Abstract
Recent studies have reported correlations between mineral intake and metabolic syndrome (MS), but accurate relationships and consistency in the results are difficult to confirm. Accordingly, this study aims to assess the dietary intakes of magnesium (Mg), manganese (Mn), and copper (Cu) to determine their relationship with MS. Data from a total of 5,136 adults (2,084 men, 3,052 women) was collected from the 2007–2008 Korea National Health and Nutrition Examination Survey (KNHANES), and the intakes of Mg, Mn, and Cu of the MS patients were compared with those of healthy adults. The relationship between the intakes of these minerals and the MS risks was analyzed. Diagnosis of MS was evaluated by the National Cholesterol Education Program's Adult Treatment Panel III (NCEP-ATP III) standards. Among all study subjects, 25.9 % (540 subjects) of the men and 24.5 % (748 subjects) of the women met diagnostic criteria for inclusion in the MS group. In the men, daily intakes of Mg and Cu in the MS group were significantly lower than those in control group, and in the women, daily intakes of energy, Mg, Mn, and Cu in the MS group were significantly lower than those of the control group. The women subjects with high blood pressure showed significantly lower intakes of Mg, Mn, and Cu than control subjects. In addition, in the women, the highest quartile of Mg and Cu was inversely associated with MS, but with adjustment were not maintained. However, in the postmenopausal women, MS was significant and inversely associated with the highest quartiles of Cu intake and the association remained significant after adjustments. Considering that MS incidence increases and dietary intake and nutrient density decrease with increasing age, and mineral intake is reduced accordingly, these results suggest that meal management with adequate mineral intake is advisable to control MS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In an aging society, people's lifestyles change and the incidence of metabolic syndrome (MS) rapidly increases worldwide. MS is a cluster of metabolic disorders that increase the risk of developing cardiovascular disease and diabetes and elevate all-cause mortality [1]. Diagnostic standards for MS were first announced by WHO in 1998 [2], then revised by the US National Cholesterol Education Program's Adult Treatment Panel III (NCEP-ATP III) in 2001 [3]. MS is now defined as the condition in which at least three of the following are present: elevated waist circumference, elevated blood pressure, elevated blood glucose, elevated triglycerides, and low HDL-cholesterol [3].
According to NCEP-ATP III standards, the prevalence rate of MS in the USA was about 23.7 % (24.0 % for men, 23.7 % for women), and 45 % of people aged 50 years and older met diagnostic criteria for MS according to the Third National Health and Nutritional Examination Survey (NHANES III) [4, 5]. In Korea, 14.2 % of men and 17.7 % of women were reported to have MS in 1998 (NCEP-ATP III definition, the standards of abdominal obesity: above 102 cm for men and 88 cm for women) [6]. However, when the standards of abdominal obesity in Asians, which are above 90 cm for men and 80 cm for women, were applied, the prevalence rate of MS increased significantly from 24.9 % in 1998 to 29.2 % in 2001, to 30.4 % in 2005, and to 31.3 % in 2007 according to the Korean NHANES (KNHANES) [7]. This shows a higher incidence than the USA; thus, a strategy to prevent and treat MS is needed.
The onset of MS is influenced by environmental factors such as genetic factors, body composition, eating habits, exercise, and insulin resistance due to visceral fat accumulation, which are known to be key mechanisms [8]. As people's interest in dietary life has risen recently, many studies on the relationship between diet and MS have been performed, but the role of diet in the syndrome has not yet been clearly determined [9–11].
Magnesium (Mg) is the second most abundant cation in the cell. It interacts with phosphate, when nucleic acid or ATP is synthesized, and acts as a coenzyme that affects more than 300 enzymatic reactions including renal calcium reuptake, vitamin D formation, neurotransmission, muscle contraction and relaxation, glucose metabolism, blood pressure, and lipid metabolism [12]. A previous study reported that blood Mg level is an independent risk factor of diabetes and that the study subjects with high blood Mg level have a higher predisposition to diabetes compared with the study subjects that have lower blood Mg [13]. There also are reports that those who take Mg supplementation daily experience a decrease in averaged 24-h blood pressure [14], total cholesterol, LDL-cholesterol, and triglycerides, but increased HDL-cholesterol compared with those who do not take Mg supplementation [15].
Lower intake of antioxidant nutrients is reported to increase coronary artery disease and oxidation stress, which affect hypertension, diabetes, and cardiovascular diseases that are related to MS [16]. Manganese (Mn) is one of the antioxidant nutrients that makes up metalloenzymes and takes part in macronutrient metabolism including carbohydrate, protein, and lipid metabolism. It is a cofactor of superoxide dismutase (SOD), an antioxidant enzyme that defends against free radicals [17]. Copper (Cu) is essential for various enzymes that catalyze redox reactions, detoxification, transfer, production, and formation reactions [18]. Cu combines with SOD to prevent cellular oxidative damage and increases blood cholesterol when it is insufficient [19]. Low antioxidant minerals damage cell stability by decreasing enzymatic activity and immunity due to lack of hormones and enzymes. This is considered to be related to MS, but the studies on this matter are limited.
Therefore, this study aims to assess the daily intake of Mg, Mn, and Cu and to determine their relationship with MS risk. The dietary Mg, Mn, and Cu intake of patients with MS was compared with that of healthy adults using the data from the 2007–2008 KNHANES. The relationship between the intakes of these minerals and the MS risks was also studied.
Materials and Methods
Study Design and Subjects
The KNHANES is a cross-sectional study, which uses a rolling sampling design that involves a complex, stratified, multistage, probability-cluster survey of a representative sample of the non-institutionalized civilian Korean population. The survey data is compiled through a health interview, health examination (physical examination and clinical measurements), and a nutrition survey. The study is approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (KCDC), and written consent is obtained from all participants [20].
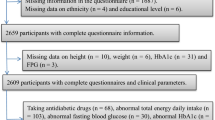
The data sets used in this study originated from the fourth KNHANES (2007–2008). A total of 14,338 individuals from 6,000 households of 300 Primary Sampling Units (PSUs) took part in the 2007–2008 KNHANES (2007, n = 4,594; 2008, n = 9,744). We excluded subjects younger than 19 years old, those who reported implausible dietary intakes (lower than 500 kcal/d or greater than 5,000 kcal/d), and those for whom we did not have data on waist circumference, blood pressure, blood glucose, insulin, triglycerides, cholesterol, high density lipoprotein (HDL)-cholesterol, and socio-demographics (smoking, alcohol intake, and physical activity). Also, subjects who took nutritional supplements were not included in the study. Finally, 5,136 subjects aged 19 years and older (2,084 men and 3,052 women) were included in the analysis.
General Characteristics, Anthropometrics, and Biochemical Variables
The health examination included anthropometric measurements (height, weight, body mass index, and waist circumference), blood pressure measurements, and blood biomarker analyses (total cholesterol, triglycerides, glucose, insulin, and HDL-cholesterol). In a health interview, a questionnaire including information on sex, age, smoking history, alcohol intake, exercise, and menopausal status was administered by trained interviewers. Metabolic Equivalent of Task values (METs) were used to classify physical activity as low, middle, or high. METs are multiples of the resting metabolic rates and were calculated using the short form (version 2.0, April 2004) of the International Physical Activity Questionnaire. Low activity was 600 > MET-min/week; middle activity was 600 ≤ MET-min/week<3,000; and high activity was 3,000 ≤ MET-min/week.
Height was measured within 0.1 cm, and weight was measured with a metric weight scale to the nearest 0.1 kg in light clothing without shoes. Body mass index (BMI) was calculated as body weight in kilograms divided by height in meters squared. Blood pressure measurements were taken three times, and the average of the second and third measurements for both systolic blood pressure (SBP) and diastolic blood pressure (DBP) was used. Fasting serum total cholesterol, triglycerides, glucose, and HDL-cholesterol were measured by an ADVIA 1650 Automatic Analyzer (Siemens, New York, USA) in 2007 and Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan) in 2008. Serum insulin was measured with the Insulin RIA Kit (BioSource, Nivelles, Belgium) in 2007 and INS-IRMA (BioSource, Nivelles, Belgium) in 2008. Among the biochemical variables used in this study, there were differences only in HDL-cholesterol levels because of non-identical analysis methods and equipment due to the change of the clinical inspection agency in 2007 and 2008. Therefore, this study used the following conversion equations to correct these level differences.
-
1)
$$ \mathrm{Conversion}\ \mathrm{equation}(2007)=\left\{\left(\mathrm{raw}\ \mathrm{data}\times 1.15\right)+3.70\right\}\times 0.86+2.98 $$
-
2)
$$ \mathrm{Conversion}\ \mathrm{equation}(2008)=\left(\mathrm{raw}\ \mathrm{data}\times 0.86\right)+2.98 $$
Diagnostic Criteria for MS
The diagnosis of MS was based on the updated NCEP criteria [21]. Participants with three or more of the following five criteria were defined as having MS:
-
1.
Abdominal obesity (defined as waist circumference ≥90 cm for men and ≥85 cm for women in Korea) [22]
-
2.
Elevated blood pressure (≥130 mmHg systolic and/or ≥85 mmHg diastolic)
-
3.
High fasting blood glucose (≥100 mg/dL)
-
4.
High triglycerides (≥150 mg/dL)
-
5.
Low HDL-cholesterol (<40 mg/dL for men and <50 mg/dL for women)
The third criterion, fasting blood glucose, was changed to 100 mg/dL from the initial cutoff point of 110 mg/dL according to the revised guideline [21]. MS risk score was determined by adding the number of risk factors among the five: elevated waist circumference, high triglycerides, low HDL-cholesterol, elevated glucose, and elevated blood pressure.
Dietary Assessment and Mineral Database
Trained dietitians interviewed each subject to collect dietary data through 24-h dietary recalls. The daily Mg, Mn, and Cu intakes from foods were estimated using a mineral database produced by previous studies [23, 24] and the food composition table of the National Rural Living Science Institute, Korea [25]. The subjects of the 2007–2008 KNHANES consumed 1,819 foods based on 24-h dietary recall data. The Mg, Mn, and Cu databases covered 73.9, 56.8, and 68.9 % of the food items consumed by the subjects of the 2007–2008 KNHANES. In terms of food intake, the database covered 92.4 % for Mg, 86.7 % for Mn, and 84.3 % for Cu. Foods not included in the mineral content database were calculated by replacing them with similar foods in the database. After calculating the intake of minerals per individual, the status of the intake was calculated in relation to the Dietary Reference Intakes (DRIs) for Koreans [26]. For estimated average requirements (EAR), the ratio of the subjects whose EAR intake is insufficient compared with its standard intake amount was calculated, while for tolerable upper intake level (UL), the ratio of the subjects that overtook UL compared with its standard was calculated. In addition, for recommended nutrient intake (RNI) adequate intake (AI), the intake rate compared with each of its standard was calculated.
Statistical Analysis
All statistical analyses were performed using SAS software (version 9.2, SAS Institute Inc., Cary, NC, USA) through a “survey procedure.” Differences in socio-demographics, body composition, and clinical and metabolic parameters between MS subjects and control subjects were evaluated by the t-test for continuous variables and the chi-square test for categorical variables. Multivariate logistic regression analysis was used to estimate the adjusted odds ratios (OR) and 95 % confidence intervals of the mineral intake, predicting MS. To determine the confounding factor in this study, MS prevalence difference according to basic factors such as age, smoking status, alcohol consumption, physical activity, and energy intake was verified. In the case of age (50 years and younger vs. 50 years and older) and energy intake (used median value, men <2070.6 vs. ≥2070.6 kcal, women <1529.3 vs. ≥1529.3 kcal), men group (age, p < 0.0001 and energy, p = 0.0074) and women group (age, p < 0.0001 and energy, p < 0.0001) showed significant difference in MS prevalence. On the other hand, as in smoking status (current smoker vs. non/past smoker) and physical activity (used median value, men <2850.5 MET vs. ≥2850.5 MET, women <1580.0 MET vs. ≥1580.0 MET), there were no significant differences in the prevalence of MS between men group (smoking status, p = 0.7553 and physical activity, p = 0.1655) and women group (smoking status, p = 0.8564 and physical activity, p = 0.4627). In the case of frequency of alcohol intake (≤once a week vs. twice a week or more), there was no significant difference in the prevalence of MS between women group (p = 0.2007); however, in men group, there was significant difference (p = 0.0021). Therefore, in women, confounders included age and energy intake, and in men, added frequency of alcohol intake. All statistical tests were two-sided, and a p-value <0.05 was considered significant.
Results
General Characteristics
Socio-demographics, body composition, and clinical and metabolic parameters in the group with and without MS are shown in Table 1. Among this study's population (N = 5,136), 1,288 (25.1 %) individuals (41.9 % men) were MS subjects meeting the NCEP-ATP III criteria. The average age of the MS group was significantly higher than that of the control group in both men and women (p < 0.0001, respectively). In men, the MS group showed significantly higher rate in frequent alcohol intake compared with the control group (p = 0.0008), while the non-smoking subjects showed significantly lower rate of the MS group (p = 0.0008). In women, the MS group had a smaller proportion of subjects who did not drink (p < 0.0001). No difference was observed in physical activity in both men and women. The MS group exhibited significantly higher weight (p < 0.0001), BMI (p < 0.0001), waist circumference (p < 0.0001), SBP (p < 0.0001), and DBP (p < 0.0001) than the control group. Subjects with MS also had significantly higher serum levels of cholesterol (p < 0.0001), insulin (p < 0.0001), triglycerides (p < 0.0001), and glucose (p < 0.0001) than subjects without MS. By contrast, serum HDL-cholesterol levels were significantly lower in subjects with MS (p < 0.0001).
Energy and Mineral Intakes
Daily energy and mineral intakes by the subjects in the group with MS and those in the group without MS are shown in Table 2. In women, daily intakes of energy, Mg, Mn, and Cu in the control group were significantly higher than those in the MS group (p = 0.0069, p = 0.0155, p = 0.0086, p = 0.0038, respectively). In men, daily intakes of Mg and Cu in the control group were significantly higher than those in the MS group (p = 0.0195, p = 0.0422), but no difference was observed in energy intake or Mn intake between the two groups. Men reached 86.3–92.0 % and women 82.1–88.2 % of the recommended Mg intake value. However, the rates of subjects with Mg intakes lower than the EAR were 50.0–57.4 % in men and 53.5–62.0 % in women. In this study, the ratio of Mn and Cu intake to the RNI or AI was above 100 %.
Dietary Mineral Intake and Diagnostic Components of MS
Dietary Mg, Mn, and Cu intakes according to the abnormalities of the individual diagnostic components of MS are shown in Table 3. In women, subjects with high blood pressure had significantly lower Mg, Mn, and Cu intakes (p = 0.0003, p < 0.0001, p = 0.0035, respectively), and in those with low HDL-cholesterol, only Mg was significantly lower (p = 0.0393) than in those without such abnormalities.
Relationship between Mineral Intake and MS Risk
The relationships between mineral intake and the risk of MS are shown in Table 4. No significant association between dietary Mn intake and the risk of MS was found. In women, dietary Mg and Cu intakes were inversely associated with the risk of MS (Model 1: 4th vs. 1st quartile, OR = 0.61, 95 % CI = 0.47–0.81, P for trend = 0.0002 in Mg; OR = 0.64, 95 % CI = 0.48–0.85, P for trend = 0.0170 in Cu), but there was no significant relation between these minerals and MS risk after adjusting for potential confounders (Model 2). In addition, the correlation of mineral intake and MS risk of women before and after menopause was analyzed, and no meaningful relation between the risk of MS and Mg and Mn intake was shown (Table 5). In the postmenopausal women, dietary Mg, Mn, and Cu intakes were inversely associated with the risk of MS (Model 1: 4th vs. 1st quartile, OR = 0.50, 95 % CI = 0.35–0.72, P for trend = 0.0028 in Mg; OR = 0.57, 95 % CI = 0.39–0.85, P for trend = 0.0179 in Mn; OR = 0.53, 95 % CI = 0.36–0.77, P for trend < .0001 in Cu), but there was no significant relation between Mg and Mn and MS risk after adjusting for potential confounders. However, in the case of Cu, its relation with MS remained even after adjusting the potential confounders.
Discussion
As the incidence of MS increases, various physiological functions of minerals and the importance of mineral intake are increasingly emphasized. Accordingly, this study was designed to assess the daily intake status of Mg, Mn, and Cu to investigate their relationship with MS risk. Our results showed that there were significant relationships between low daily intakes of Mg, Mn, and Cu and abnormality of blood pressure, and between low Mg intake and abnormality of HDL-cholesterol, especially in women. Daily Mg and Cu intakes also showed a negative and significant correlation with MS risks in women.
It has been reported that MS and dietary intake are closely related and, especially, that excessive energy intake causes obesity and results in increasing incidence of MS. However, in the case of the women in this study, the energy intake of the MS group was significantly lower than that of the control group, showing contradictory results with those of previous studies [27]. Buscemi et al. [28] reported that when conducting the food record method for 3 days, the energy intake of the MS group showed no significant difference with that of the control group and that body fat increases even though the MS subjects are taking in the same energy as the normal subjects because the MS group is in the energy-sparing metabolism state.
As found in previous studies showing that overweight and obese people report lower dietary intake, so their dietary intake is underestimated [29–31], the dietary intake of the MS group with higher BMI in women is likely to have been underestimated. Also, given that actual dietary intake is reported, through continuous KNHANES administrations, to be reduced as subjects increase in age from adulthood to old age, energy intake is thought to be low because the age of the MS group was significantly higher than that of the control group. Therefore, this study to find out the relevance between Mg, Mn, and Cu intake, and MS conducted comparison and analysis by regarding the age and energy intake of the subjects as important confounders.
Many studies showing that Mg intake is related to MS have been reported [32–34]. Mg functions as a coenzyme that takes part in glucose and insulin metabolism [35]. In a previous study, type II diabetes patients who had low Mg levels were supplemented with oral intake of Mg for 16 weeks, and the results showed an improvement in insulin sensitivity and glucose control [36]. There are also reports on healthy elders improving insulin sensitivity due to Mg supplementation [37]. McKeown et al. [34] reported that intake of a sufficient amount of Mg through ingestion of green vegetables, beans, and whole wheat was important for the elders, as their study results on 535 elders older than 60 years showed a significant relationship between low Mg intake and risk of MS. Ford et al. [32] analyzed 7,669 adult NHANES participants older than 20 years and found that there was a significant relationship between Mg intake and MS. Studies on 4,637 subjects aged 18–30 years [33] and 2,504 subjects aged 18–74 years [38], and on 11,686 subjects aged 45 years and older [30] also showed the same results. In the current study, only women with high blood pressure and low HDL-cholesterol had a lower Mg intake than normal subjects. Daily Mg intake also had a negative and significant correlation with MS risks in women. However, considering the energy intake, Mg intake showed no significant differences between the two groups and no significant relation with MS risks. Nevertheless, given that many previous studies [32–34, 38, 39] report on the relationship between Mg intake and MS, and MS incidence increases and energy intake and nutrient density decrease with increasing age, with Mg intake reduced accordingly, the findings of this study showing the relation between Mg intake and MS risks in women should be noted.
Cu deficiency is not common, but the animals with Cu deficiency exhibit hypertriglyceridemia and hypercholesterolemia, abnormal anatomy, and electrical and mechanical conditions of the heart [40]. Cu deficiency was reported to increase blood cholesterol levels in adults [41]. Currently, there are various studies on the relationship between Cu intake and MS diagnostic indicators. A study on the relationship between blood glucose levels and Cu reported that glycation was promoted in rats with Cu deficiency, whereas Cu supplementation decreased blood glucose levels in diabetic mice [42, 43]. Cu supplementation was reported to have a correlation with serum total cholesterol and LDL-cholesterol in the studies on humans and rats [44, 45]. Rats with Cu deficiency also showed a significant increase in HDL-cholesterol. This is considered to be caused by decreased HDL-binding [46]. A negative correlation between Cu deficiency and hypertension was also reported; this is decreased collagen maturity because of less activity in Cu-dependent lysyl oxidase [47]. In addition, excessive Cu intake increased the systolic blood pressure of the rat, and it is explained that this is because excess Cu increases the mature collagen amount and the degradation of vessel [48].
As in previous studies, in this study, as well, Cu intake showed a significant negative correlation with the risk of high blood pressure and MS risks in women. In previous studies, the relationship between Cu intake and MS is interpreted as due to Cu combining with SOD and inhibiting the oxidation of cells, but it is difficult to discuss such a relationship in this case, because the current study did not include oxidative stress-related biomarkers. The previous studies have reported that Cu intake is related to MS in states of insufficient or low Cu [40–43]. Bo et al. [19] reported that marginal Cu deficiency was related to abnormal metabolic changes, with the results showing a significant correlation between Cu intake and DBP, blood glucose, total cholesterol, and LDL-cholesterol. In this study, average Cu intake was enough to exceed the recommended intake but showed significant relevance to MS risks, especially for women. This result is considered to be shown because the ratio of MS subjects whose Cu intake falls short of the EAR is 4.8 % for men, while that for women was higher, 18.8 %. Also, in this study, odd ratios for MS with increasing Cu intake showed a tendency of decreasing to less than 1 even in the case of men. These findings of this study show that it is necessary to take the appropriate amount of Cu to prevent MS in a daily intake status not in the level of Cu deficiency.
Several studies show a significant correlation between MS and the intake of antioxidative minerals other than Cu, such as zinc and selenium. Li et al. [49] studied 221 MS patients aged 18–65 years and compared their Cu intake with that of 329 normal subjects. The study results showed that the Cu intake was lower in the MS patients and Mn intake decreased as the MS diagnostic indicators increased. In this study, no significant relationship was found between Mn intake and MS diagnostic indicators except high blood pressure, which was different from Cu intake. This result was also different from those of previous studies. This study did not observe antioxidation conditions or minerals in the blood other than Mn intake; thus, accurate interpretation is difficult, but the Mn intake level in our subjects is considered to not affect the metabolic process that is related to MS.
Menopause induces both the increase of insulin resistance and atherogenic changes, which can lead to increasing cardiovascular disease risk and prevalence of MS. In this study, the relationship of mineral intake and MS of women before and after menopause was analyzed and as conclusion, in the case of women after menopause, as the Cu intake increases the odd ratio of MS decreases. Considering that the studies about the relationship of mineral intake and MS was very limited until now, more systematical study of various biomarkers related with mineral and its relation with MS based on this study is necessary.
There are several limitations in this study. This study was a cross-sectional study that observes simple correlations between Mg, Mn, and Cu intake, and MS; thus, it is difficult to discuss their causal relationship. Also, this study did not measure the conditions of oxidative biomarkers or blood mineral levels that could explain the relationship with MS, other than mineral intake. Further studies that could explain the relationship between MS and mineral conditions in the human body by analyzing detailed biochemical indicators are needed. In conclusion, significant and negative correlations between the Mg and Cu intakes, and MS risks were found in women. After adjusting for energy intake, there was no significant relationship between these mineral intakes and MS risks. However, in the postmenopausal women, MS was significant and inversely associated with the highest quartiles of Cu intake and the association remained significant after adjustments.
References
Grundy SM (2006) Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol 47:1093–1100
Alberti KG, Zimmet PZ (1998) Definition diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus, provisional report of a WHO consultation. Diabet Med 15:539–553
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285:2486–2497
Ford ES, Giles WJ, Dietz WH (2002) Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA 287:356–359
Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT (2002) The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288:2709–2716
Park HS, Oh SW, Cho SI, Choi WH, Kim YS (2004) The metabolic syndrome and associated lifestyle factors among South Korean adults. Int J Epidemiol 33:328–336
Lim S, Shin H, Song JH, Kwak SH, Kang SM, Yoon JW, Choi SH, Cho SI, Park KS, Lee HK, Jang HC, Koh KK (2011) Increasing prevalence of metabolic syndrome in Korea. Diabetes Care 34:1323–1328
Day C (2007) Metabolic syndrome, or what you will: definitions and epidemiology. Diab Vasc Dis Res 4:32–38
Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC (2006) Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr 84:1489–1497
Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC (2007) Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome. Am J Clin Nutr 85:910–918
Lutsey PL, Steffen LM, Stevens J (2008) Dietary intake and the development of the metabolic syndrome: the atherosclerosis risk in communities study. Circulation 117:754–761
Wester PO (1987) Magnesium. Am J Clin Nutr 45:1305–1312
Barbagallo M, Dominguez LJ, Galioto A, Ferlisi A, Cani C, Malfa A, Pineo A, Busardo A, Paolisso G (2003) Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Aspects Med 24:39–52
Kawano Y, Matsuoka H, Takishita S, Omae T (1998) Effects of magnesium supplementation in hypertensive patients: assessment by office, home, and ambulatory blood pressures. Hypertension 32:260–265
Singh RB, Rastogi SS, Mani UV, Seth J, Devi L (1991) Does dietary magnesium modulate blood lipids? Biol Trace Elem Res 30:59–64
Roberts CK, Sindhu KK (2009) Oxidative stress and metabolic syndrome. Life Sci 84:705–712
Zhang Y, Zhang HM, Shi Y, Lustgarten M, Li Y, Qi W, Zhang BX, Van Remmen H (2010) Loss of manganese superoxide dismutase leads to abnormal growth and signal transduction in mouse embryonic fibroblasts. Free Radic Biol Med 49:1255–1262
Uauy R, Olivares M, Gonzalez M (1998) Essentiality of copper in humans. Am J Clin Nutr 67S:952S–959S
Bo S, Durazzo M, Gambino R, Berutti C, Milanesio N, Caropreso A, Gentile L, Cassader M, Cavallo-Perin P, Pagano G (2008) Associations of dietary and serum copper with inflammation, oxidative stress, and metabolic variables in adults. J Nutr 138:305–310
The Ministry of Health and Welfare, Korean Center for Disease Control and Prevention (2009) National health statistics. Korea Center for Disease Control and. Prevention, Seoul
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752
Lee SY, Kim SM, Kwon HS, Kim DY, Kim DJ, Cho GJ, Han JH, Kim SR, Park CY, Oh SJ (2006) Cut-off points of waist circumference for defining abdominal obesity in the Korean population. Kor Soc Stud Obes 15:1–9
Bae YJ, Kim MH, Choi MK (2010) Analysis of magnesium contents in commonly consumed foods and evaluation of its daily intake in Korean independent-living subjects. Biol Trace Elem Res 135:182–199
Choi MK, Kim EY (2007) Analysis of manganese content in frequently consumed foods by Koreans. Korean J Nutr 40:769–778
Institute National Rural Resources Development, Administration RD (2006) Food composition tables, 7th edn. Hyoil, Seoul
The Korean Nutrition Society (2010) Dietary Reference Intakes for Koreans. The Korean Nutrition Society, Seoul
Yang YJ, Kim YJ, Yang YK, Kim JY, Kwon O (2012) Dietary flavan-3-ols intake and metabolic syndrome risk in Korean adults. Nutr Res Pract 6:68–77
Buscemi S, Verga S, Donatelli M, D'Orio L, Mattina A, Tranchina MR, Pizzo G, Mulè G, Cerasola G (2009) A low reported energy intake is associated with metabolic syndrome. J Endocrinol Invest 32:538–541
Bandini LG, Schoeller DA, Cyr HN, Dietz WH (1990) Validity of reported energy intake in obese and nonobese adolescents. Am J Clin Nutr 52:421–425
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320:1240–1243
Livingstone MB, Black AE (2003) Markers of the validity of reported energy intake. J Nutr 133(Suppl 3):S895–S920
Ford ES, Li C, McGuire LC, Mokdad AH, Liu S (2006) Intake of dietary magnesium and the prevalence of the metabolic syndrome among U.S. adults. Obesity 15:1139–1146
He K, Liu K, Daviglus ML, Morris SJ, Loria CM, Horn LV, Jacobs DR, Savage PJ (2006) Magnesium intake and incidence of metabolic syndrome among young adults. Circulation 113:1675–1682
McKeown NM, Jacques PF, Zhang XL, Sahyoun NR (2008) Dietary magnesium intake is related to metabolic syndrome in older Americans. Eur J Nutr 47:210–216
Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A (2000) Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta 294:1–26
Rodriguez-Moran M, Guerrero-Rometo F (2003) Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: a randomized double-blind controlled trial. Diabetes Care 26:1147–1152
Paolisso G, Sgmbato S, Gambardella A, Pizza G, Tesauro P, Varrichio M, D'Onofrio F (1992) Daily magnesium supplements improve glucose handling in elderly subjects. Am J Clin Nutr 55:1161–1167
Song Y, Ridker PM, Manson JE, Cook NR, Buring JE, Liu S (2005) Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care 28:1438–1444
Mirmiran P, Shab-Bidar S, Hosseini-Esfahani F, Asghari G, Hosseinpour-Niazi S, Azizi F (2012) Magnesium intake and prevalence of metabolic syndrome in adults: Tehran Lipid and Glucose Study. Public Health Nutr 15:693–701
Madsen E, Gitlin JD (2007) Copper deficiency. Curr Opin Gastroenterol 23:187–192
Salonen JT, Salonen R, Korpela H, Suntionen S, Tuomilehto J (1991) Serum copper and the risk of acute myocardial infarction: a prospective population study in men in Estern Finland. Am J Epidemiol 134:268–276
Saari JT, Dahlen GM (1999) Early and advanced glycation end-products are increased in dietary copper deficiency. J Nutr Biochem 10:210–214
Sitasawad S, Deshpande M, Katdare M, Tirth S, Parab P (2001) Beneficial effect of supplementation with copper sulfate on STZ-diabetic mice (IDDM). Diabetes Res Clin Pract 52:77–84
Galhardi CM, Diniz YS, Rodrigues HG, Faine LA, Burneiko RC, Ribas BO, Novelli EL (2005) Beneficial effects of dietary copper supplementation on serum lipids and antioxidant defenses in rats. Ann Nutr Metab 49:283–288
Alarcón-Corredor OM, Guerrero Y, Ramírez de Fernández M, D'Jesús I, Burguera M, Burguera JL, Di Bernardo ML, García MY, Alarcón AO (2004) Effect of copper supplementation on lipid profile of Venezuelan hyperlipemic patients. Arch Latinoam Nutr 54:413–418
Lefevre M, Keen CL, Lönnerdal B, Hurley LS, Schneeman BO (1986) Copper deficiency-induced hypercholesterolemia: effects on HDLsubfractions and hepatic lipoprotein receptor activity in the rat. J Nutr 116:1735–1746
Opsahl W, Zeronian H, Ellison M, Lewis D, Rucker RB, Riggins RS (1982) Role of copper in collagen cross-linking and its influence on selected mechanical properties of chick bone and tendon. J Nutr 112:708–716
Liu CCF, Medeiros DM (1986) Excess diet copper increases systolic blood pressure in rats. Biol Trace Elem Res 9:15–24
Li Y, Guo H, Wu M, Liu M (2013) Serum and dietary antioxidant status is associated with lower prevalence of the metabolic syndrome in a study in Shanghai, China. Asia Pac J Clin Nutr 22:60–68
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, MK., Bae, YJ. Relationship between Dietary Magnesium, Manganese, and Copper and Metabolic Syndrome Risk in Korean Adults: The Korea National Health and Nutrition Examination Survey (2007–2008). Biol Trace Elem Res 156, 56–66 (2013). https://doi.org/10.1007/s12011-013-9852-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9852-z