Abstract
Obesity is associated with an alteration in zinc metabolism. This alteration may be associated with changes in gene expression of zinc transporters. In this study, we examined the leukocyte expression of zinc transporter ZnTs in response to zinc supplementation in young obese women. Thirty-five young obese women (BMI ≥ 25 kg/m2), aged 18–28 years, were randomly assigned to two groups: a placebo group or a zinc group (30 mg zinc/day for 8 weeks). Usual dietary zinc intake was estimated from 3-day diet records. Serum zinc and urinary zinc concentrations were measured by atomic absorption spectrometry. Messenger RNA (mRNA) levels of leukocyte ZnT transporters were examined using quantitative real-time PCR. Expression levels of two ZnT transporters, ZnT1 and ZnT5, in obese women, increased significantly after zinc supplementation. At the end of the study, mRNA levels of ZnT1 and ZnT5 showed no correlation with serum zinc or urinary zinc concentration in obese women. In addition, a further study was conducted to identify whether the association between the gene expression levels of leukocyte ZnT1 and ZnT5 and dietary zinc intake remained consistent in 216 healthy young adults aged 20–29 years. A positive correlation between ZnT1 and dietary zinc intake (r = 0.181, P = 0.089) was also observed in healthy men although the significance was marginal. Taken together, these results show that the gene expression levels of ZnT1 and ZnT5 may be changed by zinc intake, suggesting that zinc supplementation could potentially restore ZnT transporter expression in obese women with altered zinc metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc is an essential trace element involved in various biological functions in the human body [1]. To maintain these functions, cellular zinc levels are tightly regulated by various zinc-specific transporter proteins [2, 3]. In particular, the family of ZnT zinc transporters (solute-like carrier, SLC30) comprises zinc exporter proteins that play a key role in maintaining cellular zinc homeostasis by transporting zinc ions from the cytoplasm to either the extracellular space or intracellular compartments. To date, 10 members of the ZnT family have been identified in various mammalian tissues [4], and many of these proteins have been shown to be highly expressed in human leukocytes [5].
Previous studies reported the relationship between ZnTs expression and dietary zinc [2, 3]. In rodent models, expression levels of ZnT1 and ZnT2 increased in response to high zinc intake, and the expression level of ZnT2 decreased in response to low zinc intake [6, 7]. In human lymphocyte and monocyte cell lines such as THP-1 or Molt-4, expression levels of ZnT1, ZnT5, and ZnT6 were altered by zinc treatment or depletion [8–10]. A limited number of studies have explored changes in the expression of leukocyte ZnT transporters by dietary zinc in humans. In one previous study of healthy men aged 19–31 years [11], the expression level of leukocyte ZnT1 was significantly increased by 2~3-fold after 10-day zinc supplementation. Another study of healthy young and elderly women [9], however, reported inconsistent changes in ZnT1 expression after zinc supplementation.
Obesity, one of the major risk factors for global disease burden [12, 13], is associated with alterations in zinc metabolism [14]. Several previous studies have reported that obese individuals have lower serum/plasma or erythrocyte zinc concentrations than non-obese individuals [15–17]. Serum or erythrocyte zinc concentrations were lower in overweight or obese adults than in non-obese adults, and serum zinc concentrations showed an inverse association with BMI and waist circumference [15, 17]. Zinc concentrations in plasma and erythrocytes were significantly lower in obese children and adolescents aged 7–14 years than in non-obese counterparts [16]. Furthermore, the activity of superoxide dismutase (SOD), a zinc-dependent enzyme, was significantly lower in obese women than in non-obese women [18].
Expression levels of ZnTs in obese subjects have been identified to be different from those in non-obese subjects. Messenger RNA (mRNA) levels of several ZnTs in the adipose tissue of obese individuals were lower than those in non-obese individuals [19]. Moreover, we recently showed that the expression of some leukocyte ZnTs was significantly lower in obese women than in non-obese women, and that the expression levels of ZnTs were inversely correlated with BMI and body fat, suggesting that an alteration in zinc metabolism in obesity may be related to changes in ZnT expression [5]. Further, the ZnT expression was negatively associated with the levels of inflammatory markers such as C-reactive protein (CRP) and tumor necrosis factor-alpha (TNF-α), which were significantly increased in the obese women [5]. Thus, it is important to identify if there are changes in the expression of ZnTs in response to changes in zinc intake in obese individuals; however, little is known about this relationship. We hypothesized that zinc supplementation would restore the obesity-associated alterations in ZnT expression.
Peripheral blood cells are easier to obtain from human subjects than cells from tissue biopsies and are often used as cellular materials to assess nutritional or pathological states [20]. Leukocytes respond readily to nutritional stimuli. In particular, the gene expressions of ZnTs in leukocyte populations are changed by zinc treatment or depletion [10, 11]. Further, leukocytes are important mediators of the immune system, which make them more attractive targets in the study of obesity, a chronic inflammatory state, and ZnT gene expression [21].
Therefore, the objectives of this study were to examine the gene expression levels of leukocyte zinc transporters in response to zinc intake by zinc supplementation in young obese women. In addition, a further study was conducted to identify whether the pattern of association between gene expression levels of leukocyte zinc transporters and dietary zinc intake was also present in young healthy adults.
Methods
Subjects
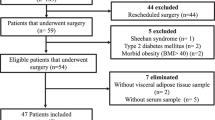
Forty obese women aged 18–28 years were recruited from the Daegu and Gyeongbuk areas as described in a previous study [18]. In brief, obesity was defined as a body mass index (BMI) ≥ 25 kg/m2. Participants completed a questionnaire on demographic characteristics, smoking, nutritional supplementation, personal medical history, family history, and medication use through a face-to-face interview. Exclusion criteria were as follows: diagnosis with any acute or chronic disease other than obesity, supplementation with vitamins or minerals or other nutritional supplements, any medications including oral contraceptives, smoking or participation in a weight-loss program. In addition, to analyze zinc transporter gene expression, participants whose RNA samples were not of good quality were excluded. Finally, data of 35 subjects (17 subjects in the zinc group and 18 subjects in the placebo group) were used for analysis. Subjects were asked to maintain their current lifestyle, such as diet and physical activity, during the study period. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Public Institutional Review Board (PIRB12-040-02) and the IRB of Kyung Hee University (KHSIRB-12-005). Written informed consent was obtained from all subjects.
Study Design
The study was a double-blinded, randomized, placebo-controlled trial. Subjects were randomly assigned to one of two groups: a zinc group that took 30 mg zinc/day as a tablet of zinc gluconate (Vitamin House, South Korea) and a placebo group that took placebo tablets (zinc-free starch) daily for 8 weeks. The zinc dose of 30 mg/day in this study was chosen because the tolerable upper intake level for Korean adults is 35 mg/day. Subjects were instructed to take the tablets between meals. To ensure compliance, zinc or placebo tablets were given with pill reminders and the staff called all subjects every day to confirm that they were taking their pills.
Anthropometric Measurements
All anthropometric measurements were conducted by trained research staff using standardized protocols before and after 8-week zinc intervention. Height was measured by anthropometry (TKK-11252, Japan). Body weight and percentage body fat were measured by bioimpedance analysis (InBody 3.0, Biospace Co., Korea). BMI was calculated as weight (kg) divided by height squared (m2).
Nutritional Assessment
Three-non-consecutive-day dietary records, including two weekdays and one weekend day, were collected at the beginning and at the end of the study to estimate usual dietary intake. At the first visit, trained dieticians gave detailed instructions to participants for the 3-day dietary record. Subjects were asked to record the amount of all food and beverages consumed for 3 days. Dietary records were checked by trained staff for completeness. Dietary intake of energy and zinc as well as nutrients known to influence zinc absorption, such as protein and iron [22], were calculated using a dietary evaluation program (Can-pro 3.0, Korean Nutrition Society).
Biochemical Analyses
Fasting blood samples and 24-h urine samples were collected before and after 8-week zinc intervention. Blood samples were collected using plastic syringes and put on ice for a maximum of 2 h, centrifuged at 1500g for 10 min at 4 °C (Allegra 6R, Beckman Colter, USA), and stored at −70 °C prior to analysis. Urine samples were collected in a polyethylene container, weighed, then stored in aliquots at −20 °C until analysis [23]. Serum and urinary zinc were measured using atomic absorption spectrometry (AAS 600, PerkinElmer, USA). Urinary creatinine was measured by the Jaffe method [24] and urinary zinc was adjusted for urinary creatinine. The activity of SOD in serum was measured using a SOD assay kit (Cayman Chemical Company, USA). Inflammatory markers such as CRP, TNF-α and interleukin-6 (IL-6) in serum were measured by enzyme immunoassay. CRP was measured using a high-sensitivity CRP ELISA kit (Immundiagonstik AG, Europe). TNF-α was measured using the Quantikine human TNF-α kit (R&D systems Inc., USA). IL-6 was measured using the Quantikine human IL-6 kit (R&D systems Inc.).
Preparation of Leukocyte Total RNA and Real-Time PCR Analysis
Total RNA was extracted from 1.5 ml whole blood using the QIAamp RNA blood mini kit (QIAGEN, USA) [9]. Concentration and purity of the extracted RNA samples were evaluated by measuring absorbance at 260 and 280 nm wavelengths using a spectrophotometer (NanoDrop, Thermo, USA), and samples with OD260/OD280 values of 1.8 or higher were used in the analysis. Complementary DNA (cDNA) was synthesized from 0.15 μg of total RNA using a PrimeScriptTM RT reagent kit (Takara, Japan) according to the manufacturer’s protocol. Gene expression levels of leukocyte zinc transporters were assessed via real-time PCR. Reactions were prepared using SYBR Premix Ex Taq II (Takara, Japan) and conducted on a MiniOpticon (Bio-Rad, USA) in duplicate. We examined gene expression levels of the following zinc transporters: ZnT1, ZnT2, ZnT5, ZnT6 and ZnT9, all of which have been shown to be influenced by dietary zinc [2, 3]. Relative expression levels of zinc transporters were determined using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the reference gene and calculated as 2− ΔCt(ΔCt = Cttarget − Ctreference) [9]. To investigate differences in zinc transporter expression levels between placebo and zinc groups at baseline and after 8 weeks, fold-changes were calculated relative to the expression level of ZnT1 in the placebo group. In addition, the relative changes between baseline and after 8 weeks in zinc group were calculated when the changes in placebo group were set as 1.0.
Further Study in Young Healthy Adults
We performed a second study in young healthy adults to examine whether the positive association between the expression levels of ZnT1 and ZnT5 and zinc intake in obese women was also present in the healthy population. One hundred sixty-two young healthy adults aged 20–29 years (92 men and 70 women) were recruited, as described in our previous report [25]. Usual dietary zinc intake data were estimated through 3-non-consecutive-day dietary data collected using one 24-h recall and a 2-day dietary record. Fasting blood samples were collected, and leukocyte ZnT1 and ZnT5 gene expression levels were determined as described above. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Review Board of Seoul National University (IRB no. 0812/001~001). Written informed consent was obtained from all subjects.
Statistical Analyses
All data analyses were conducted using Statistical Analysis Systems statistical software package 9.3 (SAS Institute, Cary, NC, USA). All data were presented as the mean and standard error of the mean (SEM). Data for obese women did not follow a Gaussian distribution because of the small sample size, so non-parametric statistical methods were used for analysis. Differences in general characteristics, dietary intake, zinc status and inflammatory status at baseline were examined by the Wilcoxon rank sum test. Significant changes in general characteristics, dietary intake, zinc status, and inflammatory status after zinc supplementation were examined by the Wilcoxon signed rank test. Because changes in the percentage of body fat differed within each group after 8 weeks, which could affect the gene expression of zinc transporters, zinc transporter expression levels and their relative changes between baseline and after 8 weeks were shown as least-square (LS) means for the percentage of body fat calculated by generalized linear model (GLM). Differences in the zinc transporter expression levels among placebo and zinc groups at baseline and after 8 weeks were examined by Tukey’s test in the GLM. Differences of the relative changes between placebo and zinc group were also examined by GLM. For young healthy adults, Pearson’s correlation analysis was conducted to examine the association between the gene expression levels of ZnT1 and ZnT5 and dietary zinc intake.
Results
Changes in General Characteristics, Dietary Intake, and Zinc Status After Zinc Supplementation in Obese Women
Characteristics of subjects in the placebo and zinc groups at baseline are shown in Table 1. BMI and percentage of body fat were not significantly different between the placebo and zinc groups at baseline. Nutrient intake, such as protein, zinc and iron intake at baseline, were also not significantly different between the two groups. Furthermore, zinc status including serum zinc, urinary zinc and serum SOD levels were not different between the placebo and zinc groups at baseline.
Changes in anthropometric measurements, dietary intake, zinc status, and inflammatory markers after the 8-week intervention are shown in Table 1. There was no significant change in BMI or percentage of body fat in the zinc group, while the percentage of body fat slightly increased in the placebo group (P = 0.024). Dietary intake of energy, protein and iron was unchanged after the 8-week trial. Serum zinc concentration in the zinc group increased by 18 % (P < 0.001). However, urinary zinc concentration and SOD activity did not change in response to zinc supplementation. The levels of CRP (P = 0.08) and IL-6 (P < 0.01) were decreased after zinc supplementation.
Changes in Gene Expression Levels of Leukocyte Zinc Transporters After Zinc Supplementation in Obese Women
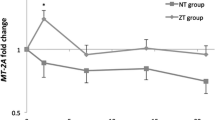
We examined whether the gene expression levels of ZnT1, ZnT2, ZnT5, ZnT6 and ZnT9 were affected by zinc supplementation (Fig. 1). Among these transporters, mRNA levels of ZnT1 and ZnT5 in the zinc group increased significantly after 8-week zinc supplementation (P < 0.01). The relative changes of ZnT1 and ZnT5 gene expression after zinc supplementation in zinc group were 1.3 and 1.2 times higher than the changes in placebo group (P < 0.05) (Table 2).
Correlation of Leukocyte Zinc Transporter Expression Levels with Dietary Zinc Intake in Young Healthy Adults
Dietary zinc intake was significantly higher in men (8.8 ± 0.2 mg/day, n = 90) than in women (7.2 ± 0.2 mg/day, n = 72) (P < 0.0001). The mRNA levels of ZnT1 were significantly higher in men [493.3 ± 10.1 for men (n = 90) and 443.5 ± 14.8 for women (n = 72) in arbitrary units, P = 0.006], but the ZnT5 mRNA levels were not significantly different between men and women. Correlations between expression levels of zinc transporters, such as ZnT1 and ZnT5, and dietary zinc intake in young healthy adults (n = 162) are shown in Table 3. mRNA level of ZnT1 had a positive correlation with dietary zinc intake in total subjects (r = 0.155, P = 0.049). mRNA level of ZnT1 was marginally correlated with dietary zinc intake in men (r = 0.181, P = 0.089), but not in women. But, mRNA level of ZnT5 was not correlated with dietary zinc intake in total subjects.
Discussion
In this study, we found that gene expression levels of leukocyte ZnT transporters increased significantly with zinc supplementation of 30 mg/day for 8 weeks in obese women aged 18–28 years. mRNA levels of ZnT1 and ZnT5 were significantly higher in the zinc supplemental group than in the placebo group after 8-week zinc supplementation. At the end of the study, mRNA levels of ZnT1 and ZnT5 showed a positive correlation with zinc intake in young obese women. Furthermore, the positive association between ZnT1 gene expression and dietary zinc intake was also present in 90 healthy young men even though the significance was marginal. Taken together, these results indicate that the gene expression levels of ZnT1 and ZnT5 are affected by zinc intake, which suggests that zinc supplementation might restore the obesity-associated shift in ZnT transporter expression in women.
Few human studies have examined the effect of zinc supplementation on the expression of ZnT transporters in obese individuals. A study by Foster et al. [26] examined alterations in zinc transporter expression in peripheral blood mononuclear cells after zinc supplementation in women aged 65.0 ± 7.8 years with type 2 diabetes mellitus (average BMI of 28.6 ± 5.1 kg/m2), but significant changes in zinc transporter expression were not detected after zinc supplementation for 12 weeks (40 mg/day as zinc sulfate). The differences between Foster et al.’s study and the present study may due to the inflammation status of subjects. In a previous study, we found that alterations in zinc transporter gene expression were associated with an increase in expression of inflammatory markers, such as CRP and TNF-α, in obese women [5]. Furthermore, zinc supplementation of 30 mg/day for 8 weeks significantly decreased inflammatory markers such as CRP and IL-6 in these obese women [18]. However, in Foster et al.’s study, zinc supplementation did not change the level of inflammatory markers such as CRP, TNF-α, and IL-6 in diabetic women. Thus, the lack of improvement in inflammatory status might have resulted in no alterations in zinc transporter gene expression in subjects, even though the subjects had type 2 diabetes mellitus, which is associated with perturbed zinc homeostasis. Furthermore, the age of subjects might have contributed to the discrepancy in results between our study and that of Foster and colleagues, because age is well-known to affect zinc metabolism [27]. These inconsistent results indicate the need for further research on the association among zinc transporter gene expression, zinc intake, and intermediate factors in obese individuals.
We found that expression of ZnT1 and ZnT5 transporters was significantly induced by zinc supplementation in obese individuals. Our findings are consistent with the critical roles of ZnT1 and ZnT5 in the regulation of zinc metabolism. For example, ZnT1 is localized mainly in the plasma membrane and mediates zinc efflux from cells [4]. When zinc supply is limited and below adequate levels, such as in the case of obesity, a decrease in ZnT1 expression would maintain intracellular zinc concentrations [5, 8, 10]. Conversely, when additional zinc is provided by zinc supplementation, an increase in ZnT1 expression would enhance zinc efflux from cells and protect cells from accumulating too much zinc within the cytosol [8, 10, 11, 13]. ZnT5 is located in the Golgi apparatus and facilitates the transport of zinc into the Golgi lumen and vesicular compartments [4]. When zinc is supplemented, ZnT5 expression could increase to store additional zinc in the Golgi lumen [28]. Importantly, ZnT5 is thought to be responsible for loading zinc on secretory proteins such as alkaline phosphatase (ALP), which requires zinc for its catalytic activity [29]. Therefore, it is plausible that when zinc levels are sufficient due to dietary supplementation, synthesis of ZnT5 could activate more ALP. When available zinc levels are too low; however, ZnT5 expression could be diminished. In our previous studies, we showed that ZnT5 mRNA levels were 40 % lower in obese women than in non-obese women [5]. Taken together, ZnT1 and ZnT5 expression might be potential indicators of functional zinc status.
The molecular mechanisms responsible for the induction of ZnT1 and ZnT5 transporter by zinc supplementation may involve the activity of metal responsive transcription factor (MTF-1) [30]. MTF-1 is a transcription factor that enhances the transcription of target genes by binding to metal responsive elements (MRE) located in the promoter regions of target genes. Previous studies have identified several MREs in the promoter regions of both ZnT1 and ZnT5 genes [31, 32]. Our findings suggest that the MREs identified in these genes are functional and are able to respond to subtle changes in dietary zinc intake that are indistinguishable in terms of plasma zinc concentrations. Both ZnT1 and ZnT5 are ubiquitously expressed in most types of tissues, indicating that the changes in leukocytes seen in this study may also apply to other tissue types. Therefore, modulation of ZnT1 and ZnT5 expression in response to changes in zinc intake is likely to have a wide-ranging impact on maintaining body zinc homeostasis.
Similar to our previous reports [5], mRNA levels of ZnT1 and ZnT5 were not correlated with zinc status markers such as serum zinc or urinary zinc concentration in both placebo group and supplemental zinc group (data not shown). This indicates that the associations between zinc transporters and circulating zinc concentration do not change in high zinc intake by zinc supplementation. Zinc transporters may be more sensitive to changes in zinc intake than circulating zinc concentrations, such as serum zinc because serum zinc concentrations are maintained within a narrow range, even when dietary zinc levels fluctuate due to zinc homeostasis [33].
The positive association between ZnT1 gene expression and dietary zinc intake was also present in 90 healthy young men even though the significance was marginal. ZnT1 gene expression responded to changes in habitual dietary zinc intake in healthy subjects with normal zinc metabolism as well as in obese women with altered zinc metabolism. These results suggest that the ZnT1 transporter may be a good indicator of dietary zinc intake in healthy subjects. In a zinc intervention study with four young healthy men aged 19–31 years [11], leukocyte mRNA level of ZnT1 increased after zinc supplementation (15 mg Zn/day as zinc sulfate) for 10 days. However, the sample size of this study was too small to generalize. Additionally, in another zinc intervention study of 15 young women aged 20–24 years and 10 elderly healthy women aged 64–75 years [9], many individuals showed an increase in ZnT1 expression after zinc supplementation (22 mg Zn/day as zinc gluconate) for 27 days, but these changes were not significant and no clear relationship was found between ZnT1 expression and dietary zinc. These previous human studies involved healthy subjects with normal zinc metabolism. Therefore, it is possible that basal levels of zinc transporter expression before intervention are relatively high, and changes in zinc transporter expressions in response to differences in dietary zinc may not be detectable in those subjects. In this study, the positive association between ZnT1 gene expression and dietary zinc intake was observed in healthy men, but not in women. This difference between men and women may be due to different zinc status of subjects. Among 162 subjects, dietary zinc intake was significantly lower in women than that of men and the prevalence of zinc deficiency in women was higher than that of men. Further studies are needed to confirm the association between ZnT transporter gene expression and zinc intake in healthy subjects.
In conclusion, this study was a first attempt to explore the effect of zinc supplementation on ZnTs gene expression in obese individuals with altered zinc metabolism. We observed that zinc supplementation with 30 mg zinc/day for 8 weeks increased the expression levels of some zinc transporters (ZnT1 and ZnT5) in young obese women. Considering that obesity is associated with altered zinc metabolism, our findings suggest a critical role of ZnT1 and ZnT5 in the regulation of zinc metabolism and additional studies to further identify the relationships between zinc transporter expression and changes in zinc status in healthy individuals as well as those with various disease states are required.
References
Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lonnerdal B, Ruel MT, Sandtrom B, Wasantwisut E, Hotz C (2004) International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 25(1 Suppl 2):S99–S203
Liuzzi JP, Cousins RJ (2004) Mammalian zinc transporters. Annu Rev Nutr 24:151–172
Lichten LA, Cousins RJ (2009) Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 29:153–176
Huang L, Tepaamorndech S (2013) The SLC30 family of zinc transporters—a review of current understanding of their biological and pathophysiological roles. Mol Aspects Med 34(2–3):548–560
Noh H, Paik HY, Kim J, Chung J (2014) The alteration of zinc transporter gene expression is associated with inflammatory markers in obese women. Biol Trace Elem Res 158(1):1–8
McMahon RJ, Cousins RJ (1998) Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc Natl Acad Sci U S A 95(9):4841–4846
Liuzzi JP, Blanchard RK, Cousins RJ (2001) Differential regulation of zinc transporter 1, 2, and 4 mRNA expression by dietary zinc in rats. J Nutr 131(1):46–52
Cousins RJ, Blanchard RK, Popp MP, Liu L, Cao J, Moore JB, Green CL (2003) A global view of the selectivity of zinc deprivation and excess on genes expressed in human THP-1 mononuclear cells. Proc Natl Acad Sci U S A 100(12):6952–6957
Andree KB, Kim J, Kirschke CP, Gregg JP, Paik H, Joung H, Woodhouse L, King JC, Huang L (2004) Investigation of lymphocyte gene expression for use as biomarkers for zinc status in humans. J Nutr 134(7):1716–1723
Overbeck S, Uciechowski P, Ackland ML, Ford D, Rink L (2008) Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J Leukoc Biol 83(2):368–380
Aydemir TB, Blanchard RK, Cousins RJ (2006) Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc Natl Acad Sci U S A 103(6):1699–1704
Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ, Comparative Risk Assessment Collaborating G (2002) Selected major risk factors and global and regional burden of disease. Lancet 360(9343):1347–1360
Caballero B (2007) The global epidemic of obesity: an overview. Epidemiol Rev 29:1–5
Astrup A, Bugel S (2010) Micronutrient deficiency in the aetiology of obesity. Int J Obes 34(6):947–948
Tungtrongchitr R, Pongpaew P, Phonrat B, Tungtrongchitr A, Viroonudomphol D, Vudhivai N, Schelp FP (2003) Serum copper, zinc, ceruloplasmin and superoxide dismutase in Thai overweight and obese. J Med Assoc Thai 86(6):543–551
Marreiro DN, Fisberg M, Cozzolino SM (2004) Zinc nutritional status and its relationships with hyperinsulinemia in obese children and adolescents. Biol Trace Elem Res 100(2):137–149
Ennes Dourado Ferro F, de Sousa Lima VB, Mello Soares NR, Franciscato Cozzolino SM, do Nascimento Marreiro D (2011) Biomarkers of metabolic syndrome and its relationship with the zinc nutritional status in obese women. Nutr Hosp 26(3):650–654
Kim J, Ahn J (2014) Effect of zinc supplementation on inflammatory markers and adipokines in young obese women. Biol Trace Elem Res 157(2):101–106
Smidt K, Pedersen SB, Brock B, Schmitz O, Fisker S, Bendix J, Wogensen L, Rungby J (2007) Zinc-transporter genes in human visceral and subcutaneous adipocytes: lean versus obese. Mol Cell Endocrinol 264(1–2):68–73
Burczynski ME, Dorner AJ (2006) Transcriptional profiling of peripheral blood cells in clinical pharmacogenomic studies. Pharmacogenomics 7(2):187–202
Sagaya FM, Hurrell RF, Vergeres G (2012) Postprandial blood cell transcriptomics in response to the ingestion of dairy products by healthy individuals. J Nutr Biochem 23(12):1701–1715
Lonnerdal B (2000) Dietary factors influencing zinc absorption. J Nutr 130(5S Suppl):1378S–1383S
Kim J, Paik HY, Joung H, Woodhouse LR, Li S, King JC (2004) Zinc supplementation reduces fractional zinc absorption in young and elderly Korean women. J Am Coll Nutr 23(4):309–315
Jacobs RM, Lumsden JH, Taylor JA, Grift E (1991) Effects of interferents on the kinetic Jaffe reaction and an enzymatic colorimetric test for serum creatinine concentration determination in cats, cows, dogs and horses. Can J Vet Res 55(2):150–154
Noh H, Paik HY, Kim J, Chung J (2013) Salty taste acuity is affected by the joint action of alphaENaC A663T gene polymorphism and available zinc intake in young women. Nutrients 5(12):4950–4963
Foster M, Petocz P, Samman S (2013) Inflammation markers predict zinc transporter gene expression in women with type 2 diabetes mellitus. J Nutr Biochem 24(9):1655–1661
Kim J, Paik HY, Joung H, Woodhouse LR, Li S, King JC (2007) Effect of dietary phytate on zinc homeostasis in young and elderly Korean women. J Am Coll Nutr 26(1):1–9
Kambe T, Narita H, Yamaguchi-Iwai Y, Hirose J, Amano T, Sugiura N, Sasaki R, Mori K, Iwanaga T, Nagao M (2002) Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J Biol Chem 277(21):19049–19055
Suzuki T, Ishihara K, Migaki H, Ishihara K, Nagao M, Yamaguchi-Iwai Y, Kambe T (2005) Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J Biol Chem 280(35):30956–30962
Jackson KA, Valentine RA, Coneyworth LJ, Mathers JC, Ford D (2008) Mechanisms of mammalian zinc-regulated gene expression. Biochem Soc Trans 36(Pt 6):1262–1266
Cragg RA, Christie GR, Phillips SR, Russi RM, Kury S, Mathers JC, Taylor PM, Ford D (2002) A novel zinc-regulated human zinc transporter, hZTL1, is localized to the enterocyte apical membrane. J Biol Chem 277(25):22789–22797
Langmade SJ, Ravindra R, Daniels PJ, Andrews GK (2000) The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem 275(44):34803–34809
Kim J, Paik HY, Joung H, Woodhouse LR, King JC (2011) Plasma zinc but not the exchangeable zinc pool size differs between young and older Korean women. Biol Trace Elem Res 142(2):130–136
Acknowledgments
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) founded by the Ministry of Education, Science and Technology (NRF-2010-0011226 and NRF2012R1A1A1012317).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Noh, H., Paik, H.Y., Kim, J. et al. The Changes of Zinc Transporter ZnT Gene Expression in Response to Zinc Supplementation in Obese Women. Biol Trace Elem Res 162, 38–45 (2014). https://doi.org/10.1007/s12011-014-0128-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0128-z