Abstract
The present study was designed to determine the effects of both Wi-Fi (2.45 GHz)- and mobile phone (900 and 1800 MHz)-induced electromagnetic radiation (EMR) on oxidative stress and trace element levels in the kidney and testis of growing rats from pregnancy to 6 weeks of age. Thirty-two rats and their 96 newborn offspring were equally divided into four different groups, namely, control, 2.45 GHz, 900 MHz, and 1800 MHz groups. The 2.45 GHz, 900 MHz, and 1,800 MHz groups were exposed to EMR for 60 min/day during pregnancy and growth. During the fourth, fifth, and sixth weeks of the experiment, kidney and testis samples were taken from decapitated rats. Results from the fourth week showed that the level of lipid peroxidation in the kidney and testis and the copper, zinc, reduced glutathione (GSH), glutathione peroxidase (GSH-Px), and total antioxidant status (TAS) values in the kidney decreased in the EMR groups, while iron concentrations in the kidney as well as vitamin A and vitamin E concentrations in the testis increased in the EMR groups. Results for fifth-week samples showed that iron, vitamin A, and β-carotene concentrations in the kidney increased in the EMR groups, while the GSH and TAS levels decreased. The sixth week results showed that iron concentrations in the kidney and the extent of lipid peroxidation in the kidney and testis increased in the EMR groups, while copper, TAS, and GSH concentrations decreased. There were no statistically significant differences in kidney chromium, magnesium, and manganese concentrations among the four groups. In conclusion, Wi-Fi- and mobile phone-induced EMR caused oxidative damage by increasing the extent of lipid peroxidation and the iron level, while decreasing total antioxidant status, copper, and GSH values. Wi-Fi- and mobile phone-induced EMR may cause precocious puberty and oxidative kidney and testis injury in growing rats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of Wi-Fi and radiation-emitting wireless devices such as mobile phones in schools and places of employment has increased dramatically in the recent century [1, 2]. The widespread use of Wi-Fi and mobile phones has drastically increased radiofrequency electromagnetic fields. Digital cellular phones using the Global System for Mobile Communications (GSM) transmit information in bursts of microwaves of 900 and 1800 MHz, while Wi-Fi functions at 2.45 GHz [3]. Different types of Wi-Fi and mobile phone stations and their sources must be placed indoors or near living/working and residential areas. The biological effects of electromagnetic radiation (EMR) and their consequences have become the subject of great public debate. However, the effects of EMRs remain unclear, and previous studies have reported conflicting results.
Electromagnetic fields can alter the energy levels and spin orientation of electrons and increase the production of reactive oxygen species (ROS). Thus, exposure to EMR is associated with enhanced ROS production [3–5]. These species and/or other free radicals may affect reproductive systems, but the cellular and molecular mechanisms involved in this process are unclear [6, 7]. The human body is equipped with a complete arsenal of defenses against ROS. ROS concentrations are maintained under strict control through the activity of a redox defense system that includes enzymatic and non-enzymatic antioxidants [8]. Vitamin E is the primary ROS scavenger in the lipid phase of cell membranes [9]. Retinol is the major circulating form of vitamin A; it also has an antioxidant role and plays an essential role in spermatogenesis and combating the oxidative toxicity associated with EMR [10, 11]. Elements that act as cofactors of several enzyme antioxidant systems have been shown to have protective effects against EMR-induced oxidative stress injury [12]. For example, zinc (Zn) plays a critical role in biological membrane stabilization, protein synthesis, and nucleic acid metabolism as well as in the growth of normal tissue [13, 14]. Zn and copper (Cu) protect against oxidation by acting as cofactors for antioxidant enzymes such as superoxide dismutase and catalase [15]. Selenium (Se) is a cofactor for glutathione peroxidase (GSH-Px), an important antioxidant enzyme that removes lipid hydroperoxides and hydrogen peroxide [16–18]. Magnesium (Mg) stabilizes DNA, RNA, and ribosomes and activates approximately 300 enzymes, including those involved in energy metabolism and ROS production [19]. The calcium (Ca) ion has a basic function in neurotransmitter secretion, oxidative stress, and apoptosis [20, 21]. Magnesium (Mg) blocks the entrance of Ca ions into cells and reduces oxidative stress levels. Cu, Se, and Zn act as cofactors of antioxidant enzymes and are essential for inhibiting the free radical production associated with EMR exposure [12, 22].
Devices that incorporate wireless technology such as laptop computers and cell phones are often used near reproductive organs and kidneys and may have harmful effects on the kidney and testis. Testes are extremely susceptible to oxidative damage induced by ROS because they contain large amounts of peroxidation-susceptible [23, 24] polyunsaturated fatty acids (PUFAs), but contain low amounts of antioxidants [23]. The kidneys generate very high levels of ROS through their very highly aerobic metabolism and blood perfusion, but also have relatively poor enzymatic antioxidant defense systems [25]. Some recent studies reported that exposure to Wi-Fi [10, 26, 27] and mobile phone [23, 28] EMR-induced oxidative stress and decreased the levels of antioxidants in the kidney and testis of experimental animals. However, whether EMR affects element levels, lipid peroxidation, or antioxidant levels in the kidney and testis during development of offspring is currently unknown and therefore warrants further investigation.
The present study was designed to determine the possible effects whole-body 2.45-GHz and 900- and 1800-MHz EMR exposure on oxidative stress and element levels in the rat kidney and testis from pregnancy up to 6 weeks of age. Young rats were used in the current study because their growing organs may be more prone to the effects of EMR, similar to children and adolescents who spend a large amount of time using mobile phone and Wi-Fi devices at school and home.
Materials and Methods
Animals
All experimental procedures in this double-blind study were approved by the Medical Faculty Experimentation Ethics Committee of Suleyman Demirel University (protocol number 2013-03/02). Wistar albino female rats (n = 32) and their 96 male offspring were used in the current experiment. Female rats were 12 weeks old and weighed 180 ± 21 g at the beginning of the experiment. Animals were maintained and used in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals prepared by the Suleyman Demirel University. The rats were housed individually in stainless steel cages in a pathogen-free environment in our laboratory at +22 °C ± 2 with light from 0800–2000 and free access to water and were fed a commercial diet.
Study Groups
The rats were exposed to EMR radiation during pregnancy. The 96 newborn male offspring of female rats were selected and randomly divided into four equal groups as follows. Female newborn rats were excluded from the study.
-
Group A (n = 24), control rats—the rats exposed to cage stress 60 min/day during gestation up to 6 weeks of age (5 days per week)
-
Group B (n = 24), rats exposed to 2.45 GHz during 60 min/day during gestation up to 6 weeks of age (5 days per week) [12]
-
Group C (n = 24), rats exposed to 900 MHz during 60 min/day during gestation up to 6 weeks of age (5 days per week) [25, 29]
-
Group D (n = 24), rats exposed to 1800 MHz during 60 min/day during gestation up to 6 weeks old (5 days per week) [29]
One-hour exposure to radiation in groups B, C, and D was executed between 0900 and 1200 each day. Control rats were exposed to cage stress without exposure to the radiofrequencies. Pregnancy of the rats was detected by the presence of sperm in the vaginal smear. Malformation or prenatal death of the offspring was not observed during the experiment. After pregnancy, female and male rats were exposed to EMR radiation up to 3 weeks of age. After determining the genders of the newborns, male newborns were exposed to EMR radiation until 6 weeks of age.
Exposure System and Design
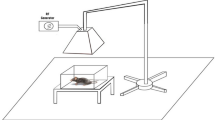
Details of the exposure system have been described in detail elsewhere [6, 30]. A generator from Biçer Electronic, Co. (Sakarya, Turkey) with a half-wave dipole antenna system was used to irradiate the cells with 900, 1,800, and 2.45 GHz radio frequencies with 217-Hz pulses. The electric field density was set at 20 dB and 11 V/m to achieve a 0.1-W/kg whole-body average specific absorption rate (SAR). The distance of the antenna from the head of rats for Wi-Fi (2.45 GHz) and mobile phone (900 and 1800 MHz) exposure was 25 and 1 cm, respectively (Fig. 1). The exposure system was kept in a specific room that contained plastic furniture such as tables and chairs for protecting the cells from possible radiation reflection. Walls of the room were completely covered by chromium–nickel sheets to protect the cells from possible external telemetric exposure. The required electrical field density (0.1-W/kg whole-body average SAR) for 900- and 1800-MHz exposures was continuously recorded every 5 min using a satellite level meter (EXTECH-480836, Extech Instruments Corporation, Nashua, NH, USA), as described in a previous study [31].
Radiation reflection and exposure were measured using a portable radio frequency survey system (Extech-480836) with a standard probe. The electromagnetic radiation dose was calculated from the measured electric field density (volts per meter). Dielectric permittivity and conductivity values of rat tissues at certain frequencies were obtained from a report by Peyman et al. [32]. SAR values at the input 12 μW/cm2 power flux density were calculated using a software program [29]. Whole-body SAR values were in the 0.01–1.2-W/kg range, with SAR mean values of 0.18 ± 0.07 W/kg for whole-body 900 and 1800 MHz, and 2.45-GHz EMR exposures, with a value of 10 V/m at the closest point in the body.
Control group rats were placed in the cylindrical restrainer with the radio frequency source switched off for time periods similar to those used for irradiation. Control animals were kept in their cages without treatment or restraints of any kind.
Preparation of Kidney and Testes Samples
Inhalation anesthesia was provided before sacrifice by decapitation followed by removal of the kidney and testis. The dissected testes were weighed and washed twice with cold saline solution, placed into glass bottles, labeled, and stored in a deep freezer (−33 °C) until processing (maximum 3 weeks). Next, kidney and testis samples were cut into small pieces using scissors and homogenized (2 min at 5,000 rpm) in five volumes (1:5, w/v) of ice-cold Tris–HCl buffer (50 mM, pH 7.4) using an ultrasonic homogenizer (Bandelin-2070, BANDELIN Electronic, GmbH & Co. KG, Berlin, Germany). All preparation procedures were performed on ice. Lipid peroxidation and enzyme activities were then immediately assessed in the kidney and testis homogenate samples. total antioxidant status (TAS) and element levels in the kidney samples were analyzed within 4 weeks. TAS and element levels were only measured in the kidney samples due to limited tissue samples.
Assessments of Lipid Peroxidation and Protein Levels
Lipid peroxidation levels in kidney and testis homogenate were measured by assaying for thiobarbituric acid-reactive substances, as described previously [33]. The pink-colored chromogen formed during the reaction of thiobarbituric acid with lipid peroxidation breakdown products was measured spectrophotometrically (Shimadzu UV-1800; Shimadzu Corp., Kyoto, Japan) at a wavelength of 535 nm. The levels of lipid peroxidation in the kidney and testis homogenate were expressed as micromoles per gram protein.
Reduced Glutathione, Glutathione Peroxidase, and Protein Assays
GSH content in the kidney and testis was measured at a wavelength of 412 nm, according to the method of Sedlak and Lindsay [34]. GSH-Px activity in the kidney and testis was measured spectrophotometrically (Shimadzu UV-1800; Shimadzu Corp., Kyoto, Japan) at 37 °C, at a wavelength of 412 nm, according to the method of Lawrence and Burk [35]. Protein content of the kidney and testis samples was measured according to the method of Lowry et al. [36], using bovine serum albumin as the protein standard.
Total Antioxidant Status Level Determinations
The kidney TAS levels were measured calorimetrically using the TAS kit (Mega Tıp Inc, Gaziantep, Turkey) [37]. The results in the serum and erythrocytes were expressed in micromoles of H2O2 equivalent per gram tissue.
Plasma Vitamin A, Vitamin E, and β-Carotene Analyses
Levels of vitamins A (retinol) and E (α-tocopherol) in the kidney and testis samples were measured using the method described by Desai [38] and Suzuki and Katoh [39] with some modifications. Approximately 0.25 g of each tissue sample was saponified by the addition of 0.3 mL of 60 % (w/v in water) KOH and 2 mL of 1 % (w/v in ethanol) ascorbic acid, followed by heating at 70 °C for 30 min. After cooling the samples on ice, 2 mL of water and 1 mL of n-hexane were added to the samples. The samples were mixed and allowed to stand for 10 min to facilitate phase separation. A 0.5-mL n-hexane extract aliquot was taken, and the vitamin A concentration was measured at 325 nm. Next, reactants were added, and the hexane absorbance value was measured at 535 nm in a spectrophotometer. Calibrations were performed using standard solutions of all-trans retinol and α-tocopherol in hexane.
The β-carotene concentration in kidney and testis was determined according to the method of Suzuki and Katoh [39]. For this, 2 mL of hexane was mixed with 0.25 g of kidney and testis. The β-carotene concentration in the hexane mixture was measured at 453 nm in a spectrophotometer.
Measurement of Element Levels in Kidney
The kidney tissue chromium (Cr), Cu, iron (Fe), Mg, manganese (Mn), Se, and Zn levels were determined using an inductively coupled plasma atomic emission spectroscopy (ICP-AES) system after digestion with nitric acid (65 % and 2.5 mL) and perchloric acid (65 % and 0.5 mL) and ashing (150–180 °C) as described in a previous study [40]. Approximately 0.5 g of tissue was obtained from each animal for this measurement. The tissue concentrations of the elements are expressed as micrograms per gram wet tissue weight.
Apparatus
The ICP-AES system used was an ICAP 6000 ICP-OES emission spectrometer equipped with the plus autosampler and was controlled by a computer (Thermo Fisher Scientific Inc., Istanbul, Turkey). The plasma operating conditions in this study were containing ICP system 15 L/min plasma gas flow rate, 0.5 argon carrier flow rate, 1.51/min sample flow rate, and elution flow rate. The speed of the peristaltic pump was 100 rpm. Transport lines were made using a 1.25-mm-i.d. polytetrafluoroethylene tubing. The analytic lines of ICP-AES measurements in Cr, Cu, Fe, Mg, Mn, Se, and Zn analyses were 267.72, 324.75, 259.94, 285.21, 257.61, 196.09, and 206.20 nm, respectively.
Reagents
All reagents were of analytical reagent grade, and deionized water was used. Stock solutions of Cr, Cu, Fe, Mg, Mn, Se, and Zn were prepared by taking appropriate amounts of standards in nitric acid solution. Working solutions were prepared immediately before use. Adjustment of pH was made with buffer (acetic acid, boric acids, and their potassium salts). Doubly distilled deionized water was used in the current study. All glassware used was washed with 10 % nitric acid for 1 day and rinsed with deionized water before use.
Statistical Analyses
All results are expressed as means ± standard deviation (SD). p values of less than 0.05 were regarded as significant. Significant values were assessed with Mann–Whitney U test. Data was analyzed using the SPSS statistical program (version 17.0 software, SPSS Inc. Chicago, IL, USA).
Results
Fourth-Week Results
Mean MDA levels (an index of in vivo lipid peroxidation), GSH, GSH-Px, and antioxidant vitamin results from the fourth week in the four groups are shown in Table 1, whereas kidney TAS and element levels from the fourth week are shown in Table 2. Mean levels of lipid peroxidation (p < 0.05 and p < 0.001) in kidney and testis and mean levels of Cu (p < 0.05), Zn (p < 0.05), GSH (p < 0.05), TAS (p < 0.05 and p < 0.001), and GSH-Px (p < 0.05) in the kidney were significantly lower in the 2.45 GHz and 900 and 1800 MHz groups than in the control group. In addition, iron concentrations in the kidney (p < 0.001) and vitamin A (p < 0.05) and vitamin E (p < 0.05 and p < 0.001) concentrations in the testis were significantly higher in the 2.45 GHz and 900 and 1800 MHz groups than in the control group. This suggests that EMR-induced lipid peroxidation levels are decreased in the kidney and testes due to increased antioxidant activities. However, Cr, Mg, Mn, Se, and β-carotene concentrations in the four groups were not statistically significant.
Fifth-Week Results
Mean kidney and testis levels of lipid peroxidation, GSH, GSH-Px, and antioxidant vitamins in the fifth week for the four groups are shown in Table 3. Kidney TAS and element levels in the fifth week are shown in Table 4. The Fe (p < 0.05), vitamin A (p < 0.05 and p < 0.01), and β-carotene (p < 0.05 and p < 0.01) concentrations in the kidney were significantly higher in the EMR groups than in the control group, although kidney GSH and TAS (p < 0.05 and p < 0.01) levels were significantly (p < 0.05) lower in the EMR groups than in the control group. An elevation in the level of lipid peroxidation indicates that lipid peroxidation caused cell membrane and intracellular component injury. However, lipid peroxidation, GSH-Px, vitamin E, and Cu, Cr, Mg, Mn, Se, and Zn values in kidney and testis of the four groups were not statistically significant.
Sixth-Week Results
Mean lipid peroxidation, GSH, GSH-Px, and antioxidant vitamin in the sixth week in the kidney and testis for the four groups are shown in Table 5. Kidney TAS and element levels in the sixth week are shown in Table 6. Testis and kidney lipid peroxidation (p < 0.05) and kidney Fe (p < 0.05 and p < 0.01) levels were higher in the 2.45 GHz and 900 and 1800 MHz groups than in the control group, although Cu (p < 0.05), TAS (p < 0.001), and GSH (p < 0.05) concentrations were significantly decreased in the EMR groups. The other parameters assessed in the four groups were not statistically significant.
Discussion
Wireless devices are widely used in today's world. Modern wireless devices such as Wi-Fi-enabled devices or wireless internet access devices use higher frequency ranges (2,400–2,500 Hz) than cell phones and typically have a longer exposure time and wider area of exposure [1, 2]. Devices using this type of wireless technology such as laptop computers are primarily used near reproductive organs and may have harmful effects on the kidney and testis.
Oxidative stress plays a role in EMR exposure effects on body tissues [8, 20, 21]. Kidney and testicular tissues are very sensitive to ROS effects because the testis is rich in PUFAs but low in antioxidants. The kidney exhibits very high metabolic activity and blood flow (oxygen). Additionally, testis and sperm membranes consist of dense unsaturated fatty acids that make sperm sensitive to oxygen-induced damage mediated by lipid peroxidation and free water-induced oxygen [23–25]. Elevated levels of ROS may damage DNA, lipids, protein, and enzymes in these tissues. Some studies showed that Wi-Fi- and mobile phone-induced EMR can generate oxidative stress in the testis and kidneys [10, 25, 27], while others suggested that EMR exposure does not affect oxidative stress markers such as MDA [26]. Certain protective enzymatic and non-enzymatic antioxidants play important roles in antioxidant defense, but increased oxidative stress in cells stemming from EMR may lead to cell injury when defense systems are overwhelmed [10]. The EMR absorption rate in tissues is directly related to dielectric properties and conductivity of the organs. Because of increased water uptake during pregnancy, whole-body electrical conductivity increases during pregnancy, making pregnant women and their fetuses more sensitive to EMR [41].
The current study showed that lipid peroxidation values in the fourth week were decreased in the 2.45 GHz as well as the 900 and 1,800 GHz EMR groups compared to levels in the control group, although antioxidant values increased. Atasoy et al. [26] reported that levels of the lipid peroxidation-associated molecule MDA were decreased in the sperm cells of rats following Wi-Fi exposure. Kismali et al. [41] reported that whole-body 1800-MHz EMR exposure for 15 min/day for 1 week did not change MDA levels in the blood of pregnant rabbits. The report supported the lipid peroxidation results obtained during the fourth week. In the fifth and sixth weeks, lipid peroxidation levels were higher in the EMR groups than in the control groups, while GSH values were decreased. The current study also showed that 4-week-old rats were more sensitive than mature (5- or 6-week-old) rats to the main oxidative stress parameters, as demonstrated by decreased lipid peroxidation levels in the kidney and testis and decreased TAS, GSH, and GSH-Px levels in the kidney. Furthermore, the normalization observed in rats after the fifth week was higher than that in the fourth week after the recovery period. Thus, 5-week-old rats more successfully dealt with oxidative stress than 4-week-old rats. Similarly, Oksay et al. [10] reported increased MDA levels in the testis of adult rats after 30-day 2.45-GHz exposure. Oktem et al. [25] and Devrim et al. [42] reported that MDA levels increased in adult rats after 900-MHz exposure, although GSH-Px activities were decreased by EMR exposure. Our results confirm those of Oksay et al. [10], Oktem et al. [25], and Devrim et al. [42].
GSH is a major thiol group-containing antioxidant and plays an important role in protecting cells against ROS-induced damage [17, 43]. It also acts as substrate in many essential enzymatic reactions involving GSH-Px and glutathione reductase. Tissue GSH levels reflect the capability of tissues to scavenge free oxygen radicals, preserve the cellular reduction–oxidation balance, and defend cells against oxidative damage. Depletion of GSH in EMR-exposed animals may be responsible for lipid peroxidation in the testis and kidney. In the current study, GSH-Px activity decreased in the testis and kidney during the fourth week, and GSH levels decreased in the fourth, fifth, and sixth weeks in the kidney, which may have been a consequence of depleted GSH stores. Similarly, increases in lipid peroxidation and a concomitant depletion in GSH level were observed in the kidney after EMR exposure, suggesting that increased lipid peroxidation levels may have been a consequence of depleted GSH stores [43].
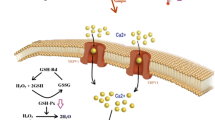
Free oxygen radicals are very reactive paramagnetic chemical species because they have several unpaired electrons and thus unpaired spins. Because the electron spin of molecules has an associated magnetic moment, the interconversion and chemical fates of singlet and triplet states are influenced by EMR [44]. Several reports indicated that EMR enhances ROS levels in cells via Fenton reactions of metals such as Zn, Cu, and Fe [29, 45, 46]. The Fenton reaction is a catalytic process that converts hydrogen peroxides into the highly toxic hydroxyl radical [8, 47]. We observed that Fe levels and the extent of lipid peroxidation were increased in the kidney following EMR exposure, due to Fe oxidation in the tissue via Fenton reaction.
Kidney Cu, Zn, and lipid peroxidation levels after the fourth week were lower in the three EMR groups than in the control group, although the values returned to control levels or were increased by EMR exposure after the fifth and sixth weeks. Cu is an essential element in biological systems. Zn is a micronutrient abundantly present in meat and seafood and serves as a cofactor for more than 80 metalloenzymes involved in DNA transcription and protein synthesis [14]. Because DNA transcription is a major factor in germ cell development, Zn is likely important for reproduction [44]. Furthermore, Zn finger proteins are implicated in the genetic expression of steroid hormone receptors [14], and zinc induces antioxidant properties and interacts with Fe molecules to inhibit oxidative stress [47]. Zn and Cu protect against oxidative stress by acting as cofactors for antioxidant enzymes such as superoxide dismutase [14, 15]. The biological functions of Cu are intimately related to its redox properties as a transition metal. Redox cycling between Cu2+ and Cu1+ can catalyze the production of highly toxic hydroxyl radicals [16].
The vitamin A active metabolite retinoic acid plays an important role in spermatogenesis and promotes the entrance of spermatogonia into the meiotic pathway. Retinoic acid appears to be responsible for differentiation of undifferentiated spermatogonia by upregulating Kit expression in germ cells [11]. Vitamin E has also been shown to suppress lipid peroxidation in testicular microsomes and mitochondria, and deficiencies in this vitamin lead to a state of oxidative stress and deterioration in both spermatogenesis and testosterone production [2]. We observed that liver vitamin A and β-carotene concentrations were decreased by the 2.45-GHz, 900- and 1800-GHz EMR exposures (unpublished data). In the current study, we observed increased concentrations of vitamin A, vitamin E, and β-carotene concretions after the fourth and fifth weeks, although lipid peroxidation levels were decreased. The increase in vitamin A, vitamin E, and β-carotene concentrations in the kidney and testis of EMR exposure groups may reflect transport from the liver to these cells, which is very important for spermatogenesis and kidney oxidative functions.
Conclusion
In conclusion, our results demonstrated that Wi-Fi (2.45 GHz) and mobile phone (900 and 1800 MHz) devices contribute to oxidative stress in the kidney and testes as demonstrated by increased lipid peroxidation and oxidizable iron content and decreased antioxidant trace elements (copper and zinc), TAS, and GSH during kidney and testis development. The present study demonstrated that Wi-Fi- and mobile phone-induced EMR may cause precious puberty and kidney oxidative injury in growing rats. This is the first study that investigated the role of Wi-Fi- and mobile phone-derived EMR exposure on growing rat kidney and testis.
References
Behari J, Kesari KK (2006) Effects of microwave radiations on reproductive system of male rats. Embryo Talk 1:81–85
Nazıroğlu M, Yuksel M, Özkaya MO, Köse SA (2013) Effects of Wi-Fi and mobile phone on reproductive systems in female and male. J Membr Biol. doi:10.1007/s00232-013-9597-9
Murphy JC, Kaden DA, Warren J, Sivak A (1993) International Commission for Protection Against Environmental Mutagens and Carcinogens. Power frequency electric and magnetic fields: a review of genetic toxicology. Mutat Res 296:221–240
Nazıroğlu M, Tokat S, Demirci S (2012) Role of melatonin on electromagnetic radiation-induced oxidative stress and Ca2+ signaling molecular pathways in breast cancer. J Recept Signal Transduct Res 32:290–297
Türker Y, Nazıroğlu M, Gümral N et al (2011) Selenium and L-Carnitine reduce oxidative stress in the heart of rat induced by 2.45-GHz radiation from wireless devices. Biol Trace Elem Res 143:1640–1650
Nazıroğlu M, Çelik Ö, Özgül C et al (2012) Melatonin modulates wireless devices (2.45 GHz)-induced brain and dorsal root ganglion injury through TRPM2 and voltage gated calcium channels in rat. Physiol Behav 105:683–692
Kim JY, Kim HT, Moon KH, Shin HJ (2007) Longterm exposure of rats to 2.45 GHz electromagnetic field: effects on reproductive function. Korean J Urol 48:1308–1314
Nazıroğlu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32:1990–2001
Nazıroğlu M, Karaoğlu A, Orhan Aksoy A (2004) Selenium and high dose vitamin E administration protects cisplatin- induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 195:221–230
Oksay T, Nazıroğlu M, Doğan S, Güzel A, Gümral N, Koşar PA (2013) Protective effects of melatonin against oxidative injury in rat testis induced by wireless (2.45 GHz) devices. Andrologia. doi:10.1111/and.12044
Hogarth C, Griswold MD (2010) The key role of vitamin A in spermatogenesis. J Clin Invest 120:956–962
Nazıroğlu M, Gumral N (2009) Modulator effects of selenium and L- carnitine on wireless devices (2.45 GHz) induced oxidative stress and electroencephalography records in brain of rat. Int J Radiat Biol 85:680–689
Ebisch IM, Thomas CM, Peters WH, Braat DD, Steegers-Theunissen RP (2007) The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod 13:163–174
Favier AE (1992) The role of zinc in reproduction. Hormonal mechanism. Biol Trace Elem Res 32:363–382
Fatmi W, Kechrid Z, Nazıroğlu M, Flores-Arce M (2013) Selenium supplementation modulates zinc levels and antioxidant values in blood and tissues of diabetic rats fed zinc-deficient diet. Biol Trace Elem Res 152:243–250
Nazıroğlu M, Özgül C, Küçükayaz M, Çiğ B, Hebeisen S, Bal R (2013) Selenium attenuates calcium ion influx and oxidative stress through voltage gated and TRPM2 cation channels in transfected cells. Basic Clin Pharmacol Toxicol 112(2):96–102
Nazıroğlu M (2009) Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem Res 34:2181–2191
Uğuz AC, Nazıroğlu M, Espino J et al (2009) Selenium modulates oxidative stress-induced cell apoptosis in human myeloid HL-60 cells via regulation of caspase-3, -9 and calcium influx. J Membr Biol 232:15–23
Gulczynska E, Gadzinowski J, Wilczynski J, Zylinska L (2006) Prenatal MgSO4 treatment modifies the erythrocyte band 3 in preterm neonates. Pharmacol Res 53:347–352
Nazıroğlu M (2011) TRPM2 cation channels, oxidative stress and neurological diseases: where are we now? Neurochem Res 36:355–366
Nazıroğlu M (2012) Molecular role of catalase on oxidative stress-induced Ca(2+) signaling and TRP cation channel activation in nervous system. J Recept Signal Transduct Res 32:134–141
Çelik MS, Güven K, Akpolat V, et al (2013) Extremely low frequency magnetic field induces manganese accumulation in brain, kidney and liver of rats. Tox Ind Health (in press)
Wathes DC, Abayasekara DR, Aitken RJ (2007) Polyunsaturated fatty acids in male and female reproduction. Biol Reprod 77:190–201
Nazıroğlu M (2003) Enhanced testicular antioxidant capacity in streptozotocin induced diabetic rats: protective role of vitamins C, E and Selenium. Biol Trace Elem Res 94:61–71
Oktem F, Ozguner F, Mollaoglu H et al (2005) Oxidative damage in the kidney induced by 900-MHz-emitted mobile phone: protection by melatonin. Arch Med Res 36:350–355
Atasoy HI, Gunal MY, Atasoy P, Elgun S, Bugdayci G (2013) Immunohistopathologic demonstration of deleterious effects on growing rat testes of radiofrequency waves emitted from conventional Wi-Fi devices. J Pediatr Urol 9:223–229
Meena R, Kumari K, Kumar J, Rajamani P, Verma HN, Kesari KK (2013) Therapeutic approaches of melatonin in microwave radiations-induced oxidative stress-mediated toxicity on male fertility pattern of Wistar rats. Electromagn Biol Med (in press)
Jelodar G, Nazifi S, Akbari A (2013) The prophylactic effect of vitamin C on induced oxidative stress in rat testis following exposure to 900 MHz radio frequency wave generated by a BTS antenna model. Electromagn Biol Med 32:409–416
Ozlem Nisbet H, Nisbet C, Akar A, Cevik M, Karayigit MO (2012) Effects of exposure to electromagnetic field (1.8/0.9 GHz) on testicular function and structure in growing rats. Res Vet Sci 93(2):1001–1005
Nazıroğlu M, Ciğ B, Doğan S, Uğuz AC, Dilek S, Faouzi D (2012) 2.45-Gz wireless devices induce oxidative stress and proliferation through cytosolic Ca(2+) influx in human leukemia cancer cells. Int J Radiat Biol 88(6):449–456
Jin Z, Zong C, Jiang B, Zhou Z, Tong J, Cao Y (2012) The effect of combined exposure of 900 MHz radiofrequency fields and doxorubicin in HL-60 cells. PLoS One 7(9):e46102
Peyman A, Rezazadeh A, Gabriel C (2001) Changes in the dielectric properties of rat tissue as a function of age at microwave frequencies. Phys Med Biol 46:1617–1629
Placer ZA, Cushman L, Johnson BC (1966) Estimation of products of lipid peroxidation (malonyl dialdehyde) in biological fluids. Anal Biochem 16:359–364
Sedlak J, Lindsay RHC (1968) Estimation of total, protein bound and non-protein sulfhydryl groups in tissue with Ellmann's reagent. Anal Biochem 25:192–205
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Erel O (2004) A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 37:277–285
Desai ID (1984) Vitamin E analysis methods for animal tissues. Methods Enzymol 105:138–147
Suzuki J, Katoh N (1990) A simple and cheap method for measuring vitamin A in cattle using only a spectrophotometer. Jpn J Vet Sci 52:1282–1284
Clegg MS, Keen CL, Lönnerdal B, Hurley LS (1981) Influence of ashing techniques in the analyses of trace elements in animal tissue. Biol Trace Elem Res 3:107–115
Kismali G, Ozgur E, Guler G, Akcay A, Sel T, Seyhan N (2012) The influence of 1800 MHz GSM-like signals on blood chemistry and oxidative stress in non-pregnant and pregnant rabbits. Int J Rad Biol 88:414–419
Devrim E, Ergüder IB, Kılıçoğlu B, Yaykaşlı E, Cetin R, Durak I (2008) Effects of electromagnetic radiation use on oxidant/antioxidant status and DNA turn-over enzyme activities in erythrocytes and heart, kidney, liver, and ovary tissues from rats: possible protective role of vitamin C. Toxicol Mech Methods 18:679–683
Nazıroğlu M, Özgül C, Çiğ B (2013) Neuroprotection induced by N-acetylcysteine against cytosolic glutathione depletion induced-Ca2+ influx through TRPV1 channels in dorsal root ganglion neuron of mice. Neuroscience 242:151–160
Zago MP, Oteiza PI (2001) The antioxidant properties of zinc: interactions with iron and antioxidants. Free Radic Biol Med 31:266–274
Pal A, Singh A, Nag TC, Chattopadhyay P, Mathur R, Jain S (2013) Iron oxide nanoparticles and magnetic field exposure promote functional recovery by attenuating free radical-induced damage in rats with spinal cord transection. Int J Nanomedicine 8:2259–2272
Nazıroğlu M, Yürekli VA (2013) Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: focus on trace elements. Cell Mol Neurosci 33:589–599
Baltaci AK, Mogulkoc R, Salbacak A, Celik I, Sivrikaya A (2012) The role of zinc supplementation in the inhibition of tissue damage caused by exposure to electromagnetic field in rat lung and liver tissues. Bratisl Lek Listy 113:400–403
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özorak, A., Nazıroğlu, M., Çelik, Ö. et al. Wi-Fi (2.45 GHz)- and Mobile Phone (900 and 1800 MHz)-Induced Risks on Oxidative Stress and Elements in Kidney and Testis of Rats During Pregnancy and the Development of Offspring. Biol Trace Elem Res 156, 221–229 (2013). https://doi.org/10.1007/s12011-013-9836-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9836-z