Abstract
Nickel constitutes about 8–60 % of orthodontic alloys. It is known as an allergenic/cytotoxic trace metal. Therefore, it should be investigated in patients undergoing orthodontic treatment which might last for 2 or 3 years. However, no controlled studies have assessed the influence of orthodontic treatments of longer than 5 months on its systemic levels. Thus, the aim of this retrospective cohort study was to evaluate systemic nickel in patients undergoing orthodontic therapy for a minimum period of 1 year. In this study, urinary nickel concentrations in 20 female and 10 male patients being treated with stainless steel appliances were measured using atomic absorption spectrophotometry. The same procedure was done on a control group of the patients’ same-gender near-age siblings (n = 30). The effect of treatment and gender on urinary nickel levels were assessed using a repeated-measures two-way analysis of variance (ANOVA) and a Tukey test (α = 0.05). The mean treatment duration was 17.1 ± 6.4 months (range, 12–21). The mean nickel concentrations in male and female patients were 9.67 ± 3.25 and 9.9 ± 3.83 μg/L, respectively. These statistics for male and female control subjects were 6.65 ± 2.57 and 8.43 ± 2.94 μg/L, respectively. The ANOVA showed a statistically significant difference between the urinary nickel levels of the treatment and the control groups (P = 0.009) but not between the genders (P = 0.194). The interaction between gender and treatment was also nonsignificant (P = 0.337). The Tukey test indicated that the increase in nickel was higher in male patients, in comparison to their brothers (P < 0.05). It could be concluded that orthodontic therapy for longer durations with stainless-steel archwires might elevate slightly, but significantly, urinary nickel levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nickel is a carcinogenic, cytotoxic, mutagenic, and allergenic trace element known to induce cancer, birth defects, and other reproductive harms [1–7]. It is the most common source of contact allergy and is a major cause of asthma [4, 7, 8]. Therefore, it has been investigated at DNA to organism levels [2, 3, 5, 6, 9], and its potential adverse effects have been publicized [2, 6]. It is a key component of nickel–titanium (NiTi) orthodontic appliances and one of the elements available in stainless steel (SS) ones, which respectively contain approximately 60 and 8 % nickel [2, 3, 5–7, 10–13]. These alloys are susceptible to corrosion and can release nickel ions into saliva through various mechanisms [2, 3, 5, 7, 8]. Under in vitro conditions, orthodontic alloys can resist corrosion by the formation of a passivation layer [2, 7]. Nevertheless, in clinical situations, these may undergo corrosion since the protective layer is simply removed by several mechanical/chemical factors [7]. These include mastication [7, 14], brushing [7, 8], biofilm layer [2, 4, 7], saliva flow [5, 7, 8, 15], thermal stresses [4, 7, 15, 16], recycling of the appliances [4, 7, 17], metal deflection caused by occlusal loadings [6, 7, 18], chloride ions and acidic conditions [5, 7, 12, 15, 16, 18] available as byproducts of local plaque microorganism biodeterioration [2, 6, 7, 17–19], fruit juices, acidic carbonated beverages, sodium chloride [2, 7, 8, 18], fluoridated toothpastes/mouthwashes, and tea [2, 7, 17]. The introduction of corroded nickel into the human body is an extra risk to health because depending on its characteristics, solubility, and the competitive effects of other metal ions, it may be released or accumulated at different levels in different tissues [3–5, 20, 21]. Accordingly, biological functions can be affected, leaving systemic and local influences [3–5, 20].
The majority of the previous studies in this matter have explored nickel ion discharge in artificial saliva or other in vitro media [1, 2, 16]. In the other few in vivo investigations, authors have evaluated the amount of nickel ion in saliva [5, 7, 9, 12, 14, 22], oral mucosa cells [3], or dental plaque [14]. The results and conclusions of in vitro analyses are less likely relevant to the clinical situations, because such methodologies are unable to reproduce highly complex and dynamic oral environment [2–7]. Moreover, none of the in vivo studies on salivary nickel concentrations can address the changes in nickel ion levels in the bloodstream, as it is demonstrated that these might be unrelated [12]. Assessment of systemic exposure to a substance can be performed using biomarkers of exposure, of which blood and urine are the most accessible ones, which can also denote acute exposures [23].
Systemic nickel alterations during orthodontic treatment have been assessed in only three investigations [12, 23, 24], two of which have evaluated short-term exposures to orthodontic appliances [23, 24]. One of the short-term studies had assessed blood nickel in 31 subjects in 4 to 5 months, but it was questionable, because all nickel levels were far below the normal range with most of them being undetectable (<0.4 μg/L) [24]. In the other short-term report, authors had investigated nickel levels in urine samples taken from a cohort of 21 patients in a 2-month longitudinal study [23]. It should be taken into account, however, that orthodontic treatment usually lasts for 2 or 3 years, and a short-term assessment—while NiTi archwires might dominate in treatment plan—cannot necessarily reflect the impact of the whole treatment in longer periods, in which SS appliances are regularly used [6, 7, 10]. The only long-term research on serum nickel was a descriptive one on five different groups of 20 patients, being under treatment for different durations up to 2 years [12]. Nonetheless, its reliability [12] was tenuous due to the absence of a cohort design [2, 7] in addition to probable contamination of serum samples by the SS venipuncture needles [12]. The few and controversial available reports on systemic nickel [12, 23, 24] and the absence of any controlled long-term examinations on this subject may identify the merit of undertaking long-term experimental or quasi-experimental setups.
Systemic nickel can be assessed in blood. Conversely, taking blood samples merely for research purposes might be unethical, while there are efficient indirect approaches for this purpose. Markers of exposure to toxic metals usually include the sites of aggregation or elimination [10]. Since conducting measurements at the internal accumulation sites such as the kidney is impossible in human, evaluation of excretion routes as exposure biomarkers seems to be of significant value [10, 25]. Urinary nickel can reflect the level of nickel in the blood [10, 23, 24, 26] and kidney [10, 23, 25]. However, it is not well explored, especially in longer durations of orthodontic therapy [17, 23]. In view of the mentioned drawbacks and debates, we aimed to evaluate comparatively the urinary nickel concentrations in orthodontic patients undergoing treatment for at least 1 year and in their age- and gender-matched siblings.
Subjects and Methods
This retrospective cohort study was performed on 30 orthodontic patients and on the same number of control subjects. As part of the inclusion criteria, all the patients must have had at least a same-gender near-age sibling without any orthodontic treatment history. In case either a patient or the matched control participant met any of the following exclusion criteria, both would be disqualified. These comprised the subjects’ unwillingness to participate, them having teeth extracted or missing (excluding the third molars), the presence of any systemic diseases, any history of allergic reactions, medication intake, alcohol consumption or smoking, and the presence of any metal restorations such as amalgam fillings or fixed prostheses, or any soldered/extraoral orthodontic appliances [7]. Since NiTi archwires can temporarily raise salivary nickel amount [5, 22], patients must have had no NiTi archwires in their set-up for at least 1 month before sampling [5]. In order to lessen the effects of biologic differences as well as dietary and hygiene habits on nickel release, the siblings must be same gender and near age to the patients; they also must have been living together with them and must not have undergone orthodontic treatment. After evaluating 380 orthodontic patients, 30 participants (20 females and 10 males) and their age- and gender-matched siblings were enrolled. The included patients’ mean age was 20.95 ± 5.3 years (females, 20.4 ± 4.7 and males, 21.5 ± 5.1). The siblings’ mean age was 21.8 ± 6.6 years, and the difference between the mean ages of the groups was not significant (P > 0.4) according to a paired-samples t test. The ethics of the study protocol were approved by the internal review board of the institution, and written consents were taken from the subjects or their parents after thorough explanation [7]. The fixed appliances at the time of sample collection consisted only of 0.016- and 0.016 × 0.022-in SS archwires, bonded 0.018-in. slot preadjusted Roth prescription SS brackets on all teeth except the molars (Discovery, Dentaurum, Pforzheim, Germany), and an average of six SS orthodontic bands (Unitek/3 M, Monrovia, California, USA). The sampling was carried out 17.1 ± 6.4 months after the initiation of fixed orthodontic treatment (range = 12–21 months).
Sample Collection
The patients and their siblings were instructed, orally and in written, to avoid consumption of a given list of foods rich in nickel, from 48 h prior to the next visit [7]. They were as well told to avoid brushing or mouth-rinsing with fluoridated products and eating/drinking in the next visit which was scheduled in the morning [7, 12]. To avoid contamination of the specimens at home, the sampling was performed in the office. The subjects were given sterile nickel-free 50-mL plastic containers and were asked to collect the urine after discarding the first flush. They were also instructed to avoid contaminating the vessels by wiping or rinsing the surfaces [23]. The specimens were stored in a low-temperature freezer [7, 14], and in the next day transferred to the Chemical Analysis Department of the Atomic Energy Organization for electrothermal atomic absorption spectrophotometry using a calibrated device (AA280Z GTA120, Varian, Mulgrave, Australia) with 0.01-μg/L accuracy limit [7]. From each container, two 1-mL urine specimens were collected and were tested. In the case of the existence of any inconsistency between the nickel levels of the two specimens from each subject, the values would be disregarded and the procedure would be repeated on another set of two 1-mL bottles [7].
Statistical Analysis
The sample size of this two-level repeated-measures design was calculated based on a pilot study to obtain test powers of >0.8. Nickel values were normally distributed according to a D’Agostino–Pearson omnibus normality test. The data were analyzed using a repeated-measures two-way analysis of variance (ANOVA) and a Tukey post hoc test of the Statistical Package for the Social Sciences (SPSS version 16, SPSS Inc, Chicago, USA). The level of significance was set at 0.05. In addition, 95 % confidence intervals (CI) for the means and differences were computed.
Results
In the majority of the patients, NiTi archwires had been replaced by SS ones after the third month of treatment, and none of the patients had NiTi archwires in their mouth after the fifth month. Urine samples of 58 subjects were consistent and were approved after two examinations, but the tests for two subjects needed to be repeated before approval.
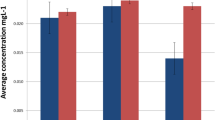
The urinary nickel concentration of each patient was higher than the matched sibling. The difference between the mean nickel levels of the cohort and the control groups was 1.98 μg/L (Fig. 1; Table 1). The repeated-measures two-way ANOVA showed that this difference was statistically significant (F = 6.723; P = 0.009), while the difference between the nickel concentrations observed in males and females was not (F = 1.450; P = 0.194). Additionally, the interaction between the influences of treatment and gender was nonsignificant (F = 0.940; P = 0.337). The Tukey test showed that the difference between the treatment and the control groups was significant in males (P < 0.05) but was only marginally significant in females (P < 0.1; Table 2).
The 95 % CI pointed to generalizable differences between the nickel levels in the patients and the control subjects, between male patients and their brothers, between female patients and their sisters, and between female and male control subjects, but not between female and male patients (Table 2).
Discussion
The most known adverse effect of nickel in orthodontics is contact allergy, especially in patients with hypersensitivity to nickel who constitute 2–5 % of men and 20–30 % of women worldwide [7, 19, 23]. It is well understood that corroded nickel may cause dermatitis and irritation through triggering soft tissue inflammation. Nonetheless, it is not clearly identified that how much released nickel is absorbed by the organism [3, 5, 12, 23, 24]. Assessment of the administration and elimination pathways of trace elements is a principal approach in comprehending reactions to such metals [23]. Nickel ions can be discharged into saliva via several mechanisms such as galvanic corrosion where two or more dissimilar metals are joined in the construction of brackets or archwires [2, 5, 8]. However, because of being small and hydrophilic, rather than being accumulated, it is typically metabolized and then excreted mostly by the kidneys. It also excretes through saliva, sweat, and keratin materials such as hair and nail [10, 17, 23, 26]. Approximately, 90 % of blood nickel is quickly exerted through urine with an elimination half time of 28 h [14, 23, 24, 26]. Therefore, renal elimination rate might reflect well the systemic nickel level as well as its acute changes [10, 23, 24, 26]. In line with our findings, the normal urinary nickel level is reported as about 4.5 μg/L (1.9–9.6 μg/L) in people without occupational exposures to nickel [23, 24, 26].
There were slight but generalizable differences between the urinary nickel levels of the two groups in the present setup. This seemed to be in contrast to some of the other studies with regard to systemic nickel concentrations [12, 24], showing no significant alterations in blood nickel. Nevertheless, similar to our findings, Menezes et al.[23] found that urinary nickel escalated about 2.2 μg/L after 2 months of fixed treatment. A reason for this resemblance might be using SS appliances in both the studies. Although NiTi archwires—which had been used in the initial stages of treatment for the patients in this sample, might accelerate galvanic corrosion caused by different metals present in the mouth, it was unlikely a confounding factor since at the time of sampling, all the patients had SS archwires in their mouth for at least 7 months. Another noticeable (though not a main) issue was the difference between the baseline urinary nickel values in the two studies. The control nickel level was much higher in their study [23] (i.e., 17.7 μg/L) which can be attributed to differences in lifestyles, socioeconomic conditions, and dietary habits [23].
All in vivo studies regarding changes in salivary nickel levels showed that the nickel release from orthodontic appliances is far below the rate of dietary nickel intake (100–800 μg/day) and, hence, probably well tolerated and nontoxic [2, 5, 7, 9–12, 14, 23]. However, it was intriguing that such subtle and insignificant increases (or even sometimes decreases [7]) might lead to generalizable increases in urinary nickel—that is about 1:7 to 1:4 of the baseline urinary nickel concentrations—either in short-term [23] or in longer periods observed in this research. This might imply that perhaps corroded products of nickel might be more absorbable than nickel available in daily food, possibly as a result of being solubilized by the biofilm microorganisms [10]. In addition, the plaque microbes can accelerate the corrosion by causing a local depletion of oxygen, a decline in pH [2, 4, 6], and taking up and metabolizing nickel [6, 10]. This nickel might be bioaccumulated with a significant rate in the biomass, by complexing with glycoproteins or ions already present in the biofilm [7, 10, 14]. Thus, despite the absence of notable increases in salivary nickel, the undetected additional nickel aggregated in the biofilm might still be absorbed into the bloodstream through intake of plaque [10].
Serum and urinary nickel concentrations might depend also on nickel excretory rates [6, 9]. The presence of other ions such as cadmium—released from silver-soldered orthodontic appliances [2, 18]—as well as methods of nickel administration can influence the routes of nickel aggregation and elimination [21]. The latter might justify, in part, the elevated urinary levels observed in this research. Moreover, whereas no significant alterations in blood nickel had been reported [12, 24], significant urinary nickel increases were observed by Menezes et al. [23] and us. Hence, the authors propose that the contrasting reports from the four studies on systemic nickel concentrations might be all together explained in the context of the extremely rapid nickel excretion process [21]. Such a fast elimination rate might effectively stabilize blood nickel at low levels (reported by studies on blood nickel [12, 24]), at the expense of significant increases in renal nickel excretion (seen in this study and the other one [23]). The same point might as well account for the absence of any correlations between salivary and serum nickel noticed by Agaoglu et al. [12], and for the inconspicuous blood nickel values remarked by Bishara et al. [24]. It might also imply the high affinity of nickel to the kidneys [6, 9, 21, 23]. Nevertheless, the debate might also have been stemmed from some errors or confounders in some of the other studies. In the study of Agaoglu et el. [12], the lack of cohort/control groups and some standardized features as well as using SS needles for blood sampling might decrease the reliability of their findings [2, 12]. As well, Bishara et al. [24] reported very low nickel levels, as 77 out of their 93 readings—for 31 patients at three intervals—showed undetected levels of nickel, and the remainder were all far below the normal range [24]. Such an inconsistency might point out the existence of false negative errors in their study. It seems that simultaneous assessment of salivary, plaque, blood, and urinary nickel might be the best and probably the only approach to prove the mechanism underlying the controversies. It should be taken into consideration, however, that even low or unchanged levels of blood nickel in orthodontic patients cannot indicate an absence of selective binding of nickel to organs [6, 10], especially to the kidneys where the nickel highly tends to localize at [6, 21, 23]. This is again relevant to patients’ health since nickel, alone or in combination with cadmium (released from silver-soldered appliances [18]), might induce single-strand DNA breakage in kidney cells [21], irrespective of nickel dosage [7, 9].
In harmony with the results of Menezes et al. [23], the effect of gender on the elevation pattern during the treatment period was not statistically significant in the current study. Nonetheless, the increase in nickel level in males was about twice as much as that in females, in a way that despite the smaller sample size of males, the difference between male patients and their brothers reached the level of significance. This might be explained by the lifelong contact with metal jewelry in females. Because, perhaps the negative influence of long-term exposures to extra nickel on intestinal adsorption [24, 27] might also apply to oral absorption. No evidence existed in similar reports to discuss this suggestion; and it might be approached with caution, as the difference between the sexes was very small. Therefore, larger samples and improved methodologies with higher powers are needed to draw decisive conclusions.
Limitations and Strengths
The present study was constrained by some factors. Evaluation of blood nickel might shed light on the links between pace of nickel elimination and remained serum nickel. However, virtually none of the assessed 380 patients agreed to give blood samples. Furthermore, reliability of the findings could be enhanced with a prospective cohort setup, in which pretreatment nickel levels were measured. Such a design would eliminate interindividual differences and diminish considerably intraindividual variations such as saliva pH. Nonetheless, it was not quite affordable in this long-term study with a rather large sample size and a long list of exclusion criteria. For instance, unlike the other studies on systemic nickel [12, 23, 24], we tried to control for additional confounders such as smoking and consuming nickel-rich diets or alcohol. Also in contrast to the study of Menezes et al. [23] where urine was collected at home, in this research, urine sampling was conducted at an office which might reduce the odds of contamination that existed in the other study [23]. In addition, enrolling pairs of matched siblings living in the same conditions was not present in none of the other studies apart from one [5]. This method could lower genetic and environmental discrepancies such as type of water pipes. The significant difference and the consistency of the results of this study in terms of greater nickel values in patients compared with their siblings implied the well control over lurking variables. Finally, instead of merely relying on statistical significance which was the only source of conclusions in almost all previous studies [3–5, 12, 14, 18, 22–24], we estimated the CI as well which can favor the validity and comparability of the findings [7].
Conclusions
Within the limitations of this long-term retrospective study, it was inferred that urinary nickel excretion might be significantly, though slightly, escalated in orthodontic patients undergoing orthodontic therapy for at least 12 months, in which no NiTi appliances are used. Gender did not have a statistically significant influence on the increase pattern, albeit this increase was somewhat more vivid in males. Future studies are warranted to assess this.
References
Eliades T, Pratsinis H, Kletsas D, Eliades G, Makou M (2004) Characterization and cytotoxicity of ions released from stainless steel and nickel–titanium orthodontic alloys. Am J Orthod Dentofac Orthop 125:24–29
House K, Sernetz F, Dymock D, Sandy JR, Ireland AJ (2008) Corrosion of orthodontic appliances—should we care? Am J Orthod Dentofac Orthop 133:584–592
Amini F, Borzabadi Farahani A, Jafari A, Rabbani M (2008) In vivo study of metal content of oral mucosa cells in patients with and without fixed orthodontic appliances. Orthod Craniofac Res 11:51–56
Matos de Souza R, Macedo de Menezes L (2008) Nickel, chromium and iron levels in the saliva of patients with simulated fixed orthodontic appliances. Angle Orthod 78:345–350
Amini F, Jafari A, Amini P, Sepasi S (2012) Metal ion release from fixed orthodontic appliances—an in vivo study. Eur J Orthod 34:126–130
Eliades T, Athanasiou AE (2002) In vivo aging of orthodontic alloys: implications for corrosion potential, nickel release, and biocompatibility. Angle Orthod 72:222–237
Amini F, Rakhshan V, Mesgarzadeh N (2012) Effects of long-term fixed orthodontic treatment on salivary nickel and chromium levels: a 1-year prospective cohort study. Biol Trace Elem Res. doi:101007/s12011-012-9457-y
Hwang CJ, Shin JS, Cha JY (2001) Metal release from simulated fixed orthodontic appliances. Am J Orthod Dentofac Orthop 120:383–391
Eliades T, Trapalis C, Eliades G, Katsavrias E (2003) Salivary metal levels of orthodontic patients: a novel methodological and analytical approach. Eur J Orthod 25:103–106
Mikulewicz M, Chojnacka K (2010) Trace metal release from orthodontic appliances by in vivo studies: a systematic literature review. Biol Trace Elem Res 137:127–138
Mikulewicz M, Chojnacka K (2011) Release of metal ions from orthodontic appliances by in vitro studies: a systematic literature review. Biol Trace Elem Res 139:241–256
Agaoglu G, Arun T, Izgi B, Yarat A (2001) Nickel and chromium levels in the saliva and serum of patients with fixed orthodontic appliances. Angle Orthod 71:375–379
Amini F, Rakhshan V, Pousti M, Rahimi H, Shariati M, Aghamohamadi B (2012) Variations in surface roughness of seven orthodontic archwires: an SEM-profilometry study. Korean J Orthod 42:129–137
Fors R, Persson M (2006) Nickel in dental plaque and saliva in patients with and without orthodontic appliances. Eur J Orthod 28:292–297
Mikulewicz M, Chojnacka K (2011) Cytocompatibility of medical biomaterials containing nickel by osteoblasts: a systematic literature review. Biol Trace Elem Res 142:865–889
Kuhta M, Pavlin D, Slaj M, Varga S, Lapter-Varga M (2009) Type of archwire and level of acidity: effects on the release of metal ions from orthodontic appliances. Angle Orthod 79:102–110
Macedo de Menezes L, Cardoso Abdo Quintão C (2010) The release of ions from metallic orthodontic appliances. Semin Orthod 16:282–292
Freitas MP, Oshima HM, Menezes LM (2011) Release of toxic ions from silver solder used in orthodontics: an in-situ evaluation. Am J Orthod Dentofac Orthop 140:177–181
Pazzini CA, Junior GO, Marques LS, Pereira CV, Pereira LJ (2009) Prevalence of nickel allergy and longitudinal evaluation of periodontal abnormalities in orthodontic allergic patients. Angle Orthod 79:922–927
Kusy RP (2004) Clinical response to allergies in patients. Am J Orthod Dentofac Orthop 125:544–547
Li Z, Gu JY, Wang XW, Fan QH, Geng YX, Jiao ZX, Hou YP, Wu WS (2010) Effects of cadmium on absorption, excretion, and distribution of nickel in rats. Biol Trace Elem Res 135:211–219
Petoumenou E, Arndt M, Keilig L, Reimann S, Hoederath H, Eliades T, Jager A, Bourauel C (2009) Nickel concentration in the saliva of patients with nickel–titanium orthodontic appliances. Am J Orthod Dentofac Orthop 135:59–65
Menezes LM, Quintao CA, Bolognese AM (2007) Urinary excretion levels of nickel in orthodontic patients. Am J Orthod Dentofac Orthop 131:635–638
Bishara SE, Barrett RD, Selim MI (1993) Biodegradation of orthodontic appliances. Part II. Changes in the blood level of nickel. Am J Orthod Dentofac Orthop 103:115–119
Fairbrother A, Wenstel R, Sappington K, Wood W (2007) Framework for metals risk assessment. Ecotoxicol Environ Saf 68:145–227
Jerrold B, Leikin FPP (2007) Poisoning and toxicology handbook. LexiComp, Chicago
Santucci B, Manna F, Cannistraci C, Cristaudo A, Capparella R, Bolasco A, Picardo M (1994) Serum and urine concentrations in nickel-sensitive patients after prolonged oral administration. Contact Dermatitis 30:97–101
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amini, F., Rakhshan, V. & Sadeghi, P. Effect of Fixed Orthodontic Therapy on Urinary Nickel Levels: A Long-term Retrospective Cohort Study. Biol Trace Elem Res 150, 31–36 (2012). https://doi.org/10.1007/s12011-012-9478-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9478-6