Abstract
Chromium(III) is considered as an essential element for carbohydrate and lipid metabolism. The aim of this clinical study was to evaluate the efficacy of Cr brewer’s yeast supplementation on body mass, carbohydrate, lipids and mineral indices in type 2 diabetic patients. Twenty adult type 2 diabetic subjects (11 males and 9 females aged 37–63) were supplemented with Cr brewer’s yeast in dosages of 500 μg Cr/person/day or placebo for 8 weeks in a double-blind, placebo-controlled crossover design. It was found that supplemental Cr did not affect body mass, blood lipid profile, resistin levels, and the serum and hair Zn, Fe, and Cu levels, but increased serum Cr (by 116%) and hair Cr (by 20.6%) concentrations and improved some blood carbohydrate indices (significant increase in the β cell function index by 18.8%) in type 2 diabetic patients. In conclusion, Cr brewer’s yeast has a weak hypoglycemic potential, but does not affect body mass, blood biochemical profile, and microelement levels in type 2 diabetic subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromium(III) has a documented role in carbohydrate, lipid, and protein metabolism [1]; however, the mechanisms of its action on the molecular level are not fully understood. Chromium is an integral part of chromodulin, the oligopeptide that plays a key role in the activation of tyrosine kinase and inhibition of phosphotyrosine phosphatase of the insulin receptor [2–4]. Recently, Penumathsa et al. [5] reported that chromium(III) supplementation increased the phosphorylation of AMP-activated protein kinase, endothelial nitric oxide synthase, and facilitated the translocation of GLUT4 to the cell membrane through regulation of caveolins in streptozotocin-induced diabetic rats.
Some reports [6, 7] showed that increased chromium losses are positively correlated with diabetes, infection, and aging. Davies et al. [6] and Morris et al. [7] reported that age-related decreases of chromium levels may increase the risk of development of impaired glucose metabolism, coronary heart disease, arteriosclerosis, and type 2 diabetes mellitus. Positive correlations were also found between low serum, hair and toenail chromium levels, and type 2 diabetes [7–10].
A classical example of the relationship between Cr(III) intakes and diabetes symptoms was provided by Jeejeebhoy et al. [11], who reported that a white female, receiving total parenteral nutrition for more than 5 years, developed unexpected 15% weight loss, impaired glucose utilization together with peripheral neuropathy after 3 1/2 years of the regimen. These symptoms were further reversed after an addition of 250 μg Cr to the total parenteral nutrition infusate.
Dietary chromium allowances have been a matter of considerable debate over the last decades. In 2001, the Food and Nutrition Board of the US National Academy of Science established an Adequate Intake of chromium at 25–35 μg/day for adult women and men, respectively [12]. Dietary chromium intakes have been decreasing due to the increasing consumption of processed foods, sugar-rich foods, and refined grain products. Some researchers believe that chromium(III) intakes may be suboptimal, which may lead to the development of diabetes and other complications; however, this hypothesis has been criticized [1].
Some clinical studies showed that pharmacological dosages of chromium(III) may be beneficial in the management of type 2 diabetes [13–22]. The efficacy of Cr(III) supplementation depends on various factors, such as the dosage, the chemical form of Cr(III) and its bioavailability, the duration of treatment, individual Cr status, and the degree of glucose intolerance.
The objective of this clinical study was to examine the efficacy of Cr(III) supplementation administered as Cr-enriched brewer’s yeasts (500 μg/day, for 8 weeks) in managing diabetes, particularly in terms of body mass, blood biochemical profile, and blood and hair concentrations of minerals (Fe, Zn, Cu, and Cr) in type 2 diabetes patients.
Material and Methods
Patients and Study Design
Subjects with recognized type 2 diabetes mellitus without serious complications, such as retinopathy and nephropathy, were enrolled in the study. They were recruited from the Poznan Diabetic Center and from the Metabolic Disorders and Hypertension Clinic. The exclusion criteria were: pregnancy or breastfeeding, vitamin–mineral supplementation in the last 3 months, thyroid hormone, estrogen, progesterone, and diuretic therapies, and alcohol and smoking addiction. The baseline characteristics of patients are presented in Table 1.
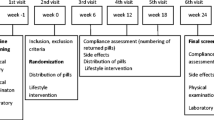
The original study protocol was approved by the Human Subjects Oversight Committee, University of Medical Sciences, Poznan (Approval No. 386/2005). The study was conducted in full conformance with the principles of the declaration of Helsinki. Written informed consent was obtained from all participants prior to entry. The study was performed according to the double-blind, crossover, randomized, placebo-controlled procedure (Scheme 1). Consenting subjects were randomly assigned to two groups receiving chromium or placebo followed by placebo or chromium for 8 weeks (with intermediate 12-week “washout” period between these treatments). The randomization list was held by the principal investigator and was available in case of emergency. The list was first opened once all the samples had been analyzed.
The Cr(III) supplement was given as Cr-enriched brewer’s yeast—provided as a commercial product “BioChromDIA” in dosages of 500 μg of elemental Cr per person/day (five tablets × 100 μg Cr(III)/tablet/day; two tablets in the morning, two tablets in the afternoon, and one tablet in the evening), while the placebo consisted of taking five placebo tablets (brewer’s yeast matrix) for 8 consecutive weeks. Both products (indistinguishable in size, color, or taste) were supplied by Pharma Nord Company (Vejle, Denmark). The BioChromDIA supplement was prepared using Saccharomyces cerevisiae enriched in chromium [20]. The content of elemental Cr in tablets was assured by the atomic absorption spectrometry method (BioChromDIA contained 100 ± 2 μg Cr/tablet, while placebo, below 1 μg Cr/tablet).
All participants were under medical supervision, regularly checked by their doctors, and were treated with oral hypoglycemic drugs (derivates of sulfonylurea and biguanides). For the purpose of this study, all subjects were asked to maintain their normal dietary habits, lifestyle, and medications during the whole period. Participants were obligated to inform the investigator in case of any medication change. Before and after each clinical phase, patients attended clinic between 8:00 and 10:00 a.m. to collect venous blood (10 ml) and scalp hair samples (approximately 0.2–0.5 g), as well as gather their dietary intake data (24-h dietary recall questionnaire). The amount of minerals in daily food ratios was processed and evaluated by Dietetic computer program. Additionally, participants were weighed in light clothes without shoes.
During two sequences of experiment, eight patients withdrew from this study. The decision of resignation was made due to personal reasons. During the experimental period in the chromium group, three persons reported subjective side effects, such as skin rash (one person), decreasing appetite (one person), and constipation (one person). These adverse effects disappeared within 7 days and supplementation followed on. In the placebo group, indigestion, deceased appetite, and frequent urination were reported by three persons.
Compliance was assessed by counting the remaining tablets and checking daily diary at monthly follow-up visits.
Methods
Blood Biochemical Parameters
Plasma fasting glucose concentration was determined by hexokinase method [23].
Plasma lipids profile (total, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, and triglyceride concentrations) were determined by standard colorimetric methods [24–26] using Olympus AU560 analyzer.
Glycated hemoglobin (HbA1C) concentration was measured by high pressure liquid chromatography (Variant; Bio-Rad) [27].
Plasma insulin concentration was determined using Microparticle Enzyme Immunoassay (IMX, Abbott Laboratories) [28], while serum resistin was determined by ELISA kit (Phoenix Pharmaceuticals, USA).
The efficacy of glucose utilization was characterized by insulin resistance and β cell function indices calculated according to the formulas of the homeostasis model (HOMA) [29]:
Trace Elements in Sera and Hair Samples
Blood sera were frozen and stored at −20°C until analyzed. For mineral analysis, 1 ml of sera, after being thawed, was transferred to plastic metal-free Eppendorf test tubes and dried overnight in 105°C using thermoblock (Thermoblock TB-941U, JWElectronic). Further, dried sera samples were treated with 0.5 ml of 65% nitric acid (Suprapur, Merck) and mineralized by gradually increasing the temperature from 50°C to 105°C until a dry residue was obtained. The digested samples were subsequently treated with 0.1 ml of nitric acid (Suprapur, Merck) and 0.9 ml of deionized water to obtain mineral solution.
The contents of Fe, Zn, Cu, and Cr in mineralized solutions were determined by the graphite furnace atomic absorption spectrometry method (AAS-5 EA, Jenoptic, Germany).
Scalp hair samples were cut from six places in the occipital region of the head. Hair samples were washed (deionized water, acetone, deionized water) and dried until a constant mass at 105°C according to the procedure advised by IAEA was obtained [30].
Hair samples (0.2 g) were subsequently transferred to the PTFE digestion vessels and treated with 5 ml of 65% nitric acid (Suprapur, Merck), then digested in the MW oven (Mars-5, CEM). After cooling to room temperature, samples were transferred to the volumetric flasks (10 ml) with deionized water and then diluted according to the analytical requirements for a given element.
The contents of Fe, Zn, and Cu in mineralized hair samples were determined by the flame atomic absorption spectrometry method (spectrometer AAS-3 with BC, Carl-Zeiss-Jena, Germany), while the content of Cr was determined using the graphite furnace atomic absorption spectrometry method (AAS-5 EA with BC, Jenoptic, Germany).
The accuracy of the mineral determinations was assured using two certified reference materials: Pig Kidney BCR 186 (Brussels) and Human serum HN2612 (Randox, UK). The recoveries for serum and hair Fe, Zn, Cu, and Cr (expressed as percent of the mean certified values) were 104%, 103%, 101% in serum and 106%, 97%, 93%, 102% in hair, respectively. In the case of serum Cr (not certified), internal standard addition method was applied, with a mean recovery of 103%.
Statistical Analyses
For statistical analyses, only data obtained from subjects who completed both supplementation phases were used. All data were presented as mean, standard deviation, and median values. Changes between baseline and endpoint in each parameter (95% confidence interval) were compared by the Wilcoxon test, at significance level α = 0.05. All statistical analyses were performed using Statistica version 7.0 software (Statsoft Inc. Tulsa, USA).
Results
In this study, the compliance rate was 98%. The baseline characteristics of participants are presented in Table 1. As can be seen, type 2 diabetes mellitus subjects were obese or overweight (BMI, 35.3 ± 9.2 kg/m2). The glycemic status assessed on the basis of glycated hemoglobin (HbA1C) concentration was above the recommended limit (>7%) for type 2 diabetics.
The baseline and endpoints of somatic and blood biochemical indices are presented in Table 2. During the course of the study, the body mass did not change significantly in either of the treatment phases.
After 8 weeks of treatment with 500 μg Cr/day, plasma glucose concentration slightly decreased by 0.82 mmol/l (8%, p = 0.08) in comparison to the baseline value, while after the placebo phase, such changes were not observed. Plasma insulin level slightly increased after Cr(III) supplementation by 4.76 mIU/l whereas it slightly decreased by 1.68 mIU/l after the placebo phase; however, these changes were not statistically different. Also, no statistically significant differences were observed in the insulin resistance index (HOMA-IR) after Cr(III) or placebo phases. However, in contrast to placebo, Cr(III) treatment significantly increased the HOMA-B index (biomarker of the β-cell functions) by 9%. Although the glycated hemoglobin concentrations slightly decreased in both phases, this trend was stronger after the Cr(III) phase (Δ = −0.53%) as compared to the placebo phase (Δ = −0.28%).
Plasma lipid profile (total, LDL and HDL cholesterol, and triglyceride concentrations) as well as serum resistin remained unchanged after the Cr(III) and placebo phases.
The effects of Cr(III) and placebo treatment on serum and hair mineral concentrations are shown in Table 3. As can be seen, supplemental Cr(III), in contrast to placebo, significantly increased serum and hair Cr concentrations (by 116% and 20.6%, respectively). On the other hand, neither Cr(III) nor placebo affected serum and hair Fe, Zn, and Cu levels in type 2 diabetic patients. In order to assess individual dietary Fe, Zn, Cu, and Cr intakes, the 24-h dietary recall was performed and the results are presented in Table 4. No significant differences in the dietary mineral intakes during Cr(III) and placebo phases were noticed.
Discussion
It is generally accepted that chromium(III) supplementation is not necessary in healthy individuals not considered to be chromium-deficient. Also, the therapeutic potential of chromium compounds in the treatment of diabetes has not been sufficiently proven [13–22, 31–33]. For these reasons, the American Diabetes Association does not recommend chromium supplementation in individuals with diabetes or obesity [34]. Also, the European Association for the Study of Diabetes does not recommend chromium supplementation as a standard treatment of diabetes [35]. Nevertheless, the chromium supplement market is growing rapidly worldwide and chromium compounds are advertised as adjuvant agents for diabetics, obesity, and appetite control.
Chromium supplementation improved at least one outcome of glycemic control in 13 out of 15 studies [36]. In several earlier studies [13, 14], it was found that supplementation of relatively high dosages of Cr picolinate (CrPic) (1,000 μg Cr/day) decreased postprandial blood glucose, HbA1c, serum insulin, and cholesterol levels as well as insulin sensitivity in Chinese and US subjects. Similar results were also observed in further studies performed by Albarracin et al. [21] and Lai [37], in which Cr(III) compounds (Cr picolinate (CrPic) and Cr yeast) were given in dosages of 600 and 1,000 μg Cr/day to US and Taiwanese type 2 diabetes mellitus (2DM) patients, respectively.
As it was mentioned above, the efficacy of Cr(III) supplementation depends also on the chemical form of Cr(III) and its bioavailability. The efficacy of chromium-enriched brewer’s yeast supplementation in the regulation of carbohydrate metabolism was examined in 10 clinical trials [36]. For example, in the Racek et al. [20] double-blind, placebo-controlled study, the application of Cr-enriched yeast (BioChromDIA, 400 μg Cr/person/day) for 12 weeks in 2DM Czech patients significantly reduced serum glucose and insulin levels, without changes in blood glycated proteins and lipid parameters. In our study conducted according to a similar clinical design, higher dosages of the same compound (500 μg Cr/person/day, BioChromDIA, PharmaNord), but given in a shorter period of time (8 weeks), were not able to improve significantly blood glucose and lipid indices. One of the reasons of the negative effect may be the difference in the total dosage of Cr(III) ingested (28 mg Cr/person in this study vs. 33.6 mg Cr/person in the study by Racek et al.).
Also in this study, like in other clinical trials with diabetics, Cr(III) did not improve significantly blood biochemistry indices [13, 17, 21, 31–33].
The mechanisms and factors determining Cr(III) action or non-action are not fully understood. Wang et al. [38] suggested that the subject’s phenotype, which determines the degree of insulin resistance, may play an important role in the response to Cr(III) [38]. Kleefstra et al. [33] were of an opinion that Western diabetic patients, in comparison to Chinese subjects, may require higher quantities of Cr(III), or may need it for a longer period of time [33]. Pattar et al. [39] suggested that the positive response to Cr(III) supplementation is possible when the combined glucose and cholesterol homeostasis is disrupted. Chen et al. [40] found that the action of Cr(III) is not mediated by insulin-related factors, such as the insulin receptor, insulin receptor substrate-1, phosphatidylinositol 3-kinase, and Akt. In another study of these authors [39], supplemental CrPic upregulated the membrane-bound transcription factors responsible for controlling cellular cholesterol balance.
A significant role of adipose-derived hormones in insulin-resistance progression has been suggested. One of these hormones is resistin, often linked to insulin resistance, obesity, and type 2 diabetes [41]. Resistin is involved in the control of the hypothalamic and peripheral lipid metabolism and regulation of food intake [42]. Wang et al. [43] studied the effects of CrPic on gene transcription and resistin and adiponectin secretion using 3T3-L1 adipocytes in vitro. Although CrPic (10 nmol/l) did not affect the expression of either of those hormones, it significantly inhibited the secretion of resistin by normal and insulin-resistant 3T3-L1 adipocytes. In this clinical study, no significant changes in resistin concentrations after Cr(III) supplementation were observed.
Insulin resistance and type 2 diabetes are associated with obesity, thus Cr(III) supplements are advertised due to the expected beneficial role in appetite regulation and body mass control. To our knowledge, Anton et al. [22] reported that supplementation with CrPic in dosages of 1,000 μg Cr/day for 8 weeks, as compared to placebo, reduced food intake, hunger levels, fat cravings, and slightly decreased body mass in overweight adult women. However, most studies published so far [14, 15, 17, 33, 37] did not confirm such effects. Also, in this study, no significant changes were found in body mass after Cr(III) supplementation.
Another aspect that should be taken into account during Cr(III) supplementation is its safety and possibility of interactions with other minerals, especially iron [44, 45]. In the study performed by Campbell et al. [46], Cr(III) supplementation (given as CrPic in dosages of 1,000 μg Cr/day for 13 weeks) did not affect hematological indices as well as serum iron, ferritin, TIBC, and transferrin saturation levels in moderately obese men. Also, a lack of a negative impact on iron metabolism was observed in women [47, 48].
Information on the interaction of Cr(III) with Cu and Zn is limited. In animal studies, Cr(III) supplementation decreased Cu, while increased Zn contents in the liver and kidneys of fat-fed and streptozotocin-treated type II diabetic rats were found [49]. In human subjects, Anderson et al. [16] did not find changes in Zn and Cu indices in type 2 diabetics after a 6-month supplementation with 400 μg Cr/day in the form of Cr(III) pidolate. Also, Volpe et al. [48] did not observe changes in serum Zn and Fe levels after a 12-week supplementation with Cr(III) in moderately obese women.
In this study, 8-week supplementation with Cr brewer’s yeast did not influence serum and hair Fe, Zn, and Cu concentrations in type 2 diabetic subjects.
There is no reliable biomarker of the Cr status. The concentration of this element in the body fluids is very low, often at the threshold of the detection limits of analytical methods [1]. A tentative assessment of the Cr status is made on the basis of its concentrations in blood, plasma, serum, urine, or hair. In this study, serum and hair Cr contents in diabetic subjects after the Cr(III) phase increased significantly, which indicate that these markers, to some extent, can reflect the Cr status; however, more sensitive biomarkers are warranted.
In conclusion, the results of this study showed that supplemental chromium given as Cr-enriched brewer’s yeast (in dosages of 500 μg/day for 8 weeks) has a weak antidiabetic potential, does not affect body mass, blood biochemistry, or mineral levels in type 2 diabetic patients.
References
Stallings D, Vincent JB (2006) Chromium. A case study in how not to perform nutritional research. Curr Topics Nutraceut Res 4:89–112
Davis CM, Vincent JB (1997) Chromium oligopeptide activates insulin receptor tyrosine kinase activity. Biochemistry 36:4382–4385
Davis CM, Sumrall KH, Vincent JB (1996) A biologically active form of chromium may activate a membrane phosphotyrosine phosphatase (PTP). Biochemistry 35:12963–12969
Vincent JB (2000) Elucidating a biological role for chromium at a molecular level. Acc Chem Res 37:503–510
Penumathsa SV, Thirunavukkarasu M, Samuel SM et al (2009) Niacin bound chromium treatment induces myocardial Glut-4 translocation and caveolar interaction via Akt, AMPK and eNOS phosphorylation in streptozotocin induced diabetic rats after ischemia-reperfusion injury. Biochim Biophys Acta 1792:39–48
Davies S, Howard JM, Hinnisett A et al (1997) Age-related decreases in chromium levels in 51, 665 hair, sweat, and serum samples from 40,872 patients: implications for the prevention of cardiovascular disease and type II diabetes mellitus. Metabolism 46:469–473
Morris BW, MacNeil S, Hardisty CA (1999) Chromium homeostasis in patients with type II (NIDDM) diabetes. J Trace Elem Med Biol 13:57–61
Kazi TG, Afridi HI, Kazi N et al (2008) Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol Trace Elem Res 122:1–18
Rajpathak S, Rimm EB, Li T et al (2004) Lower toenail chromium in men with diabetes and cardiovascular disease compared with healthy men. Diab Care 27:2211–2216
Stupar J, Vrtovec M, Dolinek F (2007) Longitudial hair chromium profiles of eldery subjects with normal glucose tolerance and type 2 diabetes mellitus. Metab Clin Exp 56:94–104
Jeejeebhoy KN, Chu RC, Marliss EB et al (1977) Chromium deficiency, glucose intolerance, and neuropathy reversed by chromium supplementation in a patient receiving long-term total parenteral nutrition. Am J Clin Nutr 30:531–538
(2001) Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, jodinc, iron, manganese, molybdenum, nickel, silicon, vanadium: chromium. National Academy Press, Washington, DC, pp 197–223
Anderson RA, Cheng N, Bryden NA et al (1997) Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes 46:1786–1791
Cefalu WT, Bell-Farrow AD, Stegner J et al (1999) Effect of chromium picolinate in insulin sensitivity in vivo. J Trace Elem Exp Med 12:71–83
Bahijiri SM, Mira SA, Mufti AM et al (2000) The effect of inorganic chromium and brewer’s yeast supplementation on glucose tolerance, serum lipids and drug dosage in individuals with type 2 diabetes. Saudi Med J 21:831–837
Anderson RA, Roussel A-M, Zouari N et al (2001) Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. J Am Coll Nutr 20:212–218
Gosh D, Bhattacharyaa B, Mukherjeeb B et al (2002) Role of chromium supplementation in Indians with type 2 diabetes mellitus. J Nutr Biochem 3:690–697
Martin J, Zhang XH, Zhong Q et al (2006) Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes. Diab Care 29:1826–1832
Pei D, Hsieh CH, Hung YJ et al (2006) The influence of chromium chloride-containing milk to glycemic control of patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Metabolism 55:923–927
Racek J, Trefil L, Rajdl D et al (2006) Influence of chromium-enriched yeast on blood glucose and insulin variables, blood lipids, and markers of oxidative stress in subjects with type 2 diabetes mellitus. Biol Trace Elem Res 109:215–230
Albarracin C, Fuqua B, Evans JL et al (2008) Chromium picolinate and biotin combination improves glucose metabolism in treated, uncontrolled overweight to obese patients with type 2 diabetes. Diabetes Metab Res Rev 24:41–51
Anton SD, Morrison CD, Cefalu WT et al (2008) Effects of chromium picolinate on food intake and satiety. Diabetes Technol Ther 10:405–412
Sacks DB, Bruns DE, Goldstein DE et al (2002) Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 48:436–472
Shephard MD, Whiting MJ (1990) Falsely low estimation of triglycerides in lipemic plasma by the enzymatic triglyceride method with modified Trinder’s chromogen. Clin Chem 36:325–329
Riesen WF (1998) Lipid metabolism. In: Thomas L (ed) Clinical laboratory diagnostics. Use and assessment of clinical laboratory results. TH-Books Verlagssesellschaft, Frankfurt (Main), pp 167–169
Miki Y (1999) A homogenous assay for the selective measurement of LDL-cholesterol in serum. Enzymatic selective protection method. Clin Lab 45:398–401
Berg AH, Sacks DB (2008) Haemoglobin A1c analysis in the management of patients with diabetes: from chaos to harmony. J Clin Pathol 61:983–987
Monti LD, Sandoli EP, Phan VC et al (1995) A sensitive and reliable method for assaying true human insulin without interaction with human proinsulin-like molecules. Acta Diabetol 32:57–63
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
International Atomic Energy Agency Reports 1984, 1985, 1987, Vienna, Coordinated research programme on the significance of hair metal analysis as a means for assessing internal body burdens of environmental exposure
Gunton JE, Cheung NW, Hitchman R et al (2005) Chromium supplementation does not improve glucose tolerance, insulin sensitivity, or lipid profile. Diab Care 28:712–714
Iqbal N, Cardillo S, Volger S et al (2009) Chromium picolinate does not improve key features of metabolic syndrome in obese nondiabetic adults. Metab Syndr Relat Disord 7:143–150
Kleefstra N, Houweling S, Jansman FGA et al (2006) Chromium treatment has no effect in patients with poorly controlled, insulin-treated type 2 diabetes in an obese Western population. Diab Care 29:321–325
American Diabetes Association (2008) A position statement of the American Diabetes Association. Nutrition recommendations and ınterventions for diabetes. Diabetes Care 31:61–78
Mann JI, De Leeuw I, Hermansen K, Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD) et al (2004) Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr Metab Cardiovasc Dis 14:373–394
Balk EM, Tatsioni A, Lichtenstein AH et al (2007) Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diab Care 30:2154–2163
Lai MH (2008) Antioxidant effects and insulin resistance improvement of chromium combined with vitamin C and supplementation for type 2 diabetes mellitus. J Clin Biochem Nutr 43:191–198
Wang ZQ, Qin J, Martin J et al (2007) Phenotype of subjects with type 2 diabetes mellitus may determine clinical response to chromium supplementation. Metab Clin Exp 56:1652–1655
Pattar GR, Tackett L, Liu P et al (2006) Chromium picolinate positively influences the glucose transporter system via affecting cholesterol homeostasis in adipocytes cultured under hyperglycemic diabetic conditions. Mutat Res 610:93–100
Chen G, Liu P, Pattar GR (2006) Chromium activates glucose transporter 4 trafficking and enhances insulin-stimulated glucose transport in 3T3-L1 adipocytes via a cholesterol-dependent mechanism. Mol Endocrinol 20:857–870
Osawa H, Ochi M, Tabara Y et al (2008) Serum resistin is positively correlated with the accumulation of metabolic syndrome factors in type 2 diabetes. Clin Endocrinol (Oxf) 69:74–80
Chen BH, Song Y, Ding EL (2009) Circulating levels of resistin and risk of type 2 diabetes in men and women: results from two prospective cohorts. Diab Care 32:329–334
Wang YQ, Dong Y, Yao MH (2009) Chromium picolinate inhibits resistin secretion in insulin-resistant 3T3-L1 adipocytes via activation of AMP-activated protein kinase. Clin Exp Pharmacol Physiol 36:843–849
Clodfelder BJ, Emamaullee J, Hepburn DD et al (2001) The trail of chromium(III) in vivo from the blood to the urine: the roles of transferrin and chromodulin. J Biol Inorg Chem 6:608–617
Lukaski HC, Siders WA, Penland JG (2007) Chromium picolinate supplementation in women: effect on body weight, composition and iron status. Nutrition 23:187–195
Campbell WW, Joseph LJ, Davey SL (1999) Effects of resistance training and chromium picolinate on body composition and skeletal muscle in older men. J Appl Physiol 86:29–39
Campbell WW, Joseph LJ, Anderson RA (2002) Effects of resistive training and chromium picolinate on body composition and skeletal muscle size in older women. Int J Sport Nutr Exerc Metab 12:125–135
Volpe SL, Huang HW, Larpadisorn K (2001) Effect of chromium supplementation and exercise on body composition, resting metabolic rate and selected biochemical parameters in moderately obese women following an exercise program. J Am Coll Nutr 20:293–306
Dogukan A, Sahin N, Tuzcu M et al (2009) The effects of chromium histidinate on mineral status of serum and tissue in fat-fed and streptozotocin-treated type II diabetic rats. Biol Trace Elem Res 131:124–132
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Król, E., Krejpcio, Z., Byks, H. et al. Effects of Chromium Brewer’s Yeast Supplementation on Body Mass, Blood Carbohydrates, and Lipids and Minerals in Type 2 Diabetic Patients. Biol Trace Elem Res 143, 726–737 (2011). https://doi.org/10.1007/s12011-010-8917-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-010-8917-5