Abstract
Nickel and chromium existing in stainless-steel crowns (SSCs, used in pediatric dentistry) might be cytotoxic and allergenic. However, no in vivo studies have examined their salivary levels in children using SSCs, or in young children without SSCs. Also, the effect of acidity on metal ion release has not yet been evaluated in any previous in vivo studies in the whole literature. Therefore, this preliminary before-after clinical trial was conducted. Salivary nickel/chromium levels of 30 children before and after 2 months of placement of SSCs were measured using atomic absorption spectrophotometry. Salivary pH was measured with a digital pH meter. The effects of treatment, pH, number of SSCs, gender, and age on salivary ions were analyzed statistically (α = 0.05, β = 0.15). Salivary nickel concentrations increased from 4.9010 ± 4.7390 to 5.6320 ± 4.7210 μg/L (P = 0.000, paired t test). Chromium increased from 0.3273 ± 0.5214 to 0.4199 ± 0.6404 μg/L (P = 0.016). Saliva pH increased from 6.81 ± 0.52 to 7.04 ± 0.47 (P = 0.000). Ion levels were not correlated with pH (P > 0.14), except chromium in the follow-up (rho = − 0.435, P = 0.016). Nickel increase (but not chromium increase) was correlated with pH increase (rho = 0.367, P = 0.046). Age was only correlated with baseline chromium (rho = 0.373, P = 0.042). Being male was associated with baseline/follow-up nickel levels (P ≤ 0.030). SSC number was not correlated with ions or pH (P > 0.36). It was shown for the first time that SSCs might increase salivary nickel and chromium concentrations and reduce saliva acidity. Nickel increase might be in line with pH elevation. The raised pH might be associated with reduced chromium release. Boys might have higher nickel levels than might girls, with or without SSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stainless-steel crown (SSC) is a prefabricated metal restoration placed over deciduous molar teeth after removing their caries; it has been proven as an effective yet economic and rapid treatment in recovering the oral function as well as maintaining the orthodontic space for permanent teeth. Therefore, they are commonly used in pediatric dentistry. SSC alloys usually contain 70–65% iron, 17–20% chromium, 13–8% nickel, and less than 2% manganese, silicon, and carbon [1, 2].
Corrosion of such alloys in the oral cavity might release nickel and chromium into the saliva and body, which can raise biocompatibility concerns: both of these trace elements are mutagenic, cytotoxic, and genotoxic and might induce contact allergy, type-IV cell-mediated hypersensitivity, asthma, birth defects, and reproductive damages [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. Chromium is usually incorporated into such alloys to produce a passive protective oxide layer [5, 23]. Nevertheless, this anti-corrosive film is usually disrupted in the oral cavity, as a result of multiple mechanisms including but not limited to thermal, physical, mechanical, and chemical stresses as well as bacterial byproducts and enzymes [4, 6,7,8,9, 15, 17, 21, 22, 24,25,26,27,28,29].
Bearing in mind the potential hazard of these metals and the high frequency of SSC usage, it seems crucial to establish the extent of their release from SSCs into saliva [18]. Despite the importance of this subject, studies on trace element release from dental materials are mostly focused on orthodontic appliances, and studies on SSC metal ion release are very rare [30,31,32,33]. Of the only four studies available on the release of metal ions from SSCs, three were in vitro [30, 31, 33], which is a design not relevant to the rapidly changing oral environment with the presence of various enzymes, acids, and stresses [4,5,6,7,8, 12,13,14, 17, 18, 23]. All the three in vitro studies were highly controversial, limited to up to 4 weeks of nickel release [30,31,32,33]. There was only one in vivo research on patients, which was a cross-sectional study on hair (systemic) levels of nickel, chromium, and iron [32]. Finally, to date, the association between salivary pH and the extent of salivary ion release or its systemic accumulation has been assessed by no in vivo studies, either in pediatric dentistry or orthodontics, etc. Only one laboratory study has assessed the effect of pH in vitro and has reported a reverse association between nickel release and pH [33].
This prospective before-after clinical trial was conducted, since (1) there is no research in the whole literature investigating the association between salivary pH and the extent of metal ion release in vivo; (2) there is no in vivo study on metal ion release from SSCs, or (3) on normal salivary values of nickel or chromium in young children; finally, (4) the only available studies on SSCs are controversial in vitro studies limited in numerous aspects (mentioned above). The null hypotheses were the following: (1) salivary nickel or chromium concentrations would not differ between the baseline (pre-treatment) and after 2 months of treatment with SSCs. (2) There would be no significant difference between the ion concentrations and pH in patients undergoing 2 months of treatment with six, seven, or eight SSCs in the mouth. (3) There would be no difference between pre-treatment and within-treatment (2 months) values of salivary pH. (4) Pre-treatment salivary pH would not be associated with pre-treatment ion concentrations. Also, there would be no association between within-treatment pH and within-treatment ion levels. (5) Age and sex would not affect pre-treatment or within-treatment ion concentrations. (6) Alterations in ion concentrations would follow a similar pattern between males and females and among people at different ages.
Subjects and Methods
This before-after clinical trial was performed on 60 observations from 30 pediatric dental patients, obtained at two time points of 30 observations each: baseline (pre-treatment) and within-treatment (60 days after the beginning of treatment). Subjects’ parents gave signed written consents, after a thorough oral and written explanation of the study, and being assured that they can leave the study at their will, any time, without any penalties, or without disruption of the free and complete treatment being delivered to them. Protocol ethics were approved by the institutional review board of the university according to the Helsinki declaration (ethical code: IR.AJUMS.REC.1395.11). Every treatment plan was in complete accordance with clinical protocols of pediatric dentistry, and no treatment plans were modified in any way for the sake of the study; therefore, no harms were inflicted by this study to the patients [21].
Sample
A total of 58 patients visiting the Department of Pediatric Dentistry, Ahvaz Jundishapur University of Medical Sciences (Ahvaz, Iran), were screened during 2017–2018 until reaching the predetermined sample size. The inclusion criteria were clinical indication for SSCs without any further need for more SSCs or space maintainers within the next 2 months, agreement to participate in the study, having sufficient unstimulated saliva in the morning, and not needing any space maintainers during the next 2 months. Also, since only patients who would undergo general anesthesia would meet the first criterion, the study was done on such patients. As other exclusion criteria, the patients had to have no history of systemic diseases or syndromes, no allergic reactions, or no history of wearing any imitation jewelry. Also, patients had to lack any intraoral metal restorations (amalgam, pins, or SSCs) or any orthodontic appliances. If any patients were dropped out of the study, they would be replaced with new patients (while their treatment would be delivered completely).
At the Department of Pediatric Dentistry, there is no need or pressure for placing all SSCs in the same session. It is quite possible to install each SSC or a maximum of two SSCs in a single session. Most patients are treated this way. However, such patients would not be appropriate for a 2-month prospective study, since (based on a pilot study and the clinical experience of the authors) almost all of them would return for treatment of other teeth, in a short time and, therefore, would be excluded from the study. This would risk delays in timing and budget wastes. The only cases appropriate for this study were those children who were indications for treatment under general anesthesia (due to such children’s lack of complying or them needing a great number of emergency treatments). In order to avoid repeats in general anesthesia, such children would receive complete dental treatments under general anesthesia. Therefore, it is almost guaranteed that they would not need new SSCs or other dental treatments within the next 2 months. Therefore, only such patients could be included. No patients’ treatment plans were changed at all, and the decision for general anesthesia was made solely on the basis of pure clinical diagnosis and treatment planning. Only patients who received SSC-only or SSC-composite treatments were included, and those receiving a combination of SSCs with other metal treatments were excluded.
Uniform Dental Treatments
Firstly, proximal surfaces of each tooth needing a crown were cut using a #69 L round bur attached to a high-speed handpiece, until the interdental contact was opened. Afterwards, the cusps and the occlusal surface were trimmed down for 1 mm using the same bur. After finishing the crown preparation, any caries was removed by a round bur attached to a low-speed handpiece. If the pulp was exposed, the access cavity was prepared using 008 fissure burs, and the pulp was treated. Afterwards, a crown (3 M, Maplewood, MN, USA) of proper size was selected and bonded to the tooth using a zinc polycarboxylate cement lacking nickel or chromium (Hoffmann’s Dental, Berlin, Germany). About half of the dental treatments underneath the SSCs were pulpotomy. Of the remainders, about two third were pulpectomy, and the rest were cavity removal only.
Saliva Sampling, pH/Ion Measurement, and Clinical Assessment
The sampling was undertaken once immediately before beginning of the treatment (as the control), and once 2 months later. Before each sampling session, a list of nickel-rich foods/drinks was handed to the parents, who were requested not to give any of them to the children from 48 h before the sampling session. They were also asked not to irrigate with fluoridated mouthwashes 24 h before the next visit, until after saliva collection [18, 19, 21, 22].
Saliva was sampled in the morning about 2 h before induction of general anesthesia (clinically needed for installing four or more SSCs) and immediately in the follow-up session. In each session, patients ejected 5 mL of their unstimulated saliva into nickel-/chromium-free polyethylene bottles irrigated beforehand with acetone and distilled water. Since the treatment would be performed under general anesthesia, the children did not rinse their mouth with distilled water, before sampling. In order to standardize both sampling methods, in the second session as well, no rinsing was performed before saliva sampling.
Saliva Acidity
In order to avoid any pH/CO2 changes [34], the pH was recorded immediately after saliva sampling, using a digital pH meter (Eutech 5500, Eutech Instruments, Queenstown, Singapore) calibrated using 4- and 7-pH buffers. This was done by immersing the glass electrode of the device into the sampled saliva for a short time until the reading stabilized. After each measurement, the electrode was irrigated using distilled water and was kept in the kit’s standard 7-pH solution [35]. Before each measurement, it was rinsed and dried.
Ion Measurement
Saliva bottles were kept for maximum a week in a refrigerator and then were shipped to the Toxicology Laboratory of the Pharmacology School of the University. At the laboratory, 1 mL of saliva was centrifuged at 5000–8000 rpm and its impurities, debris, and proteins were removed. Then, it was diluted with 0.1% nitric acid and until augmenting to 5 mL of solution. Afterwards, salivary chromium/nickel concentration of each vial was measured thrice using an atomic absorption spectrophotometer with graphite furnace (AA240FS, Varian, Sydney, Australia). The average of the three measurements per specimen was recorded as the main value in micrograms per liter (ppb) [15, 21, 36].
Clinical Assessment
In the follow-up session, a pediatric dentist carefully checked all mucosae and soft tissues for any signs of any tissue changes (e.g., allergic reactions, inflammation), using a dental mirror and under dental light. Also, the parents and children were asked about the occurrence of any disturbances in the child’s oral tissues during the last 2 months.
Statistical Analysis
The sample size was predetermined based on the values reported by a previous 2-month prospective in vivo study on orthodontic ion release [21] (since there was no comparable studies on SSCs), as 30 × 2 observations for each variable, in order to reach powers above 85% at an alpha of 0.05. The before-after differences between the ion and pH values measured at two time points (i.e., delta nickel and delta chromium) were calculated for each patient. Descriptive statistics and 95% confidence intervals were computed for ions and delta values. A paired t test was used to assess the difference between the ions and pH values measured at baseline versus 2 months after initiation. Males and females were compared in terms of age and the number of SSCs, using an independent-samples t test. The correlations between measurements at both time points were assessed using a Pearson correlation coefficient. The correlations between each ion measurement and its corresponding pH were assessed using the Spearman correlation coefficient. The correlations between the number of SSCs with delta ions and delta pH were assessed using the Spearman coefficient. The correlations between patients’ ages and ion/pH levels were assessed using the Spearman coefficient. A point-biserial correlation coefficient was used to assess the correlations between sex and ion/pH levels. A two-way repeated-measures analysis of variance (ANOVA) was used to assess the simultaneous effect of treatment and sex on ion levels. The software in use was SPSS 25 (IBM, Armonk, NY, USA). The level of significance was set at 0.05.
Results
Of the 58 screened subjects, seven were not indications of general anesthesia, six could not provide adequate unstimulated saliva, and two were agitated and not manageable, so they were excluded before the study or immediately after the first session. A patient needed an intracanal pin besides SSCs and hence was excluded. Two others needed amalgam restorations besides SSCs and therefore were excluded. One patient later needed space maintainers and thus was replaced with a new one. Three did not attend the follow-up session, so they were replaced with new patients. Also, six of the first patients who finished the whole study course were excluded once it was noted that the results of the previous spectrophotometer were not accurate. Consequently, they were replaced with new patients, and a more modern and accurate spectrophotometer was used (the one cited above) for them and the rest of subjects.
There were 19 females and 11 males with an average (SD) age of 3.9 ± 0.8 years. The average ages of boys (3.5 ± 0.7 years) and girls (4.1 ± 0.8 years) did not differ significantly (independent-samples t test, P = 0.065). The number of SSCs per patient was 6.8 ± 0.8 (range 6 to 8) without any inter-gender difference (6.8 ± 0.8 in girls and 6.8 ± 0.9 in boys, P = 0.941).
The clinical examination showed that all of children’s soft tissues were intact in the follow-up. None of the children had complained about any soft tissue disturbances during the last 2 months.
Alterations During the Study Course
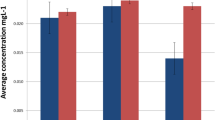
The mean nickel concentration increased from 4.90 μg/L as the control to 5.63 μg/L in the second month (Fig. 1, Table 1). According to the paired t test, this 0.7317-μg/L escalation was significant (P = 0.0004). There was a positive and perfect correlation between the nickel values measured at both time points (r [Pearson coefficient] = 0.977, P = 0.00000). Chromium levels reached from a baseline level of 0.33 to 0.42 μg/L after 2 months (P = 0.016; Fig. 1, Table 1), with a perfect correlation between the two time intervals (r = 0.962, P = 0.00000). The pH became more neutral (from 6.81 to 7.04, P = 0.0001) and the pH values were highly correlated with one another at the two time points (r = 0.850, P = 0.00000).
Associations Between pH and Ion Levels
Except a negative correlation observed between the pH and chromium measured after 60 days of treatment (rho = − 0.435, P = 0.016), no other significant correlations were observed between pH with nickel or chromium levels either at the baseline or the 60th day (all the three P values > 0.14, Spearman coefficient). The increase in pH during the course of study (i.e., the delta pH) was associated positively with the delta nickel (rho = 0.367, P = 0.046) but not with the delta chromium (rho = − 0.128, P = 0.499).
Correlations Between the Number of SSCs and Changes in Ion Levels or pH
There were no significant correlations between the number of SSCs with delta nickel, delta chromium, or delta pH (all P values > 0.36, Spearman coefficient).
Role of Age and Gender
Only the baseline chromium concentration was correlated with age (rho = 0.373, P = 0.042). The other correlations between age with ion/pH levels or with their increases over time (i.e., the delta values) were non-significant (all P values > 0.13, Spearman coefficient). Male gender was positively associated with nickel values measured at the baseline (point-biserial coefficient = 0.396, P = 0.030) or in the follow-up (point-biserial coefficient = 0.402, P = 0.027; Table 2). Gender was not correlated with chromium or pH levels, or with increases in nickel, chromium, or pH over time (all P values > 0.27, point-biserial coefficient). According to the repeated-measures two-way ANOVA, the effects of time (F = 14.306, P = 0.001) and gender (F = 5.383, P = 0.028) on nickel levels were significant (Fig. 1). The interaction of time with gender was not significant (F = 0.014, P = 0.906). The effect of time on chromium levels was significant (F = 4.713, P = 0.039; Fig. 2). The effects of gender on chromium (F = 0.359, P = 0.554) and its interaction with time (F = 1.223, P = 0.248) were not significant. Time had a significant effect on pH (F = 19.325, P = 0.000), without a significant gender effect (F = 0.262, P = 0.613) or interaction (F = 0.065, P = 0.801).
Discussion
The findings pointed out that both salivary nickel and chromium levels increased in children receiving SSCs. None of the factors “age, number of crowns, pH, or gender” were associated with metal ion release, except for male gender which might increase nickel levels irrespective of treatment. We also detected slight increases in pH after installing metal appliances in the oral cavity. The pH increase was positively associated with nickel increase; however, the level of pH measured in the follow-up session was negatively associated with chromium. In the current study, the number of SSCs was not correlated with the extents of increase of either metal, which might be attributable to the narrow range of SSCs placed. Since there were no in vivo or in vitro studies regarding the role of the number of metal appliances on metal ion release (even in the rather abundant orthodontic literature), further comparisons were not possible.
The only available studies on ion release from SSCs were highly controversial, in vitro studies limited to nickel-only release. Such studies showed levels much higher than this study or the in vivo studies on orthodontic appliances. Kulkarni et al. [30] evaluated the release of nickel ion in vitro from SSCs over 4 weeks and reported that the nickel release peaks around the first day (to 0.80 ppm) and decreases gradually afterwards; they concluded that since the release of nickel was very much below average dietary intake of nickel, it would not be hazardous [30]. Ramazani et al. [31] assessed nickel release in vitro in similar intervals over a month and asserted that the peak of nickel release is on the first day (0.0165 ppm) which reduced to less than half in the seventh day, and then reached zero in further intervals. They also reported no associations between the number of SSCs and the extent of released nickel [31]. Menek et al. [33] assessed nickel release from SSCs immersed in artificial saliva for 4 weeks in various pH values. They observed a steady increase over time (up to about 3.6 ppm) in a highly acidic pH = 2.5; on the contrary, in more neutral environments (pH = 6.25), there was a steady decrease over time—the highest amount of nickel release was in the first day (about 0.6 ppm) which reduced to about 0.2 ppm at the end of the study period; they also noted an overall increase in nickel by reducing the pH [33]. Although these studies were disputed, they all revealed a similar pattern of a steady decrease after the first day, which could be due to the formation of the passive chromium oxide layer [5, 23]. However, when the environment was acidic in the study of Menek et al. [33], probably this anti-oxide film was disrupted constantly, leading to an ever-increasing discharge of nickel. Since the number of intervals in this study was limited, we do not know the pattern of increase in these ions. However, it seems that the disruption of the chromium oxide film in the oral cavity creates a situation more similar to the in vitro study of Menek et al. [33], where they had reduced the pH to moderately acidic levels. Many mechanisms can contribute to corrosion of chromium and nickel: Galvanic corrosion happens when two dissimilar metals are in contact through a medium; however, this might apply more to orthodontic studies with different pieces of metals in contact. In the present study, the only metal parts were the SSCs. However, another mechanism is the simple redox reaction with the surroundings, which can be strengthened by acidic pH (caused by anaerobic biofilm activities or when consuming acidic foods/drinks) [4, 7, 9, 18, 25]. In the current study, the pH became more neutral after SSC placement, and this slight increase in pH was correlated positively with the increases in nickel amounts. This was not in line with expectations. A possible justification might be that much more intense changes in pH are needed to increase the redox corrosion of nickel, and that the observed increases in pH might actually be a reflection of the elevated nickel levels (as basic ions). On the other hand, in a part of this study, it was observed that more acidic pH would accompany enhanced leach of chromium. It is not known why pH did not affect the secondary nickel values (and only affected secondary chromium levels) in this study; it seems that perhaps it could affect both of the metals while its influence on chromium release might be more obvious—because the levels of chromium alterations were much narrower—and hence could hypothetically allow the detection of small alteration, whereas such small increases might be lost in the highly scattered nickel releases. Since this is the first in vivo study in the whole literature, the deductions cannot go beyond mere speculation. Since pH can both affect metal release and be affected by it, the associations would be complicated in vivo, and more studies are needed to understand them. Still, it was expected not to detect correlations between pH and baseline trace elements.
Since there was no similar in vivo studies on SSCs, we were limited to discussing our findings in the light of metal ion release from orthodontic appliances and in vitro studies on SSCs. Orthodontic in vivo studies have been quite controversial; some of them have observed elevations in salivary nickel levels after placement of orthodontic appliances [6, 15, 36, 37], whereas many studies have not pointed to such increases [14, 15, 18, 23, 25, 37], and few have reported declines in nickel [6] or chromium levels [3]. Still, even the maximum levels of increase seem insignificant in comparison to the usual intake of nickel or chromium in each day (100–800 μg/day for nickel and 50–280 μg/day for chromium) [4, 14, 18, 21, 25, 36]. For instance, the toxic dose of nickel is about 2.5 g/L, and its lethal oral dose is 50–500 mg/kg body weight [31, 38], and such doses do not seem to be reachable by the leach of nickel from SSCs. In addition, even though the reports on salivary metal ion levels pointed to trivial changes (if any), prospective studies on hair (as the biomarker of long-term trace element accumulation) have shown bolder increases in systemic nickel or chromium [4, 6, 7, 14, 15, 18, 20, 22, 23, 25, 36, 37]. There might be some explanations for this inconsistency. For example, in order to standardize the saliva sampling, it is undertaken in the morning while the patient is abstaining. Nonetheless, salivary ion release peaks after having meals, when salivary pH shifts to the acidic end [15, 17, 18, 20, 22, 23, 25]. Furthermore, a major portion of metal ions might be bioaggregated within the plaque and be absorbed through swallowing it; plaque metals might not be detected in salivary metal examinations while contributing to systemic levels [4, 6, 8, 12, 19, 20, 22]. The only in vivo study that was on systemic accumulations was that of Kodaira et al. [32] who compared hair nickel, chromium, and iron levels in 15 patients having SSCs versus 22 subjects without any SSCs and reported significant differences only in the case of chromium but not nickel or iron. On the other hand, some other concerns exist: genetic adverse effects of these metals are not always dependent on dose; moreover, chronic exposure might enhance some adverse effects; and some metals can be accumulated in certain organs and increase the local dose [5, 7, 15,16,17,18, 21, 23, 36, 39]. Small doses of these genotoxic metals might cause DNA instability and damage (including fragmentation, single-strand breakage, increases in DNA migration and comet formation, or inhibition of enzymes repairing DNA), alter cellular morphology, activate endothelial cells or monocytes, and modify the metabolism in a dose-independent fashion; and finally, since metals are not biodegradable, their sustained accumulation becomes toxic [5, 11, 16,17,18, 20, 23, 29, 36]. Besides, since young children (e.g., pediatric patients) are more active in terms of cell turnover and are much lighter than adolescents or adults (e.g., orthodontic samples of metal ion release), such small amounts of ion release might matter more in children. The most common adverse effect of nickel might be contact allergy. It is the most common cause of short- or long-term type-IV cell-mediated sensitivity. About one third of people might be hypersensitive to this metal, especially in women with 20–30% prevalence compared to males with 2–5% prevalence [10, 26, 39, 40]. Chromium is the second most common cause of contact allergy, affecting about 10% of males and 3% of females [15, 40]. Nickel might induce dermatitis and irritation [4, 12, 28] characterized by burning sensation in the mouth, lip desquamation, gingival hyperplasia, angular cheilitis, gingivitis, bleeding upon probing, periodontitis, metallic taste, discoloration, and multiform erythema [10, 26, 41]. Nickel allergic influences might be aggregated and appear after about 9 to 12 months of treatment [26], although not all researchers agree on this [4, 10, 18]. Conversely, the remaining main concern of dental practitioners might be allergic stomatitis, hypersensitivity, and perhaps periodontitis following nickel exposure [18, 20, 26, 29, 36, 39,40,41], since not all corrosion artifacts are carcinogenic or toxic and many genetic damages might be reversible [5, 16, 19, 20, 22, 29, 36, 41].
In this sample, boys had a higher baseline level of nickel compared to females. Almost the same difference existed also after 2 months of SSC treatment, which implied that the sex dimorphism in the secondary nickel concentrations might be actually a function of the intrinsic differences between the genders. Only few previous studies have assessed the role of gender: Levels of ions were not different between males and females either in saliva [21] hair [20, 22] or in urine [19]. Yet, in few studies, the patterns of increases were different between males and females in terms of salivary chromium [36] or hair chromium [22]. In this study, the patterns of chromium increases differed slightly between males and females, but it was lost to the high dispersion of the data.
The present study was the only one concerning the normal salivary concentrations of metal ions in young children (as the baseline levels of these pediatric patients). It was shown that in children, the levels of metals were in the range of previous reports on nickel (0.53 μg/L [14] to 11.9 μg/L [7] for nickel) or chromium (0.64 μg/L [6] to 3.9 μg/L [18]) excluding some studies with outlier results [40, 42]. The controversy might root in various influencing items such as variations in food quality and types, genetics, salivary composition, smoking, bacterial colonization, galvanic currents, systemic or mental health, population types, or saliva sampling differences (such as the time of day) [4, 6, 7, 12, 14, 18, 25, 39, 43, 44]. Moreover, since irrigation with distilled water before sampling might reduce salivary metal levels [23], various intervals between irrigation and sampling (e.g., 2 min [18, 21, 25] vs. 5 min [15, 40] vs. no irrigation in this study, or not disclosed in some others [3]) might play a role in the controversy. Even in some studies, saliva is stimulated and therefore would differ in composition from unstimulated saliva [14, 40]. Our results regarding the baseline nickel versus chromium were in line with most other studies [5,6,7, 15,16,17,18], while in contrast with few ones reporting higher chromium levels [14, 23, 42]. Such a difference might be due to intrinsic and food effects. It might be argued that since rinsing can affect nickel amounts more than chromium concentrations [23], it might contribute to the differences between baseline nickel and chromium [18]. However, in this study (in which there was no rinsing before sampling), nickel levels were much greater than chromium levels.
Like earlier in vivo studies, this one was limited by certain factors as well. Clinical studies cannot control many known and unknown factors relevant to ion release; although such factors make outcomes more difficult to interpret, they improve generalizability [7, 14, 17, 18, 39, 43]. Still, the significance of our findings might imply a proper control over many confounders through its longitudinal self-matched design and sample size predetermination [21]. It was not possible to assess the intraobserver agreement by sampling the saliva more than once per session. However, the high associations between the two time points in terms of each of the three variables indicated a high reliability of the findings. Furthermore, if salivary metal ion discharge fluctuates over time (depending on factors such as pH or salivary flow), momentary samplings might not reflect full-term release of such ions [17, 23], especially considering the fact that all samplings have been performed in abstained subjects to rule out the effects of food consumption and diet [15] while discharge of metal ions peaks shortly after having meals [25]. Therefore, it is possible that real rates of metal release are closer to in vitro studies (although again insignificant compared to dietary levels) [13, 14, 18, 25]. In addition, the presence of a parallel control group would improve the reliability of the study [18]. Furthermore, it would be better to also establish the systemic levels of these metal ions in various indicator tissues and fluids such as hair, blood, or urine in order to better understand the dynamism of their systemic absorption and accumulation. Nevertheless, considering the difficulty of sampling as well as expenditures, it was not possible to adopt a randomized clinical trial design and sample from various tissues and fluids. Future studies are needed to examine the systemic absorption of these ions and their associations with salivary levels, using more sophisticated designs. As advantages, this was the first study assessing the baseline levels of these trace elements in young children, examining the role of pH in vivo, and the first one researching the effects of SSCs as dental treatments.
Conclusions
Two months of treatment with SSCs might increase salivary nickel and chromium concentrations, although the increase might not be clinically noticeable. The pH might become slightly less acidic after 2 months of SSC placement. This increase in pH might be associated positively with the increase in nickel after 2 months of treatment. Slight increases of the pH (towards neutrality) after 2 months of SSC use might be associated with declines in the chromium released from SSCs. Boys might have higher nickel levels regardless of having SSCs in their mouth; the inter-gender difference might be similar in the presence or absence of SSCs, and the pattern of nickel increase after SSC use might not vary between girls and boys. Gender might not affect chromium concentrations. Age might not play a role in ion escalations or their secondary metal levels; still, aging might be associated with slightly higher baseline levels of chromium but not nickel. The effects of the number of SSCs or the effects of aging on the extent of ion release were inconclusive due to the narrow range of the variables and need more evidence. The associations with pH were complicated and warrant for further studies as well.
References
Casamassimo PS, Fields HW, McTigue DJ, Nowak A (2013) Pediatric dentistry: infancy through adolescence. Elsevier Health Sciences, Philadelphia
Seale NS, Randall R (2015) The use of stainless steel crowns: a systematic literature review. Pediatr Dent 37:145–160
Jurela A, Verzak Ž, Brailo V, Škrinjar I, Sudarević K, Janković B (2018) Salivary electrolytes in patients with metallic and ceramic orthodontic brackets. Acta Stomatol Croat 52:32–36
House K, Sernetz F, Dymock D, Sandy JR, Ireland AJ (2008) Corrosion of orthodontic appliances—should we care? Am J Orthod Dentofac Orthop 133:584–592
Amini F, Borzabadi Farahani A, Jafari A, Rabbani M (2008) In vivo study of metal content of oral mucosa cells in patients with and without fixed orthodontic appliances. Orthod Craniofac Res 11:51–56
Matos de Souza R, Macedo de Menezes L (2008) Nickel, chromium and iron levels in the saliva of patients with simulated fixed orthodontic appliances. Angle Orthod 78:345–350
Amini F, Jafari A, Amini P, Sepasi S (2012) Metal ion release from fixed orthodontic appliances—an in vivo study. Eur J Orthod 34:126–130
Eliades T, Athanasiou AE (2002) In vivo aging of orthodontic alloys: implications for corrosion potential, nickel release, and biocompatibility. Angle Orthod 72:222–237
Hwang CJ, Shin JS, Cha JY (2001) Metal release from simulated fixed orthodontic appliances. Am J Orthod Dentofac Orthop 120:383–391
Genelhu MC, Marigo M, Alves-Oliveira LF, Malaquias LC, Gomez RS (2005) Characterization of nickel-induced allergic contact stomatitis associated with fixed orthodontic appliances. Am J Orthod Dentofac Orthop 128:378–381
Faccioni F, Franceschetti P, Cerpelloni M, Fracasso ME (2003) In vivo study on metal release from fixed orthodontic appliances and DNA damage in oral mucosa cells. Am J Orthod Dentofac Orthop 124:687–693 discussion 693-684
Mikulewicz M, Chojnacka K (2010) Trace metal release from orthodontic appliances by in vivo studies: a systematic literature review. Biol Trace Elem Res 137:127–138
Mikulewicz M, Chojnacka K (2011) Release of metal ions from orthodontic appliances by in vitro studies: a systematic literature review. Biol Trace Elem Res 139:241–256
Kocadereli L, Atac PA, Kale PS, Ozer D (2000) Salivary nickel and chromium in patients with fixed orthodontic appliances. Angle Orthod 70:431–434
Agaoglu G, Arun T, Izgi B, Yarat A (2001) Nickel and chromium levels in the saliva and serum of patients with fixed orthodontic appliances. Angle Orthod 71:375–379
Natarajan M, Padmanabhan S, Chitharanjan A, Narasimhan M (2011) Evaluation of the genotoxic effects of fixed appliances on oral mucosal cells and the relationship to nickel and chromium concentrations: an in-vivo study. Am J Orthod Dentofac Orthop 140:383–388
Hafez HS, Selim EM, Kamel Eid FH, Tawfik WA, Al-Ashkar EA, Mostafa YA (2011) Cytotoxicity, genotoxicity, and metal release in patients with fixed orthodontic appliances: a longitudinal in-vivo study. Am J Orthod Dentofac Orthop 140:298–308
Amini F, Rakhshan V, Mesgarzadeh N (2012) Effects of long-term fixed orthodontic treatment on salivary nickel and chromium levels: a 1-year prospective cohort study. Biol Trace Elem Res 150:15–20
Amini F, Rakhshan V, Sadeghi P (2012) Effect of fixed orthodontic therapy on urinary nickel levels: a long-term retrospective cohort study. Biol Trace Elem Res 150:31–36
Amini F, Mollaei M, Harandi S, Rakhshan V (2015) Effects of fixed orthodontic treatment on hair nickel and chromium levels: a 6-month prospective preliminary study. Biol Trace Elem Res 164:12–17
Khaneh Masjedi M, Niknam O, Haghighat Jahromi N, Javidi P, Rakhshan V (2016) Effects of fixed orthodontic treatment using conventional, copper-included, and epoxy-coated nickel-titanium archwires on salivary nickel levels: a double-blind randomized clinical trial. Biol Trace Elem Res 174:27–31
Khaneh Masjedi M, Haghighat Jahromi N, Niknam O, Hormozi E, Rakhshan V (2017) Effects of fixed orthodontic treatment using conventional (two-piece) versus metal injection moulding brackets on hair nickel and chromium levels: a double-blind randomized clinical trial. Eur J Orthod 39:17–24
Eliades T, Trapalis C, Eliades G, Katsavrias E (2003) Salivary metal levels of orthodontic patients: a novel methodological and analytical approach. Eur J Orthod 25:103–106
Mikulewicz M, Chojnacka K (2011) Cytocompatibility of medical biomaterials containing nickel by osteoblasts: a systematic literature review. Biol trace element research 142(3):865–889
Fors R, Persson M (2006) Nickel in dental plaque and saliva in patients with and without orthodontic appliances. Eur J Orthod 28:292–297
Pazzini CA, Junior GO, Marques LS, Pereira CV, Pereira LJ (2009) Prevalence of nickel allergy and longitudinal evaluation of periodontal abnormalities in orthodontic allergic patients. Angle Orthod 79:922–927
Freitas MP, Oshima HM, Menezes LM (2011) Release of toxic ions from silver solder used in orthodontics: an in-situ evaluation. Am J Orthod Dentofac Orthop 140:177–181
Macedo de Menezes L, Cardoso Abdo Quintão C (2010) The release of ions from metallic orthodontic appliances. Semin Orthod 16:282–292
Amini F, Shariati M, Sobouti F, Rakhshan V (2016) Effects of fixed orthodontic treatment on nickel and chromium levels in gingival crevicular fluid as a novel systemic biomarker of trace elements: a longitudinal study. Am J Orthod Dentofac Orthop 149:666–672
Kulkarni P, Agrawal S, Bansal A, Jain A, Tiwari U, Anand A (2016) Assessment of nickel release from various dental appliances used routinely in pediatric dentistry. Indian J Dent 7:81–85
Ramazani N, Ahmadi R, Darijani M (2014) Assessment of nickel release from stainless steel crowns. J Dent (Tehran, Iran) 11:328–334
Kodaira H, Ohno K, Fukase N, Kuroda M, Adachi S, Kikuchi M, Asada Y (2013) Release and systemic accumulation of heavy metals from preformed crowns used in restoration of primary teeth. J Oral Sci 55:161–165
Menek N, Başaran S, Karaman Y, Ceylan G, Şen Tunç E (2012) Investigation of nickel ion release from stainless steel crowns by square wave voltammetry. Int J Electrochem Sci 7:6465–6471
Moreira AR, Passos IA, Sampaio FC, Soares MS, Oliveira RJ (2009) Flow rate, pH and calcium concentration of saliva of children and adolescents with type 1 diabetes mellitus. Braz J Med Biol Res 42:707–711
Hans R, Thomas S, Garla B, Dagli RJ, Hans MK (2016) Effect of various sugary beverages on salivary pH, flow rate, and oral clearance rate amongst adults. Scientifica 2016:1–6
Amini F, Harandi S, Mollaei M, Rakhshan V (2015) Effects of fixed orthodontic treatment using conventional versus metal-injection molding brackets on salivary nickel and chromium levels: a double-blind randomized clinical trial. Eur J Orthod 37:522–530
Petoumenou E, Arndt M, Keilig L, Reimann S, Hoederath H, Eliades T, Jager A, Bourauel C (2009) Nickel concentration in the saliva of patients with nickel-titanium orthodontic appliances. Am J Orthod Dentofac Orthop 135:59–65
Bhaskar V, Subba Reddy VV (2010) Biodegradation of nickel and chromium from space maintainers: an in vitro study. J Indian Soc Pedod Prev Dent 28:6–12
Menezes LM, Quintao CA, Bolognese AM (2007) Urinary excretion levels of nickel in orthodontic patients. Am J Orthod Dentofac Orthop 131:635–638
Singh DP, Sehgal V, Pradhan KL, Chandna A, Gupta R (2008) Estimation of nickel and chromium in saliva of patients with fixed orthodontic appliances. World J Orthod 9:196–202
Bishara SE, Barrett RD, Selim MI (1993) Biodegradation of orthodontic appliances. Part II. Changes in the blood level of nickel. Am J Orthod Dentofac Orthop 103:115–119
Kerosuo H, Moe G, Hensten-Pettersen A (1997) Salivary nickel and chromium in subjects with different types of fixed orthodontic appliances. Am J Orthod Dentofac Orthop 111:595–598
International Programme on Chemical Safety (1991) 108. Nickel. In: Environmental health criteria. World Health Organization, Geneva, pp 16–17
Wolowiec P, Chojnacka K, Loster BW, Mikulewicz M (2017) Do dietary habits influence trace elements release from fixed orthodontic appliances? Biol Trace Elem Res 180:214–222
Source of Funding
The study was funded by the authors and their institution.
Author information
Authors and Affiliations
Contributions
Leila Basir and Razieh Meshki searched the literature, conceived the assessment of effects of SSC treatment on nickel release, designed the study, supervised the experiments, and mentored the thesis. Azam Behbudi searched the literature, conceived the assessment of effects of SSC treatment on nickel release, designed and performed the experiments, and wrote the thesis. Vahid Rakhshan searched the literature, conceived the assessment of the role of salivary pH, SSC number, age, and gender as well as the extents of salivary chromium release, designed the study, specified and implemented the statistical analyses, interpreted and discussed the findings, and drafted/revised the article.
Corresponding author
Ethics declarations
Protocol ethics were approved by the institutional review board of the university according to the Helsinki declaration (ethical code: IR.AJUMS.REC.1395.11).
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Basir, L., Meshki, R., Behbudi, A. et al. Effects of Restoring the Primary Dentition with Stainless-Steel Crowns on Children’s Salivary Nickel and Chromium Levels, and the Associations with Saliva pH: a Preliminary Before-After Clinical Trial. Biol Trace Elem Res 187, 65–73 (2019). https://doi.org/10.1007/s12011-018-1376-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1376-0