Abstract
The purpose of our study was to investigate the effect of water pH in the genesis of cardiovascular injury caused by cadmium poisoning. For this study, 90 male Wistar rats were used, divided into six groups: A, 15 rats that received 400 mg/l cadmium chloride (CdCl2) in drinking water at a neutral pH of 7.0; B, 15 rats that received CdCl2 (400 mg/l) in drinking water at an acidic pH of 5.0; C, 15 rats that received CdCl2 (400 mg/l) in drinking water at a basic pH of 8.0; D, 15 rats that received water at an acidic pH of 5.0; E, 15 rats that received water at a basic pH of 8.0; and F, 15 rats that received water at a neutral pH of 7.0. All animals were euthanized after 6 months. We collected the heart and aorta from each rat for microscopic analysis. No microscopic changes were observed in the hearts. In the aorta, fatty streaks appeared in a large proportion of animals in groups A (50 %) and B (46 %), but fatty streaks appeared in a smaller minority of animals in groups C (15.3 %), D (0 %), E (7 %), and F (13.3 %) (p < 0.05). Cadmium exposure caused the development of fatty streaks in the aorta of animals and the exposure to this metal in basic pH decreased the formation of these lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is produced by a variety of sources. This heavy metal is introduced into the environment by fumes, dust, and debris from ore smelters, burning of cadmium-based products and fossil fuels, the use of tobacco, fertilizers, and related agricultural sludge, municipal waste water, and sewage discharges [1, 5]. Additional sources of cadmium include vegetables, fish, and shellfish [1, 5]. Even silver alloy dental products (used in orthodontics) and alginate (a polysaccharide found in some algae) can result in diseases associated with heavy metal intoxication [7].
The Scientific Panel on Contaminants in the Food Chain [2] noted that the average intake of cadmium in European countries is close to or slightly exceeding the limit of 2.5 μg/kg body weight [2]. Furthermore, it was noted that subgroups of the population, such as vegetarians, smokers, and people living in highly contaminated areas may exceed the tolerable weekly intake by up to two times [14].
Although the influence of cadmium on the cardiovascular system remains controversial [14], there is strong evidence that exposure to cadmium, even at low concentrations, is still a major contributor to death from a range of causes including cardiovascular disease [16].
Some studies show increased hypertension in patients exposed to cadmium [14], which may be due to the impact of cadmium on the structure and integrity of cardiac tissue and the cardiac conduction system [8]. One study showed that exposing rats to 100 ppm of cadmium in water induced hypertrophy and fusion of cardiomyocytes in addition to observed foci of cardiac fiber necrosis and severe microstructural damage [4].
Smoking habits are correlated with the concentration of cadmium in the three arterial layers. Studies show a high concentration of cadmium in the middle layer when compared with the serum, indicating that the vascular wall is also a target organ for depositing cadmium [8].
One of the main factors affecting the ability of plants to access heavy metals is the soil pH, which is generally inversely related to the availability of these elements [3]. The study from Nai et al. [12] showed that acidic pH increased the formation of precancerous lesions in the prostates of animals exposed to cadmium. However, no studies have evaluated the influence of the pH on the cardiovascular toxicity of cadmium-contaminated water.
In addition to occupational exposure, cadmium is a metal that contaminates many foods and through this route can also infect humans [5]. The risk of poisoning in patients exposed to this metal is high [5]. Thus, there is a need for simple, alternative ways to ameliorate the consequences of cadmium intoxication.
The objective of this study was to evaluate the pH effects of cadmium-contaminated drinking water on the development of cardiovascular lesions.
Material and Methods
Animals and Treatments
This study was approved by the Ethics Committee for Animal Use at the University of Oeste Paulista (CEUA-UNOESTE; Protocol no. 2010). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
For our study, we used 90 male adult Wistar rats (Rattus norvegicus albinus), weighing 200–250 g. The rats were divided into groups of four and placed in large rectangular boxes (measuring 49 × 34 × 16 cm) suitable for the accommodation of up to five adult rats. Animals were maintained under a controlled temperature of 25 ± 2 °C, relative humidity of 50 ± 15 % and normal photoperiod (12–12-h light–dark cycles).
The cadmium source used was cadmium chloride (CdCl2; Sigma Chemical Company, St. Louis, MO, USA) with a hydration of at least 98 % and water content of approximately 2.5 mol/mol. For 6 months, CdCl2 was given to the animals in their drinking water daily at a concentration of 400 mg/l (adapted from Motta et al. [11]). The pH of the water was adjusted using hydrochloric acid or sodium hydroxide. Drinking water was changed three times a week to maintain the pH. Any wastewater containing cadmium was sent to the central reservoir of the University of Oeste do Paulista (UNOESTE) and neutralized for disposal. The quantity of water remaining in the rat troughs was measured each time the solution was changed to estimate the average intake of each animal. Also, the pH of the remaining water was measured to ensure that the pH remained the same.
The animals were divided into six groups: group A, 15 rats that received cadmium chloride in their drinking water at a neutral pH 7.0; group B, 15 rats that received cadmium chloride in their drinking water at an acidic pH 5.0; group C, 15 rats that received cadmium chloride in their drinking water at a basic pH 8.0; group D, 15 rats that received drinking water at an acidic pH 5.0; group E, 15 rats that received drinking water at a basic pH 8.0; and group F, 15 rats that received drinking water at a neutral pH 7.0.
Animals of all groups were euthanized 6 months after the beginning of the experiment. Euthanasia was performed by intraperitoneal injection of thiopental (Syntec, USA) at a dose of 100 mg/kg [15]. The onset of death was inferred by the absence of breathing movements, heartbeat, and protective reflexes [15]. Necropsy was performed and the heart and aorta from each rat were removed for microscopic analysis.
Histological Study
Half of the heart and five longitudinal cross-section fragments of the aorta were fixed in 10 % formalin (Chemical Kinetics, São Paulo, Brazil) for 24 h, subjected to standard histological processing, and paraffin embedded (Dynamic Analytical Reagents, São Paulo, Brazil). Sections of 5-μm thickness were obtained and stained with hematoxylin-eosin (HE) (Dolles, São Paulo, Brazil). Histological sections of the aorta were also stained with Verhoeff-van Gienson (VVG) for better identification of the elastic lamina (Merck, Darmstadt, Germany).
Histopathologic analysis was blinded and performed by a single experienced observer (GAN) using a conventional optical microscope (NIKON Labophot, Japan). The following parameters were evaluated with the respective scoring scheme: 1. heart: interstitial inflammatory infiltrate (0 = absent, 1 = mild, 2 = moderate, 3 = severe) and inflammatory cell-type present (polymorphonuclear and/or monuclear cells); tissue congestion (0 = absent, 1 = mild, 2 = moderate, 3 = severe); changes in muscle fibers (atrophy, necrosis, hypertrophy, sclerosis - 0 = absent, 1 = present); vascular changes (arteriolosclerosis and atherosclerosis, 0 = absent, 1 = focal, 2 = diffuse); benign and malignant neoplastic lesions (0 = absent, 1 = present); and 2. Aortic atherosclerosis (0 = absent, 1 = fatty streaks, 2 = mild, 3 = moderate, 4 = severe).
Statistical Analysis
The likelihood ratio (LR) test was performed. To make comparisons 2 × 2, we used the Holm-Sidak correction to control the group error rate (α = 5 %). Statistical tests were performed at a significance level of 5 %.
Results
Five animals died during the course of our study (one rat each from groups A, C, and D and two rats from group E). The cause of death for the animals from group A and C was acute pulmonary edema, a complication associated with cadmium exposure [5]. It was not possible to establish the cause of death for the rats from groups D and E.
The average water intake per animal per day was as follows: 55 ml for group A (approximately 22 mg of cadmium), 57 ml for group B (approximately 22.8 mg of cadmium), 52 ml for group C (approximately 20.8 mg of cadmium), 60 ml for group D, 70 ml for group E, and 73 ml for group F. No statistically significant difference was found between the groups with respect to cadmium intake and water intake (p > 0.05).
Heart
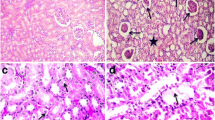
In the hearts of all animals assessed, no interstitial inflammatory infiltrate, tissue congestion, changes in muscle fibers (atrophy, necrosis, hypertrophy, sclerosis), vascular changes (arteriolosclerosis and atherosclerosis), nor benign or malignant neoplastic lesions were observed (Fig. 1).
Aorta
The only abnormality found in the aorta of the animals was fatty streaks (Fig. 2). No atheroma was observed. The incidence of fatty streaks in the animals of groups A and B differ significantly from those of animals in groups C, D, E, and F (p < 0.05) (Table 1).
Photomicroscopy of the aorta showing an elevation of the endothelium by the presence of foam cells in the intimal layer (fatty streaks). Arrows indicate transition from the normal endothelial cells (right) and the altered cells (left) (animal from group B; a, b c Hematoxylin-eosin, ×40, 100×, and ×400 magnification, respectively; d Verhoeff-van Gienson VVG, ×100 magnification)
Discussion
The main routes of human cadmium exposure are inhalation and ingestion. According to the World Health Organization (WHO), the maximum daily intake of cadmium should be 1 μg/kg body weight [17].
Atherosclerosis is the basis of most cardiovascular diseases and begins in early life. Changes in the arterial wall may begin in childhood and progress slowly over decades [8]. A study by Navas-Acien et al. [13] showed that the odds ratio of peripheral arterial disease was 2.82 for the highest concentration of blood cadmium (6.23 nmol/l or 0.70 mg/l) compared with lower doses (3.56 nmol/l or 12.40 mg/l). Cadmium exposure is considered a risk factor for developing atherosclerosis [6, 9, 10]. Endothelial cells absorb cadmium and can transport the metal to the subendothelial space. Smooth muscle cells also absorb cadmium, initiating the proliferation of these cells and thus leading to the thickening of the medial layer of the vessel wall. Cadmium disrupts the endothelial intercellular connections, thus facilitating their diffusion and allows lipids and immune cells to access the intima layer. The accumulation of smooth muscle cells in the intima, the intimal invasion by macrophages, and the death of endothelial cells promotes inflammation. The inflammation causes severe tissue remodeling, including extracellular matrix deposition and the appearance of lakes of free lipid. These events thus initiate the atherosclerotic process with plaque formation and progression of the disease [8]. In this study, we observed a higher incidence of fatty-streak formation (an initial lesion of characteristic of atherosclerosis) in animals exposed to cadmium in water with neutral or acidic pH. Those animals exposed to this heavy metal in water at basic pH showed an incidence of this injury similar to the non-exposed groups. These data suggests that the basic pH could provide a level of protection against the formation of cadmium-induced fatty streaks. Thus, the use of water with alkaline pH could be preferable for people exposed to cadmium. The appearance of fatty streaks in a portion of non-exposed animals may be due to the advanced age of the animals.
The pathophysiological effects of cadmium on the structure and integrity of cardiac tissue is still not well established. Possible mechanisms include oxidative damage, reduced coronary flow, inhibited conductivity between cardiomyocytes, and the direct interaction of cadmium with troponin and myoglobin [8]. The effects on the cardiac conduction system are based on interactions between cadmium and the systems for contraction and stimulation [8]. In our study, there was an absence of cardiac lesions in animals exposed to cadmium.
The lungs absorb from 10 to 40 % of inhaled cadmium, while ingested cadmium is poorly absorbed from the gastrointestinal tract (5–7 %) and is mostly eliminated in feces [1]. This fact may explain the absence of cardiac lesions in animals exposed to cadmium in this study as optical microscopy failed to reveal any cardiac injury. However, it is possible that functional injury associated with cardiac electrical conduction or microstructural damage can be detected only with electron microscopy. Studies evaluating the electrical function and cardiac microstructure may provide a better understanding of the pH dependence of cardiac effects due to cadmium toxicity.
Conclusion
Cadmium-contaminated drinking water caused the appearance of fatty streaks in the aorta of rats and a basic pH decreased the formation of this change. However, there were no cardiac lesions in the optical microscopy analysis, independent of the pH.
References
Bernhoft RA (2013) Cadmium toxicity and treatment. Scientific World Journal. doi:10.1155/2013/394652
CONTAM (2009) Scientific opinion of the panel on contaminants in the food chain on a request from the European commission on cadmium in food. EFSA J 980:1–139
Clemens S, Aarts MG, Thomine S, Verbruggen N (2013) Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18:92–99. doi:10.1016/j.tplants.2012.08.003
Ferramola ML, Pérez Díaz MF, Honoré SM, Sánchez SS, Antón RI, Anzulovich AC, Giménez MS (2012) Cadmium-induced oxidative stress and histological damage in the myocardium. Effects of a soy-based diet. Toxicol Appl Pharmacol 265(3):380–389
Järup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208. doi:10.1016/j.taap.2009.04.020
Knoflach M, Messner B, Shen YH, Frotschnig S, Liu G, Pfaller K, Wang X, Matosevic B, Willeit J, Kiechl S, Laufer G, Bernhard D (2011) Non-toxic cadmium concentrations induce vascular inflammation and promote atherosclerosis. Circ J 75(10):2491–2495
Menezes LM, Freitas MPM, Gonçalves TS (2009) Biocompatibility of orthodontic materials: myth or reality? Rev Dental Press Ortodon Ortop Facial 14(2):144–147
Messner B, Bernhard D (2010) Cadmium and cardiovascular diseases: cell biology, pathophysiology, and epidemiological relevance. Biometals 23:811–822. doi:10.1007/s10534-010-9314-4
Messner B, Knoflach M, Seubert A, Ritsch A, Pfaller K, Henderson B, Shen YH, Zeller I, Willeit J, Laufer G, Wick G, Kiechl S, Bernhard D (2009) Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler Thromb Vasc Biol 29(9):1392–1398. doi:10.1161/ATVBAHA.109.190082
Mlynek V, Skoczynska A (2005) The proinflammatory activity of cadmium. Postepy Hig Med Dosw 59:1–8
Motta ACF, Migliari DA, Gioso MA, Komesu MC, Sala MA, Lopes RA (2004) The carcinogenic potential of cadmium in the palatal and gingival epithelium of rats. A morphologic and morphometric analysis. Br J Vet Res AnimSci 41:183–188
Nai GA, Golghetto GM, Estrella MPS, Teixeira LDS, Moura FC, Bremer Neto H, Parizi JLS (2015) Influence of pH of water in the genesis of cancer in cadmium poisoning: an experimental study in rats. Histol Histopathol 30:61–67
Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V (2009) Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol 170:1156–1164
Nawrot TS, Staessen JA, Roels HA, Munters E, Cuypers A, Richart T, Ruttens A, Smeets K, Clijsters H, Vangronsveld J (2010) Cadmium exposure in the population: from health risks to strategies of prevention. Biometals 23:769–782. doi:10.1007/s10534-010-9343-z
Paiva FP, Mafilli VV, Santos ACS (2005) Course handling of laboratory animals. Fundação Osvaldo Cruz. Centro de Pesquisas Gonçalo Muniz. Disponível em: http://www.bioteriocentral.ufc.br/arquivos/apostilha_manipulacao.pdf. Acesso em 03 jun 2012
Tellez-Plaza M, Navas-Acien A, Menke A, Crainiceanu CM, Pastor-Barriuso R, Guallar E (2012) Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environ Health Perspect 120(7):1017–1022
WHO (1992) World Health Organization. Environmental Health Criteria, cadmium, vol 134. World Health Organization, Geneva
Acknowledgments
The authors thank Edvaldo Mamede dos Santos for solution preparation
Compliance with Ethical Standards
ᅟ
Conflict of interest
This research received no grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors declare that there is no conflict of interest.
Research involving animals
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nai, G.A., Golghetto, J.J., Estrella, M.P.S. et al. pH Dependence of Cadmium-Contaminated Drinking Water on the Development of Cardiovascular Injury in Wistar Rats. Biol Trace Elem Res 165, 81–85 (2015). https://doi.org/10.1007/s12011-014-0216-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0216-0