Abstract
Measuring toxic metal body burden is particularly important in autism spectrum disorder (ASD) because evidence indicates that children with ASD have a greater susceptibility to heavy-metal intoxication than typically developing children. The more traditional laboratory tests used to measure toxic metal levels in ASD provide a snapshot of current exposure but do not necessarily provide a measure of tissue body burden. A more recent approach is to use urinary porphyrins which provide an indirect measure of toxic metal body burden. Urinary porphyrins are not a direct measure of toxic metals in the urine but a measure of tissue body burden by the level of disruption of the heme synthesis pathway caused by the presence of toxic metals in the tissues. Recent evidence suggests that the levels of mercury-associated porphyrins are different in children with ASD as compared to controls, with significantly increased levels of pentacarboxyporphyrin (5cxP), precoproporphyrins (prcP), and coproporphyrins (cP). In addition, there is a significant relationship between the severity of the child’s autism symptoms and the elevation of mercury-associated urinary porphyrins (i.e., the higher the mercury-associated porphyrins, the more severely affected the child). The mercury-associated porphyrins can be lowered with chelation therapy, and porphyrin profile testing can be used in clinical medicine in the targeted treatment of heavy-metal toxicity.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
An autism spectrum disorder (ASD) is a neurological disorder that limits a person’s ability to function normally. Behavioral abnormalities, social limitations, sensory processing abnormalities, and impaired ability to communicate are the main issues in this multifaceted disorder. ASD can range in clinical symptoms from severe to mild among individuals diagnosed with autistic disorder (autism), pervasive developmental disorder-not otherwise specified (PDD-NOS), and Asperger’s syndrome (AS) (Frith 1997; Kern et al. 2006). To date, the disorder is behaviorally defined with no consensus as to the underlying pathology. Although there is no scientific consensus on the cause or causes of autism, there is considerable evidence implicating some external causes, such as exposure to toxic metals, particularly mercury (Hg), live viruses in vaccines, certain drugs during pregnancy, and certain pesticides. Considering that a scientific consensus on the cause or causes of autism may be years away and, in fact, may never be reached, adopting the “precautionary principle” and addressing the implicated causes of autism is warranted, particularly when there is considerable supporting evidence implicating certain factors.
There is a large and growing body of toxicological evidence indicating that Hg is either causal or contributory in the pathology of autism. For example, in a 2010 review of the research regarding toxic metals and autism, Desoto and Hitlan (2010) identified 58 research articles that provide empirical evidence relevant to the question of a link between autism and one or more heavy metals. Of those 58 articles, 43 supported a significant link between autism and exposure to toxic metals, while 15 showed no statistically significant evidence of a link between metals and autism. Thus, as of 2010, 74 % of the studies showed a significant relationship between ASD and toxic metals. Moreover, eight recent studies have shown that the greater the toxic metal body burden in a child, the worse the autism symptoms (Holmes et al. 2003; Nataf et al. 2006; Geier and Geier 2007; Adams et al. 2009; Geier et al. 2009b; Kern et al. 2010; Elsheshtawy et al. 2011; Lakshmi Priya and Geetha 2011). Although some of the studies found an association between autism and various toxic metals, such as cadmium (Cd), lead (Pb), and arsenic (As), the vast majority of the studies implicated Hg.
It is commonly found that Hg levels are higher in children with autism than they are in typically developing children (controls). Most of these studies involved a direct measure of Hg levels in hair, blood, teeth, nails, and/or urine, traditional ways of measuring heavy-metal exposure in children. Even though results from these more traditional laboratory tests have been able to show differences in toxic metal levels in children with ASD as compared to controls, they provide only a snapshot of current exposure. As a result, these laboratory tests do not necessarily provide a measure of tissue body burden. For example, Hg may remain in the blood for 12 months following exposure (Begerow et al. 1994); however, Hg has been shown to remain in the tissues, such as the liver and brain, for much longer, even decades (Falnoga et al. 2000). Figure 1 shows the principal target organs and other target areas where mercury compounds tend to accumulate.
Measuring toxic metal body burden is particularly important in ASD because evidence indicates that children diagnosed with an ASD have a greater susceptibility to heavy-metal intoxication than typically developing children (Holmes et al. 2003; Kern and Jones 2006; Rose et al. 2008; Nataf et al. 2008; James et al. 2009; Geier et al. 2009a; Majewska et al. 2010; Youn et al. 2010; Kern et al. 2011). Expressions such as “poor detoxifiers” and “poor excretors” have been used in reference to those diagnosed with an ASD (Holmes et al. 2003). Two of the main areas of susceptibility involve glutathione (GSH) and sulfate (SO4). For example, children with ASD have been found in several studies to have low plasma GSH and sulfate levels (Waring and Klovrza 2000; Kern et al. 2004; James et al. 2004, 2006, 2009; Geier and Geier 2006a; Paşca et al. 2009; Geier et al. 2009a, c; Adams et al. 2011), both of which are critically important for detoxification (Gutman 2002; Kern et al. 2004). In contrast, oxidized glutathione (GSSG) levels are significantly increased in the plasma of children with ASD, suggesting that the GSH redox system is taxed. (Reduced GSH is a major tissue antioxidant. The formation of a disulfide bond between two GSH molecules gives rise to GSSG. Under conditions of oxidative stress, GSSG accumulates and the ratio of reduced GSH to GSSG will decrease. Therefore, the reduced GSH to GSSG ratio can be used as an indicator of oxidative stress in cells and tissues).
Moreover, a recent study by Chauhan et al. (2012) compared DNA oxidation and GSH redox status in postmortem brain samples from the cerebellum and frontal, temporal, parietal, and occipital cortex in autistic subjects and age-matched normal subjects. The authors reported that levels of reduced GSH were significantly reduced and that the levels of GSSG were significantly increased in the cerebellum and temporal cortex in the brain samples from the group with autism, as compared to the corresponding levels in the control brain samples. In other words, brain GSH levels in those diagnosed with an ASD also appear to be inadequate.
In the face of toxic exposure, a consequence of an inadequate detoxification capacity in those diagnosed with an ASD would be greater toxic metal body burden, making the more traditional measures that provide a snapshot of current exposure not necessarily the optimal test. A more recent approach is to use urinary porphyrins because urinary porphyrins can provide a measure of toxic metal body burden. This is the case because, although urinary porphyrins are not a direct measure of toxic metals in the urine, some of them are a measure of tissue body burden by the level of disruption of the heme synthesis pathway (Fig. 2; Nataf et al. 2006) caused by the presence of toxic metals in the tissues. Studies have shown that urinary porphyrins (heme precursors formed in the heme synthesis pathway) can afford a measure of xenobiotic exposure and of tissue body burden, particularly with respect to Hg (Woods 1996; Pingree et al. 2001a, b). The process is summarized in Fig. 2 and in the following narrative:
A summary of the heme pathway. A summary of the heme synthesis pathway and major urinary metabolites (Nataf et al. 2006). Porphyrinogens appear in urine as porphyrin derivatives (right). Mercury can cause increased urinary 5cxP, prcP, and cP by inhibiting uroporphyrinogen decarboxylase (UROD) and/or coproporphyrinogen oxidase (CPOX); urinary uroporphyrin is not reported to alter with inhibition of these enzymatic steps
Heme (e.g., hemoglobin) is necessary for the function of aerobic cells. Heme production primarily occurs in the liver, kidneys, and erythroid cells; however, the heme synthesis pathway is active in almost every cell of the body (Wang et al. 2011). The presence of toxic metals inhibits the enzymes necessary for the heme synthesis pathway to progress forward. This inhibition or interference results in a “backlog” and an increase in the urinary export of certain porphyrins. The level of increase in these “backlogged” metabolites measured in the urine is indicative of the level of disruption of this pathway and the extent of toxic metal tissue burden. Porphyrinogens then appear in urine as porphyrin derivatives (Fig. 2, right side).
Porphyrin excretion patterns have been shown to be metal specific in both animals and humans after prolonged exposure to Hg, Pb, As, and other metals. Table 1 shows examples of porphyrin patterns from aluminum (Al), As, Pb, and Hg (Woods 1996; Pingree et al. 2001a, b). For example, specific patterns of urinary porphyrins that suggest the presence of Hg and the extent of the body burden have been demonstrated to be associated with elevations in urinary coproporphyrin (cP) and pentacarboxyporphyrin (5cxP) and by the expression of an atypical porphyrin – precoproporphyrin (prcP) (also known as keto-isocoproporphyrin) not found in the urine of unexposed controls (Woods et al. 1993; Gonzalez-Ramirez et al. 1995). Hg can cause this increase in urinary 5cxP, prcP, and cP by inhibiting the enzymes uroporphyrinogen decarboxylase (UROD) and/or coproporphyrinogen oxidase (CPOX) (Nataf et al. 2006). Woods (1996) noted that these distinct changes in urinary porphyrin concentrations were observed as early as 1–2 weeks after initiation of Hg exposure and that they increased in a dose – and time-related fashion with the concentration of Hg in the kidney, a principal target organ of Hg compounds.
For Hg in particular, research shows that there is a high degree of statistical correlation between renal Hg burden and porphyrin excretion (Fowler 2001). This correlation is shown consistently in the animal model, and the heme pathway is highly conserved across species (Gonzalez-Ramirez et al. 1995). For example, methyl-Hg-induced porphyrinuria in rats (Woods and Fowler 1977) is very similar to the porphyrin pattern of humans exposed to Hg vapor (Gonzalez-Ramirez et al. 1995).
Importantly, several studies not only show this characteristic pattern of porphyria with Hg toxicity but also show that the porphyrin profile can be normalized with chelation (Woods et al. 1991, 1993; Echeverria et al. 1995; Woods 1996; Pingree et al. 2001a; Nataf et al. 2006). In addition, Pingree et al. (2001a) found a high correlation between pre-chelation urinary porphyrins and pre-chelation Hg concentrations and also between pre-chelation urinary porphyrins and post-chelation urinary Hg levels in rats.
Urinary porphyrin profiles also correlate significantly with specific neurobehavioral deficits associated with low-level Hg exposure. Echeverria et al. (2005), for example, examined the behavioral effects of low-level exposure to Hg vapor among dentists. These investigators observed that urinary porphyrins were as sensitive as urinary Hg levels for observing adverse effects of Hg on cognitive and motor testing. Porphyrin researchers (Woods et al. 1993; Gonzalez-Ramirez et al. 1995; Cianciola et al. 1997; Woods and Fowler 1977; Pingree et al. 2001a, b; Heyer et al. 2006) conclude that urinary porphyrin profiles are a useful biomarker for Hg exposure and its potential adverse health effects in human subjects. Importantly, Woods et al. (2009) published a study supporting the sensitivity of urinary porphyrins as a biological indicator of subclinical Hg exposure in children. Finally, Geier et al. (2011) analyzed data from a randomized, prospective clinical trial that was designed to evaluate the potential health consequences of prolonged exposure to Hg from dental amalgam fillings and established the sensitivity and specificity of specific urinary porphyrins as a biomarker for low-level Hg body burden.
Although measuring urinary porphyrins is not a direct measure of mercury in the brain, studies show that the brain and kidneys are target organs for mercury following Hg exposure (Pingree et al. 2001a). For example, Pingree et al. (2001b) gave rats methyl-Hg hydroxide (MMH) in drinking water for 9 weeks and determined both inorganic (Hg2+) and organic (CH3Hg+) mercury species levels in urine and tissues by cold vapor atomic fluorescence spectroscopy (CVAFS). After the treatment, Hg2+ and CH3Hg+ concentrations were 0.28 and 4.80 μg/g in the brain and 51.5 and 42.2 μg/g in the kidney, respectively.
Urinary Porphyrins and Autism
Recent evidence suggests that the levels of Hg-associated porphyrins are different in children diagnosed with an ASD as compared to controls. Studies revealed that children with an ASD diagnosis had significantly increased levels of urinary 5cxP, prcP, and cP as compared to controls (Geier and Geier 2006b, 2007; Nataf et al. 2006; Austin and Shandley 2008; Youn et al. 2010; Kern et al. 2011). Studies that have examined urinary profiles in autism are summarized in Table 2 and explained in more detail in the following narrative:
In 2006, Geier and Geier (2006b) reported elevated cP levels in children with ASD (n = 37) as compared to their siblings (n = 7). Then, in 2007, Geier and Geier (2007) found that patients diagnosed with an ASD (n = 71) had significant elevations in urinary levels of cP, 5cxP, and prcP relative to controls (n = 14) and >50 % of patients diagnosed with an ASD had urinary cP levels more than two standard deviations above the mean values for controls. Significant reductions in urinary 5cxP and cP levels were observed in ASD patients following chelation, and a significant relationship between the severity of the child’s ASD diagnosis and the elevation of Hg-associated urinary porphyrins (i.e., the higher the Hg-associated porphyrins, the more severe the diagnosis). These two studies were conducted in the USA.
Similarly, Nataf et al. (2006) examined French children diagnosed with autism (n = 106) and found that these children had elevated prcP and cP as compared to controls (n = 12). In addition, >50 % of patients diagnosed with autism had urinary prcP levels more than two standard deviations above the mean values for controls. They also found a significant relationship between the severity of the child’s ASD diagnosis and Hg-associated urinary porphyrins. Austin and Shandley (2008) found similar results in children with ASD (n = 41) in Australia, using control data from lab reference ranges and other studies. These previously mentioned studies used Laboratoire Philippe Auguste and/or Laboratory Corporation of America (LabCorp) (CLIA-approved lab based in the US). Later, a urinary porphyrin study conducted in Korea, using Metametrix (CLIA-approved lab based in the USA), found significant increases of uP, 5cxP, prcP, cP, and total porphyrins in children diagnosed with an ASD (n = 65) as compared to controls (n = 9) (Youn et al. 2010).
Thus, these studies from four different continents have shown that children with ASDs have higher levels of Hg-associated porphyrins (5cxP, prcP, and cP) than typically developing children (Nataf et al. 2006, 2008; Geier and Geier 2006b, 2007; Austin and Shandley 2008; Youn et al. 2010). In addition, prcP and cP levels were lowered in the children with autism who had been chelated.
These aforementioned initial studies were criticized because the controls were not age and gender matched to the children diagnosed with an ASD. However, in 2011, Kern et al. addressed these criticisms in a study that evaluated urinary porphyrin biomarkers of Hg toxicity in the porphyrin pathway in a cohort of children diagnosed with an ASD (n = 20) as compared to age-, gender-, race-, region-of-residency-, and year-of-sample-collection-matched controls (n = 20) using clinically available lab testing. As with the previous studies, participants diagnosed with an ASD had significantly increased levels of 5cxP, prcP, and cP in comparison to controls (Kern et al. 2011). No significant differences were found in non-Hg-associated urinary porphyrins: uroporphyrins (uP), hexacarboxyporphyrin (6cxP), and heptacarboxyporphyrin (7cxP). There was a significantly increased odds ratio for an ASD diagnosis, relative to controls, among study participants with prcP (odds ratio = 15.5, p < 0.01) and cP (odds ratio = 15.5, p < 0.01) levels in the second through fourth quartiles, compared to the first quartile.
Further, it was observed when excluding a single participant in that study among the controls with urinary porphyrin values that were significant outliers from other controls (more than two standard deviations above the mean) and the corresponding matched participant diagnosed with an ASD, there were significant increases in mean 5cxP (1.3-fold, p < 0.05, ASD = 5.14 ± 1.34 nmol/g creatinine, control = 4.11 ± 1.4 nmol/g creatinine), prcP (1.3-fold, p < 0.01, ASD = 21.2 ± 6.96 nmol/g creatinine, control = 16.5 ± 6.86 nmol/g creatinine), and cP (1.4-fold, p < 0.001, ASD = 255 ± 76 nmol/g creatinine, control = 184 ± 76 nmol/g creatinine) levels among participants diagnosed with an ASD in comparison to the corresponding values for the controls (Kern et al. 2011).
Recently, two studies, Woods et al. (2010) and Heyer et al. (2012), specifically examined urinary 5cxP and cP as biological indicators of ASD. Woods et al. (2010) examined enrolled 278 children 2–12 years of age. They evaluated three groups: autism, PDD-NOS, and controls. Elevated cP (p < 0.009), 6cxP (p < 0.01), and 5cxP (p < 0.001) concentrations were significantly associated with autism, but not with PDD-NOS. Then Heyer et al. (2012) specifically examined urinary 5cxP and cP among 76 male children comprised of 30 with autism, 14 with PDD-NOS, and 32 controls. ASD children (autism and PDD-NOS) had higher mean urinary 5cxP (p < 0.006) and cP (p < 0.006) concentrations compared with same-age neurotypical control children.
Urinary Mercury-Associated Porphyrins and Severity of Autism Symptoms
As mentioned briefly, two of the earlier studies that examined porphyrins in autism found that elevations in mercury-associated porphyrin levels correlated with autism severity. First, Nataf et al. (2006) found a significant relationship between the severity of the child’s ASD diagnosis (AS < PDD < autism < autism + epilepsy) and the elevation of Hg-associated urinary porphyrins (i.e., the higher the Hg-associated porphyrins, the more severe the diagnosis). This finding was later corroborated by Geier and Geier (2007). Since those two studies were conducted, two subsequent studies have also shown that Hg-associated porphyrins correlated with autism severity.
The first of these two additional studies examined the relationship between autism symptom severity using the Childhood Autism Rating Scale (CARS; Schopler et al. 1994) and single urinary porphyrin levels among the participants (n = 22) (Geier et al. 2009b). There were significant correlations between CARS scores and the Hg-associated porphyrins 5cxP/uP ratios (τb = 0.32, p < 0.05) and prcP/uP ratios (τb = 0.31, p < 0.05). By contrast, no significant correlation was observed between CARS scores and the non-Hg-associated porphyrins 7cxP/uP ratios (τb = 0.11, p = 0.47). In addition, there was a significant correlation between CARS scores and the (5cxP + prcP)/(7cxP + uP) ratio (τb = 0.35, p < 0.05), which is a specific biomarker of Hg body burden. In other words, the higher the Hg-associated porphyrins, the more severe the symptoms of autism. There was also no correlation between autism symptom severity and non-Hg-associated porphyrins.
The second study looked at the relationship between autism symptom severity using the Autism Treatment Evaluation Checklist (ATEC; Rimland and Edelson 1999) and single urinary porphyrin levels among the participants (n = 24) (Kern et al. 2010). Researchers found that the participants’ overall ATEC scores and their scores on each of the ATEC subscales (Speech/Language, Sociability, Sensory/Cognitive Awareness, and Health/Physical/Behavior) were linearly related to urinary porphyrins associated with Hg toxicity.
External Influence or a Disordered Porphyrin Metabolism?
Woods et al. (2010) and Heyer et al. (2012), who found elevated levels of 5pxP and cP Hg-associated porphyrins in autism, concluded that their findings “demonstrate that porphyrin measures are strong predictors of both autism and PDD-NOS, and support the potential clinical utility of urinary porphyrin measures in ASD.” This conclusion is consistent with previous researchers. However, they attributed the higher levels of 5pxP and cP found in ASD to a “disordered porphyrin metabolism.” This conclusion is not consistent with previous research. Previous researchers have attributed these findings to heavy-metal body burden which is supported by research in the animal model. In the animal model, it has been consistently shown that heavy-metal toxicity (predominately Hg but also lead) can increase these porphyrin parameters and that detoxification can then reduce the levels back to normal. Importantly, research shows that in ASD, similar to the animal model, these elevated porphyrin levels can also be reduced when these children undergo detoxification. As mentioned earlier, three studies have shown that detoxification reduces the level of mercury-associated porphyrins in children with ASD (Nataf et al. 2006; Geier and Geier 2006b, 2007). The fact that the Hg-associated porphyrins that are found to be elevated in autism are readily reduced with chelation suggests that these elevated porphyrin parameters are not intrinsic to the children but a result of external factors, i.e., exposure.
Another biological parameter that suggests that the elevated Hg-associated porphyrins are a result of exposure is the correlation between Hg-associated porphyrin levels and plasma-oxidized glutathione levels. Geier et al. (2009a) conducted a prospective, blind study to evaluate urinary porphyrins and glutathione levels (reduced and oxidized) among a cohort of participants diagnosed with ASDs (n = 28). A significant correlation was found between urinary porphyrins, those associated with mercury intoxication, and increasing CARS scores as well as increasing plasma-oxidized GSH. In contrast, the other urinary porphyrins did not show such correlations. Since mercury induces oxidative stress and since the mercury-associated porphyrins correlate with the level of oxidative stress in children with ASD, this finding suggest pro-oxidative exposure, not an intrinsic metabolic imbalance.
Clinical Use
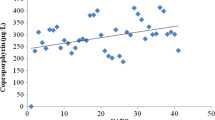
Although not currently widespread, urinary porphyrin testing is currently being used in some clinical practices as an assessment tool in the targeted treatment of heavy-metal toxicity in autism. Table 3 describes how urinary porphyrin profile testing can be used in clinical medicine. Urinary porphyrins can provide a noninvasive way to aide in the decision-making process as to determine whether or not chelation therapy is appropriate. Urinary porphyrins can also be used to monitor detoxification. Figure 3 shows the relationship between urinary mercury levels and urinary porphyrin levels during chelation therapy.
Summary
Many studies have shown that children diagnosed with an ASD are poor detoxifiers and have a higher toxic metal body burden, particularly a higher Hg burden. These studies also found that the higher the toxic metal burden in children diagnosed with an ASD, the worse were the severity of their symptoms. Hg-associated porphyrins are higher in children with ASD than in control children and are also correlated with autism symptom severity. Thus, urinary porphyrin profile evaluation can be used both to estimate toxic metal burden and as a tool to monitor Hg body burden during the targeted treatment of heavy-metal toxicity in those diagnosed with an ASD.
Key Terms
-
Urinary porphyrins. Porphyrins help form several substances in the body including hemoglobin, the protein that carries oxygen in the blood. Porphyrins can be found in urine, and a urine porphyrin test measures the amount of porphyrins in the urine. This includes uroporphyrins (uP), pentacarboxyporphyrin (5cxP), hexacarboxyporphyrin (6cxP), heptacarboxyporphyrin (7cxP), precoproporphyrins (prcP) (also known as keto-isocoproporphyrin), and coproporphyrins (cP).
-
Mercury-associated porphyrins. pentacarboxyporphyrin (5cxP), precoproporphyrins (prcP), and coproporphyrins (cP).
-
Heavy metals. Metallic compounds of certain elements such as arsenic, cadmium, lead, mercury, and nickel.
-
Chelation therapy. Treatment designed to bind heavy metals in the body in a manner that they are excreted in order to reduce heavy-metal body burden and treat heavy-metal toxicity.
-
Body burden. Refers to the total amount of a chemical (e.g., mercury) that is present in the human body at a given point in time.
-
Mercury. Silvery-white poisonous metallic element that is a liquid at room temperature.
Key Facts
-
Children diagnosed with an ASD have a greater susceptibility to heavy-metal intoxication than typically developing children.
-
Forty-three studies (as of 2010) support a significant link between autism and exposure to toxic metals.
-
Eight recent studies have shown that the greater the toxic metal body burden in a child, the worse the autism symptoms.
-
Although some of the studies found an association between autism and various toxic metals, such as cadmium, lead, and arsenic, the vast majority of the studies implicated mercury.
-
It is commonly found that Hg levels are higher in children with autism than they are in typically developing children (controls).
-
Studies reveal that children with ASD have significantly increased levels of urinary mercury-associated porphyrins (5cxP, prcP, and cP) as compared to the levels in controls, and these levels decrease with chelation therapy.
-
Mercury-associated porphyrin levels correlate with autism symptom severity.
Summary Points
-
Many studies have shown that children with ASD are poor detoxifiers and have a higher toxic metal burden, particularly a higher mercury body burden.
-
Studies have also shown that the higher the toxic metal burden in children diagnosed with an ASD, the worse is the severity of their symptoms.
-
Mercury-associated porphyrins are higher in children diagnosed with an ASD than in control children and also correlate with autism symptom severity.
-
Urinary porphyrin profile testing can be used both to estimate toxic metal burden and as a tool to monitor mercury body burden during the targeted treatment of heavy-metal toxicity in those diagnosed with an ASD.
References
Adams JB, Baral M, Geis E, et al. The severity of autism is associated with toxic metal body burden and red blood cell glutathione levels. J Toxicol. 2009;532–640.
Adams JB, Audhya T, McDonough-Means S, et al. Nutritional and metabolic status of children with autism vs neurotypical children, and the association with autism severity. Nutr Metab (Lond). 2011;8:34.
Austin DW, Shandley K. An investigation of porphyrinuria in Australian children with autism. J Toxicol Environ Health. 2008;71:1349–51.
Baker S. Detoxification and healing. New Canaan: Keats Publishing; 1997.
Begerow J, Zander D, Freier I, et al. Long-term mercury excretion in urine after removal of amalgam fillings. Int Arch Occup Environ Health. 1994;66:209–12.
Chauhan A, Audhya T, Chauhan V. Brain region-specific glutathione redox imbalance in autism. Neurochem Res. 2012;37(8):1681–9.
Cianciola ME, Echeverria D, Martin MD, et al. Epidemiologic assessment of measures used to indicate low-level exposure to mercury vapor (Hg). J Toxicol Environ Health. 1997;52:19–33.
Desoto MC, Hitlan RT. Sorting out the spinning of autism: heavy metals and the question of incidence. Acta Neurobiol Exp (Wars). 2010;70:165–76.
Echeverria D, Heyer NJ, Martin MD, et al. Behavioral effects of low-level exposure to elemental Hg among dentists. Neurotoxicol Teratol. 1995;17:161–8.
Echeverria D, Woods JS, Heyer NJ, et al. Chronic low-level mercury exposure, BDNF polymorphism, and associations with cognitive and motor function. Neurotoxicol Teratol. 2005;27:781–96.
Elsheshtawy E, Tobar S, Sherra K, et al. Study of some biomarkers in hair of children with autism. Middle East Curr Psychiatry. 2011;18:6–10.
Falnoga I, Tusek-Znidaric M, Horvat M, et al. Mercury, selenium, and cadmium in human autopsy samples from Idrija residents and mercury mine workers. Environ Res. 2000;84:211–8.
Fowler BA. Porphyrinurias induced by mercury and other metals. Toxicol Sci. 2001;61:197–8.
Frith U. Autism: explaining the enigma. Oxford: Blackwell; 1997.
Garcia-Vargas GG, Del Razo LM, Cebrian ME, et al. Altered urinary porphyrin excretion in a human population chronically exposed to arsenic in Mexico. Hum Exp Toxicol. 1994;13:839–47.
Geier DA, Geier MR. A clinical and laboratory evaluation of methionine cycle-transsulfuration and androgen pathway markers in children with autistic disorders. Horm Res. 2006a;66:182–8.
Geier DA, Geier MR. A prospective assessment of porphyrins in autistic disorders: a potential marker for heavy metal exposure. Neurotox Res. 2006b;10:57–64.
Geier DA, Geier MR. A prospective study of mercury toxicity biomarkers in autistic spectrum disorders. J Toxicol Environ Health. 2007;70:1723–30.
Geier DA, Kern JK, Adams JB, et al. A prospective study of oxidative stress biomarkers in autistic disorders. J Applied Psychol. 2009a;5:2–10.
Geier DA, Kern JK, Geier MR. A prospective blinded evaluation of urinary porphyrins verses the clinical severity of autism spectrum disorders. J Toxicol Environ Health A. 2009b;72:1585–91.
Geier DA, Kern JK, Garver CR, et al. Biomarkers of environmental toxicity and susceptibility in autism. J Neurol Sci. 2009c;280:101–8.
Geier DA, Carmody T, Kern JK, et al. A significant relationship between mercury exposure from dental amalgams and urinary porphyrins: a further assessment of the Casa Pia children’s dental amalgam trial. Biometals. 2011;24:215–24.
Gonzalez-Ramirez D, Maiorino RM, Zuniga-Charles M, et al. Sodium 2,3-dimercaptopropane-1-sulfonate challenge test for mercury in humans: II. Urinary mercury, porphyrins and neurobehavioral changes of dental workers in Monterrey, Mexico. J Pharmacol Exp Ther. 1995;272:264–74.
Gutman J. Glutathione – your bodies most powerful protector. 3rd ed. Montreal: Communications kudoca; 2002.
Heyer NJ, Bittner AC Jr, Echeverria D, et al. A cascade analysis of the interaction of mercury and coproporphyrinogen oxidase (CPOX) polymorphism on the heme biosynthetic pathway and porphyrin production. Toxicol Lett. 2006;161:159–66.
Heyer NJ, Echeverria D, Woods JS. Disordered porphyrin metabolism: a potential biological marker for autism risk assessment. Autism Res. 2012;5:84–92.
Holmes AS, Blaxill MF, Haley BE. Reduced levels of mercury in first baby haircuts of autistic children. Int J Toxicol. 2003;22:277–85.
James SJ, Cutler P, Melnyk S, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80:1611–7.
James SJ, Melnyk S, Jernigan S, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:947–56.
James SJ, Rose S, Melnyk S, et al. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 2009;23:2374–83.
Kern JK, Jones AM. Evidence of toxicity, oxidative stress, and neuronal insult in autism. J Toxicol Environ Health B. 2006;9:485–99.
Kern JK, Waring RH, Ramsden DB, et al. Abnormal sulfation chemistry in autism. In: Columbus F, editor. Progress in autism research. Hauppauge: Nova Science; 2004.
Kern JK, Trivedi MH, Garver CR, et al. The pattern of sensory processing abnormalities in autism. Autism. 2006;10:480–94.
Kern JK, Geier DA, Adams JB, et al. A Biomarker of mercury body-burden correlated with diagnostic domain specific clinical symptoms of autistic disorders. Biometals. 2010;23:1043–51.
Kern JK, Geier DA, Adams JB, et al. Toxicity biomarkers related to autism spectrum disorder: a blinded study of urinary porphyrins. Pediatr Int. 2011;53:147–53.
Lakshmi Priya MD, Geetha A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol Trace Elem Res. 2011;142:148–58.
Majewska MD, Urbanowicz E, Rok-Bujko P, et al. Age-dependent lower or higher levels of hair mercury in autistic children than in healthy controls. Acta Neurobiol Exp (Wars). 2010;70:196–208.
Nasiadek M, Chmielnicka J, Subdys J. Analysis of urinary porphyrins in rats exposed to aluminum and iron. Ecotoxicol Environ Saf. 2001;48:11–7.
Nataf R, Skorupka C, Amet L, et al. Porphyrinuria in childhood autistic disorder: implications for environmental toxicity. Toxicol Applied Pharmacol. 2006;14:99–108.
Nataf R, Skorupka C, Lam A, et al. Porphyrinuria in childhood autistic disorder is not associated with urinary creatinine deficiency. Pediatr Int. 2008;50:528–32.
Ng JC, Wang JP, Zheng B, et al. Urinary porphyrins as biomarkers for arsenic exposure among susceptible populations in Guizhou province, China. Toxicol Appl Pharmacol. 2005;206:176–84.
Paşca SP, Dronca E, Kaucsár T, et al. One carbon metabolism disturbances and the C677T MTHFR gene polymorphism in children with autism spectrum disorders. J Cell Mol Med. 2009;13:4229–38.
Pingree SD, Simmonds PL, Rummel KT, et al. Quantitative evaluation of urinary porphyrins as a measure of kidney mercury content and mercury body burden during prolonged methylmercury exposure in rats. Toxicol Sci. 2001a;61:234–40.
Pingree SD, Simmonds PL, Woods JS. Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on tissue and urine mercury levels following prolonged methylmercury exposure in rats. Toxicol Sci. 2001b;61:224–33.
Rimland B, Edelson M. Autism treatment evaluation checklist. San Diego: Autism Research Institute; 1999. www.ARI-ATEC.com and https://www.autismeval.com/ari-atec/report1.html.
Rose S, Melnyk S, Savenka A, et al. The frequency of polymorphisms affecting lead and mercury toxicity among children with autism. Am J Biochem Biotechnol. 2008;4:85–94.
Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale. Los Angeles: Western Psychological Services; 1994. p. 90025–1251.
Wang L, Angley MT, Gerber JP, et al. A review of candidate urinary biomarkers for autism spectrum disorder. Biomarkers. 2011;16:537–52.
Waring RH, Klovrza LV. Sulphur metabolism in autism. J Nutr Environ Med. 2000;10:25–32.
Woods JS. Altered porphyrin metabolism as a biomarker of mercury exposure and toxicity. Can J Physiol Pharmacol. 1996;74:210–5.
Woods JS, Fowler BA. Renal porphyrinuria during chronic methyl mercury exposure. J Lab Clin Med. 1977;90:266–72.
Woods JS, Bowers MA, Davis HA. Urinary porphyrin profiles as biomarkers of trace metal exposure and toxicity: studies on urinary porphyrin excretion patterns in rats during prolonged exposure to methyl mercury. Toxicol Appl Pharmacol. 1991;110:464–76.
Woods JS, Martin MD, Naleway CA, et al. Urinary porphyrin profiles as a biomarker of mercury exposure: studies on dentists with occupational exposure to mercury vapor. J Toxicol Environ Health. 1993;40:235–46.
Woods JS, Martin MD, Leroux BG, et al. Urinary porphyrin excretion in normal children and adolescents. Clin Chim Acta. 2009;405:104–9.
Woods JS, Armel SE, Fulton DI, et al. Urinary porphyrin excretion in neurotypical and autistic children. Environ Health Perspect. 2010;118:450–7.
Youn SI, Jin SH, Kim SH, et al. Porphyrinuria in Korean children with autism: correlation with oxidative stress. J Toxicol Environ Health A. 2010;73:701–10.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this entry
Cite this entry
Kern, J.K., Geier, D.A., Sykes, L., Geier, M. (2014). Urinary Porphyrins in Autism Spectrum Disorders. In: Patel, V., Preedy, V., Martin, C. (eds) Comprehensive Guide to Autism. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4788-7_72
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4788-7_72
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4787-0
Online ISBN: 978-1-4614-4788-7
eBook Packages: Behavioral ScienceReference Module Humanities and Social SciencesReference Module Business, Economics and Social Sciences