Abstract
Cadmium is a heavy metal of increasing environmental concern that has long been associated to several human pathological processes. Recent population surveys have correlated cadmium non-occupational exposure to widespread idiopathic pathologies. Food and tobacco are reported to be the main exposure sources of cadmium to the general population, as phosphate fertilizers are rich in such a metal, thus contaminating the crops. Although its mechanisms of toxicity are not a consensus in the literature, it is well established that reactive oxygen species play a key role in this process, leading to the oxidation of several biological molecules. We have therefore assessed whether three environmentally realistic doses of cadmium alter the oxidative status of Wistar rat testis and eventually result in histological damages. Our results show that even the lowest environmental dose of cadmium was able to disturb the endogenous antioxidant system in Wistar testis, although an increase in lipid peroxidation was observed only within the group exposed to the highest environmental dose. Despite that no remarkable morphological changes were observed in any group, significant alterations in blood vessel lumen were reported for some cadmium-exposed animals, suggesting that endothelium is one of the primary targets involved in cadmium toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium is a widespread toxic metal that has increasingly been introduced into the environment due to anthropogenic activities. Industrial waste, smelting, and combustion of coal and fossil fuel are among the major contributors to the increase in cadmium concentration in soil, water, and air [1]. Food and tobacco seem to be the main sources of cadmium exposure to human [2, 3], since phosphate fertilizers significantly increase the percentage of cadmium in soil, hence rising cadmium uptake by crops and vegetables grown for human consumption [3–5]. Potato, cereal, and vegetables contribute with approximately 83 % of the daily intake of cadmium from food, followed by meat, egg, dairy products, and fish muscle [3, 6]. Tobacco represents another important exposure source, as well, not only because tobacco leaves are rich in such a metal [7] but also because cadmium is more efficiently absorbed by the lungs than through the gastrointestinal tract [1]. Worldwide population surveys have shown a consistent link between environmental exposure to cadmium and several idiopathic pathologies among non-occupationally exposed subjects. Increased breast and endometrial cancer incidence among postmenopausal women in Sweden [8, 9], raised blood pressure in South Korean adults [10], and higher odds of periodontal diseases in the US population [11] are some of the diseases that have been correlated to environmental exposure to cadmium. The mechanistic pathways through which cadmium exerts its toxicity are not totally understood, although the literature has shown that acute cadmium exposure increases free radical generation, which in turn is known to oxidize a wide range of biological molecules [12, 13].
The testes are particularly sensitive to cadmium, playing an important role in assessing the early stages involved in cadmium toxicity, and therefore are a useful experimental model for determining the environmental safety threshold. In relation to cadmium, it has been shown that it caused a significant decrease in sperm concentration, motility (%), weight of testes, and epididymis, and increase in dead and abnormal sperm in rats that were orally given cadmium (5 mg/kg body weight (BW)) [14]. Taking all these into account, the purpose of the present study was to investigate whether three environmentally realistic doses of cadmium could disturb the testicular oxidative status and eventually result in histological damages [1, 15, 16].
Material and Methods
Chemicals
Cadmium chloride (CdCl2) was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Testis samples were fixated using a Merck glutaraldehyde solution 25 % (Darmstadt, Germany) diluted in cacodylate buffer obtained from Electron Microscopy Sciences (Hatfiels, PA, USA), and samples were embedded in Leica Historesin (Heidelberg, Germany). Biochemical assays were performed using biochemistry kits from Sigma-Aldrich Co. (St. Louis, MO, USA).
Animals
Male Wistar rats were obtained from the Multidisciplinary Center for Biological Investigation (Universidade Estadual de Campinas, SP, Brazil). Animals were housed five per cage under controlled conditions of temperature (24 °C) with a 12-h dark/light cycle and provided with water and food ad libitum. This research was approved by the Institutional Committee for Ethics in Animal Research of this University (Protocol no 2178-1).
Treatment Protocol
Twenty male adult Wistar rats (70 days old) were divided into four different groups containing five animals each: control group (cadmium 0 mg/l), Cd25 group (cadmium 25 mg/l), Cd50 group (cadmium 50 mg/l), and Cd75 group (cadmium 75 mg/l). Except for control, each group was orally exposed to different concentrations of cadmium. Metal salts were diluted in drinking water and given to the animals ad libitum over 30 consecutive days. In order to make the water more attractive and assure that different groups would consume the same amount of water in a time range, sucrose 10 % was added to the water (adaptation from [1]). In order to estimate the total cadmium intake at the end of the treatment, water consumption was measured every 24 h and animals weighed every week.
Fixation and Processing of the Tissue
At the end of the experiment, the animals were anesthetized with xylazine and ketamine (5 and 80 mg/kg BW, respectively). The left testis was dissected free and promptly frozen in liquid nitrogen and stored at −80 °C until assayed. The animals were subsequently perfused via the thoracic aorta using a solution of glutaraldehyde 5 % in 0.05 M cacodylate buffer at pH 7.4 [17]. The right testis fragments were fixed overnight and subsequently processed for light microscopy using routine techniques. For histological analysis, the testis fragments were embedded in Historesin, sectioned at a thickness of 3 μm and hematoxilin-eosin-stained.
Stereological Analyses
Representative areas of testicular tissue were photographed with an Olympus Bx-40 microscope and subjected to stereological analyses with an image system: Pro-Plus software version 4.5 (Media Cybernetics). A grid mask system was placed over the images and points were classified as one of the following: seminiferous tubule, intertubular space, blood vessel lumen, macrophage, Leydig cell cytoplasm, and Leydig cell nucleus. The volumetric proportions between seminiferous tubule and intertubular space were assessed by using a grid mask with 850 points placed over 10 fields (8500 points) for each animal at × 200 magnification. The volumetric proportions of the intertubular space components were assessed by using a grid mask of 850 points placed over 10 fields (8500) for each animal at × 1000 magnification. The volume, expressed in milliliters, of each component described above was determined as the product of the testicular volume and volumetric proportions. Since the specific gravity of the testis is nearly 1.0, its volume was considered the same as its weight [18]. To obtain a more precise liquid testis volume, 6.5 % of its weight, relative to the tunica albuginea, was excluded from this organ’s weight [19].
Leydig Cell Stereology
The proportion between nucleus and cytoplasm of Leydig cell was assessed by using a grid mask with 850 points placed over images at × 1000 magnification. One thousand points per animal were counted either over nuclei or cytoplasm of Leydig cells. The nuclear diameter of Leydig cells was obtained by measuring 10 nuclei/animal. The nuclear volume was calculated by using the 4/3πr 3 formula, where r was the mean nucleus radius. The individual volume of Leydig cells was obtained from the nucleus volume and the proportion between nucleus and cytoplasm. The number of Leydig cells per testis was obtained by dividing the total nuclear volume of these cells by the average individual nuclear volume.
Preparation of Rat Testis Homogenate
Rat testis (0.3 g) was weighed and added to 300 μl of 5 % 5-sulfosalicylic acid (SSA), followed by homogenization and subsequent addition of the same amount of SSA. The mixture was kept on ice for 10 min and further centrifuged at 10,000g for 10 min at 4 °C. The pellet was discarded and the volume of the supernatant was measured and kept on ice until use.
Assay of Lipid Peroxidation
Lipid peroxidation was assayed by the generation of thiobarbituric acid-reactive substances (TBARS) [20]. Briefly, samples of 1 mg/ml were mixed with 0.4 ml of 1 % TBA in 50 mM NaOH, 0.2 ml of 20 % H3PO4, and 40 μl of 10 N NaOH. The mixture was heated at 80–90 °C for 15 min. After cooling, 1.5 ml of butanol was added to the solution. The mixture was shaken and centrifuged at 3000 rpm during 5 min. The optical density of the organic layer was determined at 535 nm in an SLM Aminco DW2000 spectrophotometer. Under these conditions, the molar extinction coefficient used to calculate TBARS concentration was 1.56 × 105 M−1 cm−1.
Catalase Activity
Initially, a standard curve with increasing concentrations of H2O2 was made. After obtaining the curve, the assay was performed accordingly to the manufacturer (Catalase Assay Kit, Sigma Aldrich®, catalog #: CAT100). Briefly, testis samples prepared as described above and diluted 1:8 were added to 600 μl of the Enzyme Dilution Solution and then centrifuged for 2 min at 1500 rpm, and the supernatant was used for the enzymatic measurement. The reaction was initiated by the addition of 25 μl Colorimetric Assay Substrate Solution and stopped by the addition of 900 μl Stop Solution. Subsequently, was added to 10 μl of this solution, 1 ml of the Color Reagent, and after 15 min, the absorbance was read at 520 nm.
Determination of total glutathione content
Initially, a standard curve with increasing concentrations of glutathione (GSH) was made. After obtaining the curve, the assay was performed accordingly to the manufacturer (Glutathione Assay kit, Sigma-Aldrich®, catalog #: CS0260). Briefly, 10 μl testis homogenate was added to the working mixture containing assay buffer 1×, glutathione reductase, and NADPH as described by the manufacturer. After 5 min, the absorbance was measured at 412 nm.
Statistical Analysis
All data were presented as the mean ± standard deviation and analyzed via ANOVA followed by Tukey test using the program for statistical analysis (Statistica). The significance level was p < 0.01.
Results and Discussion
Although the main route of exposure to cadmium among the general population refers to low oral chronic doses, current understanding of cadmium toxicity is usually based on experimental models employing unrealistic highly concentrated injected doses. Epidemiological investigations and experimental models simulating human chronic exposure to environmental cadmium are therefore of major importance for establishing a relationship between cadmium and several pathologies of unspecific etiology.
The present study was thus designed to parallel human chronic oral exposure to environmental cadmium, i.e., by consuming contaminated food and water. Animals were randomly divided into three different cadmium groups (Cd25, Cd50, and Cd75) and subjected to increasing metal doses ranging between low to moderate environmentally realistic doses [1]. Based on average water consumption over 30 consecutive days, the estimated daily intake of cadmium was 3.65, 7.36, and 10.6 mg/kg BW for the Cd25, Cd50, and Cd75groups, respectively.
The biometric data (Table 1) show a significant increase in body weight for the Cd75 group with respect to the remaining groups. Observational surveys showed that these animals were apparently fatter, although the surgical procedure for testis removal did not show any evident increase in body fat. The increased body weight gain is likely to be due to water retention in the body, particularly in lymphatic vessels, which showed a significant increase in all cadmium-treated groups (Table 2). These results are supported by past studies, which have established a consistent correlation between cadmium and edema in several body tissues in animal models [21, 22].
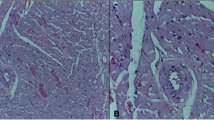
Further morphological changes were also revealed by the stereological analyses (Table 2). Both Cd25 (Fig. 1B, inset b) and Cd50 (Fig. 1C, inset c) groups exhibited a significant increase in both blood vessel lumen volume and volumetric proportion, whereas the Cd75 group (Fig. 1D, inset d) did not show any significant change in this parameter with respect to the control (Fig. 1A, inset a). However, microscopic observations showed a sharp decrease in blood vessel lumen for the Cd75 group, although only a few fields exhibited such morphology. These results suggest a trend toward vasoconstriction, as shown in a previous study [23], and are likely to be accentuated when measured over a longer period of time. No other significant morphological change was observed for all Cd-treated groups with respect to the control group (Tables 2 and 3).
Light microscopy of Wistar rat testicular tissue. A, inset a Representative areas of control animals showing a well-organized seminiferous epithelium with normal blood vessels and Leydig cell clusters; B, inset b Representative areas of the Cd25 animals with a control-like morphology, exhibiting a well-organized seminiferous epithelium, normal blood vessels, and Leydig cell clusters; C, inset c Representative areas of the Cd50 animals with a control-like morphology, exhibiting a well-organized seminiferous epithelium, normal blood vessels, and Leydig cell clusters; D, inset d Representative areas of the Cd75 animals with a control-like morphology, exhibiting a well-organized seminiferous epithelium, normal blood vessels, and Leydig cell clusters. Also, a few constricted blood vessel are seen within some testicular areas in this group. ST seminiferous tubule, Ls lymphatic space, Lc Leydig cell, V blood vessels, (arrow) Sertoli cell. HE-stained
In a previous in vitro study, aortic stripes pretreated with cadmium showed a significant increase in both phenylephrine-induced contraction and · NO-mediated and B-adrenoceptor-mediated relaxation [24]. These results suggest that both vasocontractile and vasorelaxation responses could be enhanced in cadmium-exposed subjects, although either condition might take place depending on the dose. Sutoo and Akiyama [25], for example, demonstrated that cadmium exposure at nanomole concentration increases dopamine levels, hence resulting in reduced blood pressure in spontaneously hypertensive rats. Exposure to higher doses of cadmium (200 ppm), on the other hand, is reported to result in decreased serum · NO concentration in rats, hence reducing its availability in vessel walls [26].
A review of the literature shows that the mechanisms through which cadmium affects the vascular tonus in animal models are therefore broad and not totally comprehended, although reactive oxygen species (ROS) may play an important role in this process. Cadmium is known for increasing ROS in vivo and in vitro, such as superoxide, hydrogen peroxide, hydroxyl radicals (·OH), and lipid-derived radicals from enhanced lipid peroxidation, probably initiated by · OH radicals [13, 27]. Several studies have evidenced the role of ROS as signaling molecules controlling vascular smooth muscle cell (VSMC) contractile activity and growth under physiological condition [28–30]. However, overproduction of ROS is normally associated with several pathological conditions, such as endothelial dysfunctions, increased contractility, VSMC growth and apoptosis, and lipid peroxidation [28, 31, 32].
According with an extensive literature, lipid peroxidation is one of the primary events involved in cadmium toxicity [13, 33]. Our results, however, show that both Cd25 and Cd50 groups did not exhibit any rise in lipid peroxidation with respect to the control (Fig. 2), whereas a sharp enhancement in both catalase activity (Fig. 3) and total glutathione content (Fig. 4) was observed for both groups.
Reduced glutathione (GSH) is reported to provide a first-line defense against cadmium-induced toxicity, complexing with the metal ions and thus reducing its availability in the organism [34, 35]. However, cadmium exposure is also related to the oxidation of protein sulfhydryl groups in a time-dependent and dose-dependent fashion [36], hence resulting in decreased content of GSH in the testis [37, 38]. Nzengue et al. [27] on the other hand showed a significant increase in total glutathione in keratinocyte cell line culture incubated with cadmium at a low concentration (0.2 mg/l) during 24 h, whereas no change in lipid peroxidation was verified. These results strongly suggest that cadmium could induce the transcription of the genes involved in GSH biosynthesis in some cell types, hence preventing the metal from inducing cellular oxidative stress, according to the same author. Based on the conclusions of the above authors, our results strongly suggest that rather than being depleted, glutathione is directly involved in protecting cells against cadmium-induced early cytotoxicity.
As mentioned above, a significant increase in catalase activity was also observed for both Cd25 and Cd50 groups. Our results are supported by a previous study, in which animals exposed to an environmentally realistic dose of Cadmium during 2 months (25 mg/ml) showed a significant increase of catalase activity [37]. Ola-Mudathir et al. [38], on the other hand, reported a significant decrease in catalase activity in Wistar rat testis exposed to oral cadmium at a dose slightly higher than ours, i.e., 15 mg/kg BW/day during 3 weeks. Additionally, a consistent number of studies have also shown a significant decrease in this parameter after cadmium exposure, although it should be considered that the majority of them have employed unrealistic exposure regimes, such as high doses and/or cadmium-solution body injection [13, 33]. Our data thus suggest that catalase was sufficiently activated by the increased generation of ROS, hence playing a crucial role during the early events involved in cadmium environmental exposure. Therefore, despite not observing any significant change in lipid peroxidation in both Cd25 and Cd50 groups, a slight unbalanced increase in ROS production could be enough to trigger cell signaling and thus justify the alterations in the vascular tonus.
Unlike both Cd25 and Cd50 groups, the animals exposed to the highest dose of cadmium (75 mg/l) showed a significant increase in lipid peroxidation (Fig. 2). Moreover, a significant increase in both catalase activity (Fig. 3) and total glutathione was also observed (Fig. 4). Since these animals did not show any significant change in vascular tonus with respect to the control group, we suggest that they exhibit a trend toward vasoconstriction, which is likely to eventually result in hypertension. In a previous study, a significant decrease in serum · NO concentration was observed in animals subjected to oral cadmium during 3 months at a dose of 200 mg/l, suggesting that cadmium can influence · NO sequestration and/or degradation and therefore reduces its bioavailability in vessel walls [26]. Cadmium exposure has also been shown to inhibit · NO production in cultured endothelial cells by blocking eNOS phosphorylation [39]. It is therefore reasonable to suggest that our results are linked to a reduced · NO synthesis, which in turn leads to vasoconstriction. Further assays, including long-term exposure regimes, must be performed in order to confirm this hypothesis.
In conclusion, the present study shows that environmentally realistic doses of cadmium are able to modify the antioxidant status in Wistar rat testis. However, only the animals subjected to the highest dose of cadmium (75 mg/l) exhibited a significant increase in lipid peroxidation, whereas the remaining doses should be tested for longer periods in order to assess whether they would eventually overcome the endogenous antioxidant system. Moreover, the stereological analyses show that the vascular system is a major target of cadmium, being affected prior to other tissue components. Our experimental findings corroborate with several population surveys linking cadmium to vascular diseases and therefore are consistent to justify further epidemiological studies aiming to correlate cadmium with several idiopathic pathologies.
References
Benoff S, Auborn K, Marmar J, Hurley I (2008) Link between low-dose environmentally relevant cadmium exposures and asthenozoospermia in a rat model. Fertil Steril 89:73–79. doi:10.1016/j.fertnstert.2007.12.035
Norvell WA, Wu J, Hopkins DG, Welch RM (2000) Association of cadmium in durum wheat grain with soil chloride and chelate-extractable soil cadmium. Soil Sci Soc Am J 64:2162–2168. doi:10.2136/sssaj2000.6462162x
Järup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208. doi:10.1016/j.taap.2009.04.020
Lambert R, Grant C, Sauvé S (2007) Cadmium and zinc in soil solution extracts following the application of phosphate fertilizers. Sci Total Environ 378:293–305. doi:10.1016/j.scitotenv.2007.02.008
Al-Faiyz Y, El-Garawany M, Assubaie F, Al-Eed M (2007) Impact of phosphate fertilizer on cadmium accumulation in soil and vegetable crops. Bull Environ Contam Toxicol 78:358–362. doi:10.1007/s00128-007-9025-x
Olsson IM, Bensryd I, Lundh T, Ottosson H, Skerfving S, Oskarsson A (2002) Cadmium in blood and urine-Impact of sex, age, dietary intake, iron status, and former smoking - Association of renal effects. Environ Health Perspect 110(2):1185–1190
Tsadilas CD, Karaivazoglou NA, Tsotsolis NC, Stamatiadis S, Samaras V (2004) Cadmium uptake by tobacco as affected by liming, N form, and year of cultivation. Environ Pollut 134:239–246. doi:10.1016/j.envpol.2004.08.008
Akesson A, Julin B, Wolk A (2008) Long-term dietary cadmium intake and postmenopausal endometrial cancer incidence: a population-based prospective cohort study. Cancer Res 68:6435–6441. doi:10.1158/0008-5472
Julin B, Wolk A, Bergkvist L, Bottai M, Akesson A (2012) Dietary cadmium exposure and risk of postmenopausal breast cancer: a population-based prospective cohort study. Cancer Res 72(6):1459–1466. doi:10.1158/0008-5472.CAN-11-0735
Eum KD, Lee MS, Paek D (2008) Cadmium in blood and hypertension. Sci Total Environ 407:147–153. doi:10.1016/j.scitotenv.2008.08.037
Arora M, Weuve J, Schwartz J, Wright O (2009) Association of environmental cadmium exposure with periodontal disease in U.S. adults. Environ Health Perspect 117(5):739–744. doi:10.1289/ehp.0800312
Wang Y, Fang J, Leonard SS, Rao MK (2004) Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med 36(11):1434–1443. doi:10.1016/j.freeradbiomed.2004.03.010
Liu J, Qian SY, Guo Q, Jiang J, Waalkes MP, Mason RP, Kadiiska MB (2008) Cadmium generates reactive oxygen- and carbon-centered radical species in rats: insights from in vivo spin-trapping studies. Free Radic Biol Med 45:475–481. doi:10.1016/j.freeradbiomed.2008.04.041
El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH (2004) Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and b-carotene. Food Chem Toxicol 42:1563–1571. doi:10.1016/j.fct.2004.05.001
Griffin JL, Walker LA, Shore RF, Nicholson JK (2001) Metabolic profiling of chronic exposure in the rat. Chem Res Toxicol 14:1428–1434. doi:10.1021/tx015521u
Brzóska M, Supernak-Bobko M, Moniuszko-Jakoniuk K (2003) Changes in the structure and function of the kidney of rats chronically exposed to cadmium. Biochemical and histopathological studies. Arch Toxicol 77:344–352. doi:10.1007/s00204-003-0451-1
Sinha Hikim AP, Lue Y, Swerdloff RS (1997) Separation of germ cell apoptosis from toxin-induced cell death by necrosis using in situ end-labeling histochemistry after glutaraldehyde fixation. Tissue Cell 29(4):487–493. doi:10.1016/S0040-8166(97)80034-1
Mori H, Christensen AK (1980) Morphometric analysis of Leydig cells in the normal rat testis. J Cell Biol 84:340–354
Russel LD, França LR (1995) Building a testis. Tissue Cell 27:129–147. doi:10.1016/S0040-8166(95)80016-6
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. In: Packer L, Fleischer S (eds) Methods in enzymology, biomembranes (Part C, Vol. 52). Academic, New York, pp 302–310
Hallare AV, Schirling M, Luckenbach T, Köhler H-R, Triebskorn R (2005) Combined effects of temperature and cadmium on developmental parameters and biomarker responses in zebrafish (Danio rerio) embryos. J Therm Biol 30:7–17. doi:10.1016/j.jtherbio.2004.06.002
Klaassen CD, Liu J, Diwan BA (2009) Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol 238:215–220. doi:10.1016/j.taap.2009.03.026
Leite RP, Predes FS, Monteiro JC, Freitas KM, Wada RS, Dolder H (2013) Advantage of Guaraná (Paullinia cupana Mart.) supplementation on cadmium-induced damages in testis of adult Wistar rats. Toxicol Pathol 41:73–79. doi:10.1177/0192623312447541
Takahashi Y, Poteser M, Masui H, Koizumi N, Wakabayashi I (2004) Effects of cadmium in vitro on contractile and relaxant responses of isolated rat aortas. Environ Health Prev Med 9:251–256
Suto D, Akiyama K (2000) Effect of cadmium or magnesium on calcium-dependent central function that reduces blood pressure. Arch Toxicol 74:1–4
Skoczynska A, Martynowicz H (2005) The impact of subchronic cadmium poisoning on the vascular effect of nitric oxide in rats. Hum Exp Toxicol 24:353–361. doi:10.1191/0960327105ht536oa
Nzengue Y, Steiman R, Garrel C, Lefebvre E, Guiraud P (2008) Oxidative stress and DNA damage induced by cadmium in the human keratinocyte HaCaT cell line: role of glutathione in the resistance to cadmium. Toxicol 243:193–206. doi:10.1016/j.tox.2007.10.005
Rao GN, Berck BC (2012) Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res 70:593–599. doi:10.1161/01.RES.70.3.593
Consentino F, Sill JC, Katusic ZS (1994) Role of superoxide anions in the mediation of endothelium-dependent contractions. Hypertension 23:229–235. doi:10.1161/01.HYP.23.2.229
Consentino F, Barker JE, Brand MP, Heales SJ, Werner E, Tippins JR, West N, Channon KM, Volpe M, Luscher TF (2001) Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler Thromb Vasc Biol 21:496–502. doi:10.1161/01.ATV.21.4.496
Harrison DG (1997) Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 100(9):2153–2157. doi:10.1172/JCI119751
Touyz RM, Schiffrin EL (2004) Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 122:339–352. doi:10.1007/s00418-004-0696-7
Nemmiche S, Chabane-Sari D, Guiraud P (2007) Role of -tocopherol in cadmium-induced oxidative stress in Wistar rat’s blood, liver and brain. Chem Biol Interact 170:221–230. doi:10.1016/j.cbi.2007.08.004
Singhal RK, Anderson ME, Meister A (1987) Glutathione, a first line of defense against cadmium toxicity. FASEB J 1(3):220–223
Mah V, Jalilehvand F (2010) Cadmium(II) complex formation with glutathione. J Biol Inorg Chem 15:441–458. doi:10.1007/s00775-009-0616-3
Figueiredo-Pereira ME, Yakushin S, Cohen G (1998) Disruption of the intracellular sulfhydryl homeostasis by cadmium-induced oxidative stress leads to protein thiolation and ubiquitination in neuronal cells. J Biol Chem 273(21):12703–12709. doi:10.1074/jbc.273.21.12703
Salama AF, El-Bahr SM (2007) Effect of curcumin on cadmium-induced oxidative testicular damage in rats. J Med Res Inst 28(2):167–173
Ola-Mudathir KF, Suru SM, Fafunso MA, Obioha UE, Faremi TY (2008) Protective roles of onion and garlic extracts on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem Toxicol 46:3604–3611. doi:10.1016/j.fct.2008.09.004
Majumder S, Muley A, Kolluru GK, Saurabh S, Tamilarasan KP, Chandrasekhar S, Reddy HB, Purohit S, Chatterjee S (2008) Cadmium reduced nitric oxide production by impairing phosphorylation of endothelial nitric oxide synthese. Biochem Cell Biol 86:1–10. doi:10.1139/o07-146
Acknowledgments
We would like to thank Luis Henrique Gonzaga Ribeiro for excelent technical assistance. This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico e Fundação de Amparo à Pesquisa do Estado de São Paulo. R.P.L. is a student supported by Capes fellowship and E.F.P. by FAPESP fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leite, R., Peloso, E.F., Gadelha, F.R. et al. Environmentally Realistic Doses of Cadmium as a Possible Etiologic Agent for Idiopathic Pathologies. Biol Trace Elem Res 168, 133–140 (2015). https://doi.org/10.1007/s12011-015-0322-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0322-7