Abstract
Obesity, a chronic inflammatory state, is associated with altered zinc metabolism. ZnT and Zip transporters are involved in the regulation of zinc metabolism. This study examined the relationships among obesity, zinc transporter gene expression, and inflammatory markers in young Korean women. The messenger RNA (mRNA) levels of leukocyte zinc transporters between obese (BMI = 28.3 ± 0.5 kg/m2, n = 35) and nonobese (BMI = 20.7 ± 0.2 kg/m2, n = 20) women aged 18–28 years were examined using quantitative real-time polymerase chain reaction. Inflammatory markers, such as C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and interleukin (IL)-6, were measured in serum by enzyme immunoassay. ZnT1 and Zip1 were the most abundantly expressed zinc transporters in leukocytes. The mRNA levels of many zinc transporters (ZnT4, ZnT5, ZnT9, Zip1, Zip4, and Zip6) were significantly lower in obese women, and expression of these genes was inversely correlated with BMI and body fat percentage. In addition, inflammatory markers (CRP and TNF-α) were significantly higher in obese women. The mRNA levels of ZnT4, Zip1, and Zip6 were inversely correlated with CRP (P < 0.05), and mRNA levels of ZnT4 and ZnT5 were inversely correlated with TNF-α (P < 0.05). In standardized simple regression models, levels of TNF-α and CRP were negatively associated with mRNA levels of zinc transporters such as ZnT4, ZnT5, Zip1, and Zip6 (P < 0.05). These results suggest that the expression of zinc transporters may be altered in obese individuals. Changes in zinc transporters may also be related to the inflammatory state associated with obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a worldwide public health problem. The prevalence of obesity has been increasing throughout the world, even in developing countries [1]. Obesity is a chronic inflammatory state characterized by altered adipokine production and increased levels of inflammatory cytokines. In obese individuals, plasma levels of inflammatory markers, such as C-reactive protein (CRP), tumor necrosis factor (TNF)-α, and interleukin (IL)-6, are elevated [2].
Zinc metabolism is also altered in obesity. Obese individuals have lower blood zinc concentrations than nonobese individuals [3, 4]. In obese women, erythrocyte zinc concentration is inversely associated with waist circumference and body mass index (BMI) [5]. Low serum or plasma zinc in obese individuals may be associated with the redistribution of zinc among various tissues [6–8]. In particular, a recent study showed that the reduction of body fat percentage by dietary intervention in obese adolescents led to an increase in the level of intraerythorcytic zinc and a decrease in urinary zinc excretion [9]. Zinc redistribution can be regulated by zinc transporters, which fall into two classes: zinc transporters or ZnT [also known as solute-linked carrier (SLC) 30] and Zir- and Irt-like proteins, or Zip (also known as SLC39) [10]. ZnT transporters decrease intracellular zinc levels by transporting zinc ions from the cytoplasm to either the extracellular space or intracellular vesicles, whereas Zip transporters increase intracellular zinc levels through the reverse route. Members of the ZnT family and the Zip family (10 and 14, respectively) have been identified in various mammalian tissues [10]. It has been shown that zinc supplementation or depletion alters zinc transporter expression levels in cultured human leukocytes [11, 12] and leukocytes isolated from human blood [11–13]. However, whether zinc transporter expression is altered in obese individuals is not known, and potential mechanisms for such changes remain unclear. To our knowledge, only one study examined zinc transporter expression in obese subjects [14], but the study did not look into the relationship with inflammatory markers, which might be the possible mechanism for the shift of zinc transporters in obesity.
This study examined gene expression levels of zinc transporters between obese and nonobese Korean women and investigated the association of zinc transporter mRNA levels and inflammatory markers.
Methods
Study Design and Subjects
Nonobese and obese women aged 18–28 years (20 and 35, respectively) were recruited from the Daegu and Gyeongbuk areas. Participants answered questions about demographic characteristics, smoking, nutritional supplementation, personal medical history, family history, and medication use in a face-to-face interview. Exclusion criteria included vitamin–mineral or other nutritional supplements and any medications including oral contraceptives, smoking, or participation in a weight-loss program. Obesity [15] was defined as a BMI of ≥25 kg/m2. Anthropometric measurements, blood pressure (BP), a fasting blood sample, and 24-h urine samples were collected. Also, 3-day diet records were obtained to assess usual dietary intake. Written informed consent was obtained from all participants. All procedures were approved by the Public Institutional Review Board (IRB; PIRB12-040-02) and the IRB of Kyung Hee University (KHSIRB-12-005).
Anthropometric Measurements and BP
All anthropometric measurements were performed by trained research staff using standardized protocols. Height was measured by anthropometry (TKK-11252, Japan). Body weight and body fat were measured by bioimpedance analysis (Inbody 3.0, Biospace, South Korea). BMI was calculated as weight (kg) divided by height squared (m2). Waist circumference was measured at the midpoint between the lower border of the rib cage and the top of the iliac crest using stretch-resistant tape [16]. Systolic BP (SBP) and diastolic BP (DBP) were measured twice using an automatic sphygmometer (HEM-770A, Japan) after the subject had been resting for 10 min in a sitting position. Average SBP and DBP were used for the analysis [17].
Nutritional Assessment
To estimate usual dietary intake, a three-nonconsecutive-day dietary record including two weekdays and one weekend day was collected. At the first visit, detailed instructions for the 3-day dietary record were given to subjects by trained dietitians. The subjects were instructed to record the type and amount of all foods and beverages they consumed for 3 days. The dietary records were checked for completeness by trained staff. Daily energy and nutrient intakes were calculated using a dietary evaluation program (Can-pro 3.0, Korean Nutrition Society).
Biochemical Analyses
Fasting blood samples and 24-h urine samples were collected. Blood samples were collected using plastic syringes and put on ice for a maximum of 2 h, centrifuged at 1,500 × g for 10 min at 4 °C (Allegra 6R, Beckman Colter, USA), and stored at −70 °C prior to analysis. Urine samples were collected in a polyethylene container, weighed, then stored in aliquots at −20 °C until analysis [18]. Serum and urinary zinc were measured using atomic absorption spectrometry (AAS 600, Perkin-Elmer, USA). Urinary creatinine was measured using the Jaffe method [19]. The activity of SOD was measured using an SOD assay kit (Cayman Chemical, USA). Inflammatory markers including CRP, TNF-α, and IL-6 were measured in serum by enzyme immunoassay. Specifically, CRP [20] was measured using a high-sensitivity CRP ELISA kit (Immundiagonstik, Europe). TNF-α [21] was measured using the Quantikine human TNF-α kit (R&D Systems, USA). IL-6 [22] was measured using the Quantikine human IL-6 kit (R&D Systems). Fasting triacylglyceride (TG) and total cholesterol (TC) were measured using an automated analyzer (ADVIA 2400, Japan). Fasting insulin was measured using a chemiluminescent immunoassay. Leptin concentration was measured using a human leptin kit (Linco Research, USA) [23], and adiponectin concentration was determined by immunoassay using a human adiponectin kit (R&D Systems).
Preparation of Leukocyte total RNA and Real-Time PCR Analysis
Total RNA was extracted from 1.5 ml whole blood using the QIAamp RNA blood mini kit (Qiagen, USA) [11]. The concentration and purity of extracted RNA was examined by measuring absorbance at 260- and 280-nm wavelength using a spectrophotometer (Nanodrop, Thermo, USA). RNA samples with OD260/OD280 values of 1.8 or higher were used in the analysis. cDNA was synthesized from 0.15 μg of total RNA using a PrimeScriptTM RT reagent kit (Takara, Japan) according to the manufacturer’s protocol. Zinc transporter gene expression levels in leukocytes were assessed via real-time polymerase chain reaction (PCR). Reactions were prepared using SYBR Premix Ex Taq II (Takara) and conducted on a Mini-Opticon (Bio-rad, USA) in duplicate. We examined the expression of a wide range of zinc transporters expressed in leukocytes [7, 12]. The relative expression levels of zinc transporters were determined using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as reference gene and expressed as 2−ΔCt (ΔCt = Cttarget-Ctreference) per 10−4 GAPDH [11].
Statistical Analysis
All data analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC, USA). Differences in anthropometric measurements, zinc status, inflammatory markers, biochemical parameters, and mRNA levels of leukocyte zinc transporters between nonobese and obese women were identified using the Wilcoxon rank–sum test. Spearman correlations between mRNA levels of leukocyte zinc transporters and anthropometric measurements were also calculated. In addition, the relationships among mRNA levels of leukocyte zinc transporters, zinc status, inflammatory markers, and other biochemical parameters were examined using Spearman’s partial rank correlation coefficients, with adjustment for BMI. Standardized regression analysis was conducted to explore the effects of BMI and inflammatory markers on mRNA levels of zinc transporters after calculation of z scores for each value of BMI and inflammatory markers.
Results
Differences in Anthropometric Measurements, Zinc Status, Inflammatory Markers, and Biochemical Parameters between Nonobese and Obese Women
Anthropometric measurements, zinc status, inflammatory markers, and biochemical parameters are shown in Table 1, stratified by obesity status. BMI, percentage body fat, and waist circumference were significantly higher in obese women (P < 0.0001). SBP and DBP were also significantly higher in obese women (P < 0.01). Dietary zinc intake and serum and urinary zinc concentrations were not significantly different between the two groups. Serum SOD activity was significantly lower in obese women (P = 0.02), while inflammatory markers, such as CRP (P < 0.0001) and TNFα (P < 0.001), were significantly higher in obese women. The levels of serum TG, TC, and insulin were significantly higher in obese women as well. Finally, adiponectin was significantly lower (P = 0.04) and leptin was significantly higher (P < 0.0001) in obese women.
Zinc Transporter mRNA Levels in Nonobese and Obese Women
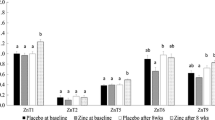
Zinc transporter mRNA levels in leukocytes are shown in Fig. 1. The zinc transporters with the highest expression levels were ZnT1 and Zip1. The differences in zinc transporter mRNA levels between nonobese and obese women are shown in Table 2. The mRNA levels of zinc transporters, such as ZnT4, ZnT5, ZnT9, Zip1, Zip4, and Zip6, were significantly lower in obese women. Zip1 mRNA levels were 70.6 % lower in obese women (P < 0.0001). By contrast, ZnT7 mRNA levels were 78.1 % higher in obese women (P < 0.0001).
Association of Zinc Transporter Expression Levels with Anthropometric Measurements, Zinc Status, Inflammatory Markers, and Biochemical Parameters
The correlations among leukocyte zinc transporters, anthropometric measurements, zinc status, inflammatory markers, and biochemical parameters are shown in Table 3. The mRNA levels of zinc transporters, such as ZnT4, ZnT5, ZnT9, Zip1, Zip4, and Zip6, which were significantly lower in obese women, were inversely correlated with BMI and percentage body fat. By contrast, ZnT7 mRNA levels, which were significantly higher in obese women, were positively correlated with BMI and percentage body fat (P < 0.001). Zinc transporter mRNA levels were not correlated with either dietary zinc intake or serum zinc concentration. However, the mRNA levels of ZnT9 and Zip6 were positively correlated with urinary zinc concentration (P < 0.05).
Even after adjustment for BMI, inflammatory markers were significantly associated with the mRNA levels of zinc transporters that had lower expression in obese women. The levels of ZnT4, Zip1, and Zip6 mRNA were inversely correlated with CRP (P < 0.05), and the mRNA levels of ZnT4 and ZnT5 were inversely correlated with TNF-α (P < 0.05). However, mRNA levels of other zinc transporters were not associated with inflammatory markers (data not shown).
After adjustment for BMI, TG, and TC were not correlated with zinc transporter mRNA levels. Similarly, serum leptin, adiponectin, and insulin showed no correlation with zinc transporter expression after adjustment for BMI.
For further analysis, the results of standardized simple regression analyses to examine the influence of BMI and inflammatory markers on mRNA levels of zinc transporters are shown in Table 4. BMI and inflammatory markers were inversely associated with mRNA level of zinc transporters. BMI had the negative association with mRNA level of ZnT4 (standardized β = -0.29, P < 0.0001), ZnT5 (standardized β = -0.20, P < 0.0001), and Zip1 (standardized β = -0.43, P < 0.0001). The level of TNF-α was negatively associated with mRNA level of ZnT4 (standardized β = -0.24, P < 0.0001), ZnT5 (standardized β = -0.15, P < 0.001), and Zip1 (standardized β = -0.30, P < 0.001), even though they were a little smaller than those of BMI. Furthermore, TNF-α level was negatively associated with mRNA level of ZnT4 (standardized β = -0.12, P = 0.03) even after adjustment for BMI in a standardized multiple regression model (data not shown). In addition, CRP had the largest negative association with mRNA level of Zip6 (standardized β = -0.11, P = 0.01).
Discussion
In this study, we found that zinc transporter expression was altered in the leukocytes of obese women. Leukocyte mRNA levels of ZnT4, ZnT5, ZnT9, Zip1, Zip4, and Zip6 were significantly lower in obese women, and expression of these zinc transporters was inversely correlated with BMI and body fat. Furthermore, expression levels of some zinc transporters (ZnT4, ZnT5, Zip1, and Zip6) were inversely associated with inflammatory markers such as TNF-α and CRP. These results suggest that zinc metabolism is altered in obese individuals via changes in the expression of various zinc transporters; such changes in zinc metabolism could be related to the elevated level of inflammation found in obesity.
The zinc transporters with the highest level of expression in leukocytes were ZnT1 and Zip1. Previous studies report similar findings. Aydemir et al. [13] reported that in the monocytes of three young, healthy men aged 19–31 years, mRNA levels of ZnT1 and Zip1 were much higher than those of all other zinc transporters examined (ZnT5, ZnT7, Zip3, and Zip8). Another study, by Overbeck et al. [12], also found that, among all ZnT proteins, ZnT1 mRNA was the most highly expressed in peripheral blood mononuclear cells from seven young, healthy donors.
In our study, with the exception of ZnT7, expression levels of zinc transporters were lower in obese women. Moreover, expression of these zinc transporters was inversely correlated with BMI and body fat, suggesting zinc metabolism might be altered in obese subjects. Several studies have demonstrated altered zinc status in obese individuals [4, 5, 24]. For example, among 73 premenopausal women aged 20–50 years, erythrocyte zinc concentration was lower in obese women, displaying an inverse association with BMI and waist circumference [5]. Similarly, among 48 children aged 5–15 years [25], monocyte zinc concentration was lower in obese individuals, showing a significant inverse correlation with body weight and arm fat area. In dietary intervention study through nutritional education about balanced diet with 15 obese adolescent girls aged 14–18 years, the decreased body fat resulted in the increased levels of erythrocyte zinc with no change of zinc intake. That is, the change of zinc status in obese was affected by the proportion of body fat, not zinc intake [9]. The altered zinc status reported in obesity could be related to the changes in zinc transporter expression levels we identified in leukocytes. To the best of our knowledge, only one prior study [14] has examined zinc transporter expression in obese subjects. It compared the expression of eight ZnT (ZnT 1–8) and eight Zip (Zip 1–8) levels in the adipose tissue of 12 obese women (mean BMI 44.5 ± 5.3 kg/m2) and 12 nonobese women (mean BMI 23.1 ± 2.6 kg/m2). Consistent with our findings, mRNA levels of ZnT2, ZnT3, ZnT6, and ZnT8 were significantly lower in the subcutaneous fat of obese women. Moreover, mRNA levels of all Zip transporters examined (Zip 1–8) were significantly lower in the subcutaneous fat of obese women, indicating that changes in zinc transporter expression are an important feature of obesity.
We found that, unlike other ZnT transporters, ZnT7 mRNA levels were higher in the leukocytes of obese individuals. Huang et al. [26] found low weight gain and fat mass in ZnT7-knockout mice, suggesting ZnT7 is involved in the regulation of body composition including fat mass. In the present study, ZnT7 mRNA levels were positively correlated with BMI and percentage body fat. Therefore, an increase in body fat mass may be associated with elevated expression of ZnT7 in obese women.
Importantly, we found that mRNA levels of some zinc transporters (ZnT4, ZnT5, Zip1, and Zip6) were inversely correlated with inflammatory markers. This result suggests that obesity-related inflammation could be linked to alterations in zinc transporter expression. In the present study, mRNA levels of ZnT4 and ZnT5 were significantly lower (~50 %) in obese women and were significantly inversely correlated with serum TNFα and/or CRP. Consistent with our findings, a marked downregulation of ZnT4 and ZnT6 has been reported in mouse models of allergic inflammation [27]. The ZnT family primarily functions as a zinc exporter protein, decreasing cytosolic zinc concentrations [10, 28]. For example, in mammary and intestinal epithelial cells, a mutation in ZnT4 can result in reduced expression, leading to decreased zinc secretion [29, 30]. In light of the anti-inflammatory effects of zinc, it is possible that reduced expression of ZnT4 and ZnT5 might exert a protective role against inflammation by increasing retention of intracellular zinc [31, 32]. By contrast, expression of ZnT1 and ZnT2 was not associated with inflammatory markers in this study. These transporters are chiefly responsible for intracellular compartmentalization, playing a crucial role in the delivery of zinc to zinc-dependent proteins important for cell function such as SOD and alkaline phosphatase [30]. It is likely that expression of these crucial transporters is not downregulated by obesity-related inflammation.
The mechanism by which obesity-related inflammation is associated with Zip1 and Zip6 expression is unclear. Some Zip proteins are located in the trans-Golgi network, where they are involved in the influx of zinc from the Golgi apparatus to the cytoplasm [33]. Thus, the downregulation of these Zip proteins could facilitate the conservation of intracompartmental zinc. Similar to our findings, Zip6 is downregulated in response to LPS treatment in dendritic cells [34]. Zip1 and Zip6 can also be located in the plasma membrane, where they are involved in zinc influx from the extracellular space. In this case, decreased Zip expression could lead to a reduction in intracellular zinc concentrations. Further studies are needed to better understand the relationship between changes in Zip expression and cellular zinc concentration in obesity.
Although serum or urine zinc concentration did not differ between obese women and nonobese women in the present study, the activity of superoxide dismutase, an index of zinc status, was significantly lower in obese women than in nonobese women. No changes in serum zinc concentration may be due to zinc homeostasis. Serum concentrations are maintained stable within a narrow range even when dietary zinc levels fluctuate [35].
The present study has several limitations. First, zinc transporter protein levels were not determined. While a number of ZnT/Zip transporter genes are transcriptionally regulated [36], posttranscriptional and translational regulation cannot be ruled out and should be investigated. Second, the study cannot reveal causal relationships because of its cross-sectional design. A longitudinal study of obesity and zinc transporter expression, with a larger sample size, would be helpful in clarifying the direction of causality.
In conclusion, the present study clearly demonstrated that expression of multiple zinc transporters was significantly lower in the leukocytes of obese women. In addition, and expression of several zinc transporters (ZnT4, ZnT5, Zip1, and Zip6) were inversely associated with inflammatory markers. These findings suggest zinc transporters as an important link between altered zinc metabolism and inflammation in obesity. Further intervention studies are warranted to fully elucidate the role of zinc transporters in modulating zinc metabolism and inflammation associated with obesity.
Reference
Caballero B (2007) The global epidemic of obesity: an overview. Epidemiol Rev 29:1–5
Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B (2006) Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17(1):4–12
Marreiro DN, Geloneze B, Tambascia MA, Lerario AC, Halpern A, Cozzolino SM (2006) Effect of zinc supplementation on serum leptin levels and insulin resistance of obese women. Biol Trace Elem Res 112(2):109–118
Tungtrongchitr R, Pongpaew P, Phonrat B, Tungtrongchitr A, Viroonudomphol D, Vudhivai N, Schelp FP (2003) Serum copper, zinc, ceruloplasmin and superoxide dismutase in Thai overweight and obese. J Med Assoc Thai 86(6):543–551
Ennes Dourado Ferro F, de Sousa Lima VB, Mello Soares NR, Franciscato Cozzolino SM, do Nascimento Marreiro D (2011) Biomarkers of metabolic syndrome and its relationship with the zinc nutritional status in obese women. Nutr Hosp 26(3):650–654
Begin-Heick N, Dalpe-Scott M, Rowe J, Heick HM (1985) Zinc supplementation attenuates insulin secretory activity in pancreatic islets of the ob/ob mouse. Diabetes 34(2):179–184
Foster M, Samman S (2012) Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients 4(7):676–694
Garcia OP, Ronquillo D, Caamano Mdel C, Camacho M, Long KZ, Rosado JL (2012) Zinc, vitamin A, and vitamin C status are associated with leptin concentrations and obesity in Mexican women: results from a cross-sectional study. Nutr Metab (Lond) 9(1):59
Freire SC, Fisberg M, Cozzolino SM (2013) Dietary intervention causes redistribution of zinc in obese adolescents. Biol Trace Elem Res 154(2):168–177
Lichten LA, Cousins RJ (2009) Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 29:153–176
Andree KB, Kim J, Kirschke CP, Gregg JP, Paik H, Joung H, Woodhouse L, King JC, Huang L (2004) Investigation of lymphocyte gene expression for use as biomarkers for zinc status in humans. J Nutr 134(7):1716–1723
Overbeck S, Uciechowski P, Ackland ML, Ford D, Rink L (2008) Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J Leukoc Biol 83(2):368–380
Aydemir TB, Blanchard RK, Cousins RJ (2006) Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc Natl Acad Sci U S A 103(6):1699–1704
Smidt K, Pedersen SB, Brock B, Schmitz O, Fisker S, Bendix J, Wogensen L, Rungby J (2007) Zinc-transporter genes in human visceral and subcutaneous adipocytes: lean versus obese. Mol Cell Endocrinol 264(1–2):68–73
World Health Organization (WHO) (2000) The Asia Pacific perspective: redefining obesity and its treatment
World Health Organization (WHO) (2008) Waist circumference and waist-hip ratio: report of a WHO expert consultation
Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ (2005) Recommendations for blood pressure measurement in humans and experimental animals: Part 1. Blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 45(1):142–161
Kim J, Paik HY, Joung H, Woodhouse LR, Li S, King JC (2004) Zinc supplementation reduces fractional zinc absorption in young and elderly korean women. J Am Coll Nutr 23(4):309–315
Jacobs RM, Lumsden JH, Taylor JA, Grift E (1991) Effects of interferents on the kinetic Jaffe reaction and an enzymatic colorimetric test for serum creatinine concentration determination in cats, cows, dogs and horses. Can J Vet Res 55(2):150–154
Koenig W, Lowel H, Baumert J, Meisinger C (2004) C-reactive protein modulates risk prediction based on the Framingham score: implications for future risk assessment: results from a large cohort study in southern Germany. Circulation 109(11):1349–1353
Idriss HT, Naismith JH (2000) TNF alpha and the TNF receptor superfamily: structure–function relationship(s). Microsc Res Tech 50(3):184–195
Murakami M, Hibi M, Nakagawa N, Nakagawa T, Yasukawa K, Yamanishi K, Taga T, Kishimoto T (1993) IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science 260(5115):1808–1810
Ozata M, Mergen M, Oktenli C, Aydin A, Sanisoglu SY, Bolu E, Yilmaz MI, Sayal A, Isimer A, Ozdemir IC (2002) Increased oxidative stress and hypozincemia in male obesity. Clin Biochem 35(8):627–631
Marreiro DN, Fisberg M, Cozzolino SM (2004) Zinc nutritional status and its relationships with hyperinsulinemia in obese children and adolescents. Biol Trace Elem Res 100(2):137–149
Marotta A, Todisco N, Di Toro A, Toraldo R, Ponte G, Perrone L (1995) Zinc content of lymphomonocytes in obese children. Nutr Res 15(10):1411–1415
Huang L, Yu YY, Kirschke CP, Gertz ER, Lloyd KK (2007) Znt7 (Slc30a7)-deficient mice display reduced body zinc status and body fat accumulation. J Biol Chem 282(51):37053–37063
Lang C, Murgia C, Leong M, Tan LW, Perozzi G, Knight D, Ruffin R, Zalewski P (2007) Anti-inflammatory effects of zinc and alterations in zinc transporter mRNA in mouse models of allergic inflammation. Am J Physiol Lung Cell Mol Physiol 292(2):L577–L584
Palmiter RD, Huang L (2004) Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch 447(5):744–751
Lee DY, Shay NF, Cousins RJ (1992) Altered zinc metabolism occurs in murine lethal milk syndrome. J Nutr 122(11):2233–2238
Murgia C, Vespignani I, Rami R, Perozzi G (2006) The Znt4 mutation inlethal milk mice affects intestinal zinc homeostasis through the expression of other Zn transporters. Genes Nutr 1(1):61–70
Cassel GH (1978) Zinc: a review of current trends in therapy and our knowledge of its toxicity. Del Med J 50(6):323–328
Kelishadi R, Hashemipour M, Adeli K, Tavakoli N, Movahedian-Attar A, Shapouri J, Poursafa P, Rouzbahani A (2010) Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metab Syndr Relat Disord 8(6):505–510
Huang L, Kirschke CP, Zhang Y, Yu YY (2005) The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J Biol Chem 280(15):15456–15463
Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, Yamashita S, Kaisho T, Akira S, Murakami M, Hirano T (2006) Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol 7(9):971–977
Kim J (2013) Dietary zinc intake is inversely associated with systolic blood pressure in young obese women. Nutr Res Pract 7(5):380–384
Cousins RJ, Liuzzi JP, Lichten LA (2006) Mammalian zinc transport, trafficking, and signals. J Biol Chem 281(34):24085–24089
Acknowledgments
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) founded by the Ministry of Education, Science, and Technology (NRF-2010-0011226 to J.C. and NRF2012R1A1A1012317 to J.K.).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Noh, H., Paik, H.Y., Kim, J. et al. The Alteration of Zinc Transporter Gene Expression Is Associated with Inflammatory Markers in Obese Women. Biol Trace Elem Res 158, 1–8 (2014). https://doi.org/10.1007/s12011-014-9902-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-9902-1