (1992). Anti-oxidative therapy of pancreatitis—An 18-month interim evaluation. Zeitschrift fur die Gesamte Innere Medizin und Ihre Grenzgebiete, 47(6), 239–245.
Li, S., Bañuelos, G. S., Wu, L., & Shi, W. (2014). The changing selenium nutritional status of Chinese residents. Nutrients, 6, 103–1114.
Li, J., Ren, S., & Chen, D. (1982). A study of Kashin–Beck disease associated with environmental selenium in Shanxi area. Acta Scientiae Circumstantiae, 2(2), 129–134.
Li, J. M., & Shah, A. M. (2004). Endothelial cell superoxide generation: Regulation and relevance for cardiovascular pathophysiology. American Journal of Physiology-Regulatory Integrative and Comparative Physiology, 287, R1014–R1030.
Lorenzo Alonso, M. J., Bermejo, B. A., Ja, C. D. J., et al. (2005). Selenium levels in related biological samples: Human placenta, maternal and umbilical cord blood, hair and nails. Journal of Trace Elements in Medicine and Biology, 19(1), 49–54.
Mehdi, Y., Hornick, J. L., Istasse, L., & Dufrasne, I. (2013). Selenium in the environment, metabolism and involvement in body functions. Molecules, 18(3), 3292.
Niu, Z., Wang, Y., & Liu, G. (2016). Investigation on hair mercury concentration of people in 8 typical regions of Anhui Province and its influence factors. Environmental Chemistry, 35(3), 533–539.
Ognjanović, B. I., Djordjević, N. Z., Matić, M. M., et al. (2012). Lipid peroxidative damage on cisplatin exposure and alterations in antioxidant defense system in rat kidneys: A possible protective effect of selenium. International Journal of Molecular Sciences, 13(2), 1790–1803.
Pinheiro, M. C. N., Muller, R. C. S., Sarkis, J. E., et al. (2005). Mercury and selenium concentrations in hair samples of women in fertile age from Amazon riverside communities. Science of the Total Environment, 349, 284–288.
Qian, D. (2016). Mercury exposure and relationship between selenium and mercury at low selenium area of Yongshou, Shanxi Province. Master Thesis, China West Normal University.
Rocha, A. V., Almondes, K. G., Almeida, I. S., et al. (2014). Selenium nutritional status of women living in risk area of mercury contamination. Conference: Bioavailability, 39, 1–57.
Sakamoto, M., Yasutake, A., & Kakita, A. (2013). Selenomethionine protects against neuronal degeneration by methylmercury in the developing rat cerebrum. Environmental Science and Technology, 47(6), 2862–2868.
Tan, J. (1990). Chemico-geography of some life elements and endemic diseases with an emphasis on China. Environmental life elements and health (pp. 145–157). Beijing: Science Press.
Taylor, D., Dalton, C., Hall, A., et al. (2009). Recent developments in selenium research. British Journal of Biomedical Science, 66(2), 107–116.
Thomas, V. V., Knight, R., Haswell, S. J., et al. (2013). Maternal hair selenium levels as a possible long-term nutritional indicator of recurrent pregnancy loss. BMC Womens Health, 13(1), 1–6.
Thomson, C. D., McLachlan, S. K., Parnell, W. R., et al. (2007). Serum selenium concentrations and dietary selenium intake of New Zealand children aged 5–14 years. British Journal of Nutrition, 97(02), 357–364.
United States Environmental Protection Agency (USEPA). (1997). Mercury study report to the congress, volume V: Health effects of mercury and mercury compounds. Washington, DC, USA: USEPA.
USEPA. (2007). Method 7473: Mercury in solids and solutions by thermal decomposition, amalgamation, and atomic absorption spectrophotometry. Washington, DC.
Vendeland, S. C., Deagen, J. T., Butler, J. A., & Whanger, P. D. (1994). Uptake of selenite, selenomethionine and selenate by brush border membrane vesicles isolated from rat small intestine. BioMetals, 7, 305–312.
Wang, J., Li, H., Yang, L., et al. (2016). Distribution and translocation of selenium from soil to highland barley in the Tibetan plateau Kashin–Beck disease area. Environmental Geochemistry and Health, 39(1), 1–9.
Whanger, P. D. (2001). Selenium and the brain: A review. Nutritional Neuroscience, 4(2), 81–97.
Xu, S., Zhang, X., Lv, H., et al. (2013). Selenium levels inside and outside environment of Jinlin Province Kashin–Beck disease area. Chinese Journal of Control of Endemic Diseases, 28, 340–342. (in Chinese).
Yang, D. Y., Chen, Y. W., Gunn, J. M., & Belzile, N. (2008). Selenium, mercury in organisms: Interactions and mechanisms. Environmental Review, 16, 71−92.
Yang, J., Tong, W., Wu, C., & Liu, C. (2010). Selenium level surveillance for the year 2007 of Keshan disease in endemic areas and analysis on surveillance results between 2003 and 2007. Biological Trace Element Research, 138(1–3), 53–59.
Zhang, L. (2009). Study on the human hair mercury levels in the residents living in the different function regions in Qingdao and influence factors. Journal of Safety & Environment, 9(4), 95–97.
Zhang, H., Feng, X., Chan, H. M., & Larssen, T. (2014). New insights into traditional health risk assessments of mercury exposure: Implications of selenium. Environmental Science and Technology, 48(2), 1206–1212.
Zhang, H., Feng, X., Zhu, J., et al. (2012). Selenium in soil inhibits mercury uptake and translocation in rice (Oryza sativa L.). Environmental Science and Technology, 46, 10040–10046.
Zhou, J., Feng, X., Liu, H., et al. (2013). Examination of total mercury inputs by precipitation and litterfall in a remote upland forest of Southwestern China. Atmospheric Environment, 81, 364–372.
Zhou, J., Wang, Z., Sun, T., et al. (2016). Mercury in terrestrial forested systems with highly elevated mercury deposition in southwestern China: The risk to insects and potential release from wildfires. Environmental Pollution, 212, 188–196.
Zhu, J. H., Yang, J., & Xin-Ke, H. E. (2010). Investigation on selenium levels of food supplies and people in Keshan disease area of Shaanxi Province. Chinese Journal of Control of Endemic Diseases, 6, 438–440. (in Chinese with English Abstract).
Zhu, W., Li, R., Yang, L., et al. (1995). Study on the Kashin–Beck disease and selenium in ecological environment of Ankang, Shaanxi. Chinese Journal of Endemiology, 3, 191–193.
Acknowledgements
This research was funded by the National Basic Research Program of China (No. 2013CB934302) and the National Science and Technology Support Plan (2015BAD05B01). The authors acknowledge the participants and D. Qian in College of Chemistry and Chemical Engineering, China West Normal University, for the help of providing samples and the basic data.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Du, B., Zhou, J. & Zhou, J. Selenium status of children in Kashin–Beck disease endemic areas in Shaanxi, China: assessment with mercury. Environ Geochem Health 40, 903–913 (2018). https://doi.org/10.1007/s10653-017-0033-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-017-0033-4