Abstract
Purpose
This randomised controlled trial assessed the acute and long-term effects of daily supplementation of kanuka honey, formulated with cinnamon, chromium and magnesium on glucose metabolism, weight and lipid parameters in individuals with type 2 diabetes.
Methods
Twelve individuals with type 2 diabetes received 53.5 g of a formulated honey and a control (non-formulated) kanuka honey in a random order for 40 days, using cross-over design. Fasting glucose, insulin, HbA1c, lipids and anthropometric measures were measured at baseline and end of treatment. A meal tolerance test was performed at baseline to assess acute metabolic response.
Results
There was no statistically significant difference in acute glucose metabolism between treatment groups, as measured by the Matsuda index and AUC for glucose and insulin. After the 40-day intervention with honey, fasting glucose did not differ significantly between the two treatments (95 % CI −2.6 to 0.07). There was no statistically significant change in HbA1c or fasting insulin. There was a statistically significant reduction in total cholesterol by −0.29 mmol/L (95 % CI −0.57 to −0.23), LDL cholesterol by −0.29 mmol/L (95 % CI −0.57 to −0.23) and weight by −2.2 kg (95 % CI −4.2 to −0.1). There was a trend towards increased HDL and reduced systolic blood pressure in the intervention treatment.
Conclusion
The addition of cinnamon, chromium and magnesium supplementation to kanuka honey was not associated with a significant improvement in glucose metabolism or glycaemic control in individuals with type 2 diabetes. Use of the formulated honey was associated with a reduction in weight and improvements in lipid parameters, and should be investigated further.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last two decades, there has been increasing focus on the use of complementary therapies in the management of type 2 diabetes (T2DM). Honey, in particular, has been extensively investigated. Honey has long been acknowledged as having useful medicinal purposes, with descriptions of its healing powers in the Holy Qur’an [1]. In more recent times, honey has been recognised as a useful ‘sugar substitute’ in patients with both type 1 and type 2 diabetes, with slower absorption and less plasma glucose excursions [2–6]. This may be due to the unique composition of honey, with high concentrations of fructose and various oligosaccharides, resulting in a low glycaemic index (GI). There may also be prebiotic or antioxidant properties influencing glucose metabolism [4, 7].
Other natural substances have been found to have beneficial effects on glucose metabolism in animal and human studies. In particular, recent meta-analyses have analysed the effects of supplementation of cinnamon, chromium and magnesium in individuals with T2DM, and have shown potential improvements in insulin sensitivity and glycaemic control [8–11].
Taking these findings into account, a kanuka honey has been formulated, with the addition of cinnamon, chromium and magnesium, to create a honey dietary supplement which may deliver positive effects on blood glucose and insulin levels. This study aimed to determine the acute and longer-term effects of daily supplementation of three tablespoons (53.5 g) of this formulated kanuka honey when compared with regular kanuka honey over a 40-day period, on glucose metabolism, glycaemic control and lipids in men and women with T2DM.
Methods
This study was an open-label, randomised cross-over trial comparing the impact of regular kanuka honey and formulated kanuka honey on glucose metabolism, glycaemic control and lipid levels in patients with T2DM. The study was approved by the Ministry of Health Central Regional Ethics Committee (13/CEN/91) and was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12613000624785).
Inclusion criteria included participants with T2DM, treated with metformin or diet alone. Participants using sulphonylureas or insulin were excluded, due to the impact of these medications on insulin measurements, as were participants treated with other medications which could affect glucose tolerance, such as oral glucocorticoids. Participants with suboptimal glycaemic control (HbA1c > 80 mmol/mol or 9.5 %) were excluded, due to the potential risk of worsening hyperglycaemia with administration of honey products. Participants taking supplements containing cinnamon, chromium or magnesium were not excluded from the study; however, a washout period of 20 days was required prior to commencement of the first treatment arm. Participants were recruited through attendance at a secondary care diabetes clinic, or via advertisement.
The study was divided into two treatments—a control treatment, consisting of regular kanuka honey, and an intervention treatment, consisting of 53.5 g of a formulated honey, comprised of 4.5 g food grade cinnamon, 200 µg chromium polynicotinate and 120 mg magnesium citrate mixed with 100 % kanuka honey. Participants received both control and intervention treatments in a random order, using computer-generated randomisation. Due to the colour, aroma and taste of the cinnamon, it was not possible to blind study participants to the honey type; however, investigators remained blinded throughout.
The study design allowed for the assessment of the acute effects and impact of longer-term supplementation of the treatment honeys.
Acute treatment
At baseline, participants attended the Centre for Endocrine Diabetes and Obesity Research Wellington (CEDOR Wellington) in a fasted state, after withholding their morning oral hypoglycaemic agents. Blood samples were taken at time 0 for fasting insulin and glucose, as well as HbA1c and lipids. To assess the acute effects of the honey treatments on glucose metabolism in the immediate postprandial phase, a meal tolerance test was performed after ingestion of 53.5 g of the randomised honey on two pieces of wholemeal bread (70.4 g of carbohydrate in total). After insertion of an intravenous cannula, blood samples were taken at 30-min intervals for 120 min (a total of five samples), for glucose and insulin measurements, to allow calculation of an integrated measure of insulin sensitivity using the same formula as the Matsuda index derived from an oral glucose tolerance test [12]. This formula is shown as:
where g represents the plasma glucose concentration at the different time points and i represents the plasma insulin concentration at the different time points. The areas under the curve (AUC) for insulin and glucose during the meal test were also calculated using the trapezoidal rule.
Longer-term treatment
At a baseline visit, after completion of the meal tolerance test, subjects received a supply of honey to take home. They were instructed to consume 53.5 g (three tablespoons) of this honey each day for 40 days, measured in individual containers. Subjects were able to include the honey in their diet as per individual preferences. Subjects returned for assessment at day 40. At this visit, all containers of honey were returned and assessed for quantity of honey remaining, as a marker of compliance.
At baseline and at day 40, blood tests to measure fasting insulin and glucose, HbA1c and lipids were performed. Anthropometric measures of blood pressure, heart rate and weight were collected at each visit. Subjects were required to complete a food diary of the 3 days prior each visit (baseline and day 40). Three-day food diaries were analysed using the FoodWorks7 programme (Xyris Software, Australia), providing a breakdown of dietary intake parameters.
After completion of the first 40-day treatment, there was a 20-day washout period before commencement of the second.
Blood samples were batched and processed at an accredited laboratory (Diabetes and Lipid Laboratory, University of Otago, Dunedin) using standard commercial laboratory assays (Roche Diagnostics).
Statistical analysis
A sample size of 12 participants had 80 % power, with type 1 error of 5 %, to detect a paired difference in fasting glucose of 1.6 mmol/L (similar to that seen in a study by Khan et al. [13]), with an assumed standard deviation of 1.8.
Statistical analysis was performed using a mixed linear model with order of treatment, baseline value of the variable and randomised treatment as fixed effects, and the particular participant as a random effect, to enable the cross-over design to be taken into account. SAS version 9.3 was used for this analysis.
Results
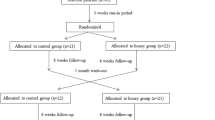
Thirty individuals were assessed for eligibility and 12 were recruited to the study (Fig. 1). Baseline characteristics of participants are shown in Table 1. Participants were predominantly of New Zealand European ethnicity with a mean age of 61.7 years (range 51.2–68.1). Mean duration of T2DM was 5.4 years (range 1–13.9). All participants were taking metformin.
Mean compliance with honey supplementation was 85 % for the control honey and 86 % for the intervention honey. No participants were lost to follow-up; however, one participant declined the second treatment with honey due to deterioration in glycaemic control during the first treatment. This participant underwent full testing as per protocol, with results analysed on an intention to treat basis.
Dietary intake as derived from 3-day food diaries at commencement and completion of each treatment is shown in Table 2. There were no significant differences between study treatments for any dietary parameter; however, the increase in total sugar intake after 40 days of honey treatment neared significance for participants for both the control treatment (p = 0.09) and intervention treatment (p = 0.08).
Acute treatment
The Matsuda index of insulin sensitivity was calculated for each participant at the commencement of each treatment, from the insulin and glucose measurements during each meal tolerance test (Table 3). There was no difference between each honey preparation with a mean (SD) Matsuda index of 2.87 (1.72) for the control honey and 2.75 (2.05) for the intervention honey. After adjusting for baseline, the Matsuda index was 0.12 (95 % CI −0.95 to 0.71, p = 0.76) lower with the intervention treatment. The AUC of glucose and insulin were also calculated from the meal tolerance test results (Table 3). There was no statistically significant difference between treatment arms for AUC glucose, at 0.7 mmol/L/120 min (95 % CI −0.9 to 2.3; p = 0.38), or for AUC insulin, at −7.41 µU/mL/120 min (95 % CI −16.7 to 1.8, p = 0.11).
Longer-term treatment
Table 4 demonstrates fasting glucose and insulin results at the commencement and completion of both treatments for all 12 participants. Using a mixed linear model, after adjusting for baseline, fasting glucose did not differ significantly after 40 days between the two treatments (95 % CI −2.6 to 0.07, p = 0.06). Neither fasting insulin nor HbA1c differed significantly after 40 days between the two honey treatments (95 % CI −4.3 to 6.5, p = 0.67 and 95 % CI −6.0 to 0.7, p = 0.11, respectively). After consumption of the formulated honey, there was a reduction in total cholesterol by −0.37 mmol/L (95 % CI −0.073 to −0.008, p = 0.046) and LDL cholesterol by −0.29 mmol/L (95 % CI −0.57 to −0.23, p = 0.039) compared to the control honey (Table 4). There was a trend towards increased HDL in the intervention treatment; however, this did not reach statistical significance.
After adjusting for baseline, there was a statistically significant reduction in weight of 2.2 kg (95 % CI −4.2 to −0.1, p = 0.039) in the intervention treatment. The point estimate for systolic blood pressure favoured the intervention treatment with a reduction in blood pressure of −10 mmHg (95 % CI −22.1 to 2.0, p = 0.09); however, the confidence intervals were consistent with no difference.
Discussion
In this study, no beneficial effects of the use of kanuka honey formulated with cinnamon, chromium and magnesium were found on immediate glucose metabolism or on fasting plasma glucose, fasting insulin or HbA1c after 40 days of daily supplementation, although changes in fasting plasma glucose did near statistical significance. Statistically significant reductions in total body weight, total cholesterol and LDL were found in participants supplemented with the formulated honey, and weak evidence of a reduction in systolic blood pressure and increased HDL was also noted; however, these did not reach statistical significance.
Acute glucose metabolism
Assessment of the acute effects of the consumption of the formulated honey on immediate glucose metabolism was assessed in this study by measuring the changes in glucose and insulin over 2 h after ingestion of each of the honey preparations. No statistically significant difference was seen in either the AUC glucose or insulin, or using an integrated measure of both. Whilst several studies have assessed changes in glycaemic control in patients after supplementation of chromium, cinnamon and magnesium, fewer have examined the impact of these supplements on glucose and insulin in the acute setting as in our present study. Only one RCT has demonstrated a statistically significant reduction in post-glucose load glycaemia in patients supplemented with chromium [14], whilst others were unable to find a statistically significant difference in AUC glucose [15–20]. Magistrelli et al. [21] demonstrated a reduction in AUC glucose (p = 0.008) after ingestion of 6 g of ground cinnamon during a cereal-based glucose tolerance test. This dose is significantly greater than the current formulated honey and may imply an important dose–response effect. In addition, the impact on acute absorption of adding these supplements to a honey solution is unknown and may have impacted on the findings.
Impact of 40-day honey supplementation on glycaemic control
Neither honey preparation reduced fasting blood glucose or HbA1c, or altered insulin levels significantly after 40 days supplementation, suggesting a lack of benefit of the honey itself. The relatively short study period may have precluded demonstration of any clinically significant change in HbA1c level. Several studies have demonstrated variable effects of honey on fasting plasma glucose levels and HbA1c. In animal studies, there is some evidence that short- and long-term ingestion of honey leads to improvement in plasma glucose levels and HbA1c in diabetic rats in a dose-dependent manner [22, 23]. The administration of honey in addition to glibenclamide or metformin has been shown to significantly reduce serum glucose and fructosamine, and increase insulin in diabetic rats, over and above treatment with these medications alone [5]. Despite this, other studies in rats have failed to show differences in glucose and insulin levels with longer-term intake of either a high honey or high sugar diet [24].
Several small studies in human subjects have looked at the impact of honey on fasting glucose, insulin levels and HbA1c, with mixed results. Bahrami et al. [25] assessed the effect of 8 weeks of honey supplementation compared to usual diet in patients with T2DM, with no difference in fasting blood glucose at the end of the study. This study remains the only study to date to assess HbA1c in participants with T2DM after prolonged honey supplementation. Their results demonstrated a significant increase in HbA1c in the group treated with honey, from a mean HbA1c of 7.1 % to 7.7 % (p < 0.01). In this study, overall dietary intake of sugars was increased in the intervention group which may have attenuated any improvement in fasting blood glucose, and may have contributed to the HbA1c findings. Whilst no increase in HbA1c was noted in the present study, a non-statistically significant increase in dietary sugar intake was noted, which may have reduced the likelihood of improved HbA1c in participants. Indeed, one participant declined the formulated honey due to deterioration in glycaemic control with the control honey.
In a cross-over study of children and adolescents with type 1 diabetes, Abdulrhman et al. [26] assessed the impact of honey versus usual diet on glucose metabolism. Honey significantly reduced fasting glucose and increased both fasting and postprandial C-peptide levels, which is of interest in a type 1 population. A study of healthy subjects showed no reduction in fasting glucose [3], whilst others in overweight and obese patients suggested an improvement of −4.2 % or 0.22 mmol/L (p = 0.042); however, this was unlikely to be clinically significant [27].
A variety of mechanisms have been proposed in the literature to explain potential effects of honey on glycaemic control. Fructose and oligosaccharides may lead to delayed gastric emptying, slower digestion and reduced intestinal absorption of glucose [4, 7]. In addition, there is increasing evidence from animal studies of the impact of gut microbiota on obesity and insulin resistance. Studies in both animals and humans suggest an increase in beneficial gut microorganisms after ingestion of certain oligosaccharides found in honey, suggesting that these components act as prebiotics [7]. Oligosaccharides may also increase glucose-stimulated insulin release from the pancreas and may be associated with increased plasma levels of GLP-1 and peptide YY [4, 7]. Honey is also known to have antioxidant properties, which may lead to improvements in beta cell function and hepatic insulin sensitivity [4].
Several explanations for the lack of improvement in parameters in the present study may be hypothesised. The small increase in dietary sugar intake in both study arms may have attenuated any effect; however, previous studies with positive findings have used greater quantities of honey [26, 27]. In addition, participants were instructed to incorporate the honey into their diet as they saw fit, which may have resulted in inconsistencies in absorption or interactions with other dietary components. Whilst kanuka honey is understood to be chemically similar to the more common manuka honey, GI has not been formally tested and could be different. However, GI and insulinaemic index (II) have been found to strongly correlate with fructose content, making this less likely [28].
Impact of 40-day formulated honey supplementation on glycaemic control
This study also assessed whether the addition of cinnamon, chromium and magnesium to honey had an effect on glucose metabolism. In in vitro and animal models, cinnamon supplementation has insulin-like properties, stimulating glucose uptake into skeletal muscle and adipose tissue, and up-regulating GLUT4 expression. In addition, the polyphenol content has an antioxidant effect [29]. However, the evidence for benefit in man is conflicting. A recent meta-analysis assessed 10 RCTs of cinnamon on glycaemic control in participants with T2DM [9]. There was significant heterogeneity between cinnamon preparations, doses (120 mg–6 g/day) and duration of supplementation (4–18 weeks). A statistically significant mean reduction in fasting plasma glucose of −1.36 mmol/L (95 % CI −2.25 to −0.48 mmol/L) was described; however, no improvement in HbA1c was seen.
Chromium deficiency in the critically unwell has been demonstrated to lead to reversible insulin resistance and diabetes [30]. The effects of supplementation in patients with T2DM who are not deficient in this micronutrient are less well understood; however, it is proposed that chromium supplementation may lead to improved glycaemic control by stimulating insulin receptors, increasing the activity of glucokinase in the liver and enhancing beta-islet function [31, 32]. Two meta-analyses assessing chromium supplementation have yielded variable results. A recent meta-analysis of 7 RCTs assessing the effect of chromium supplementation in patients with T2DM demonstrated a small decrease in fasting plasma glucose of −0.95 mmol/L (95 % CI −1.42 to −0.49 p < 0.0001) [32]. A larger meta-analysis of 41 studies assessing patients with normoglycaemia, impaired fasting glucose and T2DM was performed [10]. A significant reduction in fasting plasma glucose of 1 mmol/L (95 % CI −1.4 to −0.5) in patients with T2DM was demonstrated; however, no change was noted in participants with no diabetes or impaired fasting glucose. Half of the studies included were of poor quality, and there appeared to be a significant variation in effect depending on dose and preparation which could not be further drawn out in the analysis. Meta-analyses assessing the impact of chromium on HbA1c have been conflicting, with one showing no effect and one showing a small reduction of −0.6 % (95 % CI −0.9 to −0.2) [10, 32].
Magnesium is an important co-factor for metabolic reactions, including insulin-dependent activation of tyrosine kinase in the metabolism of glucose [33]. Magnesium supplementation is purported to assist with improved first- and second-phase insulin secretion [34]. A tendency towards lower average serum magnesium levels has been noted in patients with T2DM, compared with healthy controls [33]. A meta-analysis of nine RCTs assessing the use of magnesium supplementation in patients with T2DM demonstrated a small reduction in fasting plasma glucose of −0.56 mmol/L (95 % CI −1.1 to −0.01, p = 0.02) after 4–16 weeks supplementation to treatment; however, no statistically significant effect on HbA1c was seen [11].
Despite the evidence to support the supplementation of these additives in patients with T2DM, there was no benefit of the addition of these to honey in the present study. Benefit of these additives may be variable depending on dose and preparation effects and may not have been optimised in the formulated honey. The effect on absorption and the bioavailability of these additives when prepared in a honey-based solution is currently unknown.
Secondary outcome measures
A statistically significant reduction in LDL cholesterol and a small significant reduction in total cholesterol were noted with the formulated honey, with a trend towards increased HDL cholesterol. The evidence surrounding this remains conflicting. It is purported that cinnamon improves lipid profiles by inhibiting hepatic 3-hydroxy-3-methylglutaryl CoA reductase activity [35]. Cinnamon supplementation has been found to significantly reduce total cholesterol, triglycerides and LDL, and increase HDL in one meta-analysis [9], however not in one other [35]. In RCTs specifically designed to assess the effect of cinnamon on lipids, only one of four showed an improvement in lipid levels [36]. This occurred at high dose of cinnamon supplementation. Two meta-analyses in patients supplemented with chromium were unable to determine a significant effect on lipid profiles, although some small studies do appear to demonstrate significant increase in HDL levels [10, 32]. A meta-analysis of the use of magnesium suggested a small increase in HDL; however, this was not statistically significant [11]. In the present study, it is not possible to determine which active ingredient led to improvements in the lipid profile. Larger studies, looking at this in particular detail, may be of benefit.
In the present study, a statistically significant reduction in weight was noted with use of the formulated honey despite no specific instruction on energy intake. Food diary data did not show any significant difference in energy intake in this group. Accepting the limitations of food diaries, this suggests the possibility that weight loss may have been due to an effect of formulated honey on energy expenditure. There is a lack of evidence in the literature to explain this. Pooled mean data from animal studies have suggested statistically significant weight gain in rats treated with cinnamon, felt secondary to improvement in glycaemic control and therefore improvements in catabolic state [37]. Akilen et al. [38] performed a double-blinded RCT of cinnamon use in patients with T2DM and demonstrated a trend towards lower BMI; however, this did not reach statistical significance. Others have found increased lean mass and decreased body fat in patients with metabolic syndrome who are treated with cinnamon [39]. No statistically significant change in weight has been seen in meta-analyses using chromium or magnesium supplementation in a population with T2DM [11, 32]. One small RCT has shown reduced hunger and reduced food intake in overweight women supplemented with chromium for 8 weeks [40]; however, cinnamon use has been found to not have an effect on satiety [41]. In the present study, it is likely that the food diary data were not sensitive enough to detect small changes in total energy intake from other dietary components. However, more controlled studies using calorimetry may be worthwhile to explore this further.
The impact of the formulated honey treatment on blood pressure is equivocal. Whilst it did not reach statistical significance, there was a trend towards lower blood pressure in concordance with some literature findings. A meta-analysis of the effects of cinnamon supplementation on blood pressure demonstrated significant reductions in both systolic (by 5.39 mmHg, 95 % CI −6.89 to −3.89) and diastolic blood pressure (by 2.6 mmHg, 95 % CI −4.53 to −0.66) [42]. No effect on blood pressure was seen with magnesium supplementation [11] and has not clearly been assessed with chromium supplementation. It is again difficult to determine the significance of the findings in this present study or indeed which active component, if any, is responsible for this trend in blood pressure.
Overall, the positive findings of the present study are in keeping with those described in the literature. Findings regarding improvements in fasting blood glucose and insulin sensitivity are highly variable in the literature, and the present study continues to reflect this. Significant differences between this study and others with regards to dosing regimen, preparation and duration of treatment are likely to have impacted greatly on this. In particular, the properties of kanuka honey as compared to other honey preparations are not fully understood, and the impact of ingesting cinnamon, chromium and magnesium in a honey-based solution is unclear. In addition, ethnic differences cannot be discounted, with many published studies assessing populations of predominantly Middle Eastern and Southern Asian origin.
There were several strengths of this study. The cross-over design allowed participants to act as their own control. The study was adequately powered to observe an effect, and study dropout was low with 100 % follow-up. Whilst one participant did not take the second honey treatment, analysis was completed in an intention-to-treat manner. The use of dietary data illustrated that any effect seen was not secondary to statistically significant changes in dietary intake across the study.
The inability to blind participants to the honey formulations was a significant weakness. The ability for patients to ingest their daily dose of honey in a manner of their choosing also introduced potential heterogeneity in terms of bioavailability of the dose administered, whilst participant compliance may often be difficult to guarantee when interventions are performed in ‘real-life’ settings. In addition, the short duration of the study meant that potential statistically significant changes in HbA1c and anthropometric measures were less likely to become apparent. The multiple statistical analyses also increased the risk of type 1 error.
Conclusion
In conclusion, the addition of cinnamon, chromium and magnesium supplementation to kanuka honey does not appear to have a significant impact on glucose metabolism or glycaemic control in patients with T2DM. There is much inconsistency in the literature as to the relative effects of each of these supplements and the findings of this study further reflect this. There was evidence of a statistically significant change in body weight and improvement in lipid parameters with use of the formulated honey, and a larger trial with a longer duration of supplementation is required to further elucidate this. On the basis of this study, there is no evidence to support the supplementation of kanuka honey or additional formulation of kanuka honey with cinnamon, chromium and magnesium for the management of patients with T2DM on metformin therapy; however, an improvement in body weight and lipid parameters cannot be discounted.
References
The Holy Qur'an. Surat An-Nahl [16:69]. http://quran.com/16/69. Accessed 15 Oct 2014
Agrawal OP, Pachauri A, Yadav H et al (2007) Subjects with impaired glucose tolerance exhibit a high degree of tolerance to honey. J Med Food 10:473–478
Al-Waili NS (2004) Natural honey lowers plasma glucose, C-reactive protein, homocysteine and blood lipids in healthy, diabetic and hyperlipidemic subjects: Comparison with dextrose and sucrose. J Med Food 7:100–107
Erejuwa OO, Sulaiman SA, Ab Wahab MS (2012) Honey: a novel antidiabetic agent. Int J Biol Sci 8:913–934
Erejuwa OO, Sulaiman SA, Ab Wahab MS et al (2001) Glibenclamide or metformin combined with honey improves glycemic control in streptozotocin-induced diabetic rats. Int J Biol Sci 7:244–252
Samanta A, Burden AC, Jones AR (1985) Plasma glucose responses to glucose, sucrose and honey in patients with diabetes mellitus: an analysis of glycaemic and peak incremental indices. Diabet Med 2:371–373
Erejuwa OO, Sulaiman SA, Ab Wahab MS (2012) Oligosaccharides might contribute to the antidiabetic effect of honey: a review of the literature. Molecules 17:248–266
Akilen R, Tsiami A, Devendra D, Robinson N (2012) Cinnamon in glycaemic control: systematic review and meta-analysis. Clin Nutr 31:609–615
Allen R, Schwartzman E, Baker W et al (2013) Cinnamon use in Type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med 11:452–459
Balk EM, Tatsioni A, Lichtenstein A et al (2007) Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care 30:2154–2163
Song Y, He K, Levitan B et al (2006) Effects of oral magnesium supplementation on glycaemic control in Type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med 23:1050–1056
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470
Khan A, Safdar M, Khan MMA et al (2003) Cinnamon improves glucose and lipids of people with Type 2 diabetes. Diabetes Care 26:3215–3218
Anderson RA, Cheng N, Bryden NA et al (1997) Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with Type 2 diabetes. Diabetes 46:1786–1791
Grant AP, McMullen JK (1982) The effect of brewer’s yeast containing glucose tolerance factor on the response to treatment in type 2 diabetics: a short controlled study. Ulster Med J 51:110–114
Bahijiri SM, Mira SA, Mufti AM, Ajabnoor MA (2000) The effects of inorganic chromium and brewer’s yeast supplementation on glucose tolerance, serum lipids and drug dosage in individuals with Type 2 diabetes. Saudi Med J 21:831–837
Rabinowitz MB, Gonick HC, Levin SR, Davidson MB (1983) Effects of chromium and yeast supplements on carbohydrate and lipid metabolism in diabetic men. Diabetes Care 6:319–327
Offenbacher EG, Pi-Sunyer FX (1980) Beneficial effects of chromium-rich yeast on glucose tolerance and blood lipids in elderly subjects. Diabetes 29:919–925
Uusitupa MI, Kumpulainen JT, Voutilainen E et al (1983) Effect of inorganic chromium supplementation on glucose tolerance, insulin response and serum lipids in noninsulin-dependent diabetics. Am J Clin Nutr 38:404–410
Sherman L, Glennon JA, Brech WJ et al (1968) Failure of trivalent chromium to improve hyperglycaemia in diabetes mellitus. Metabolism 17:439–442
Magistrelli A, Chezem JC (2012) Effect of ground cinnamon on postprandial blood glucose concentration in normal-weight and obese adults. J Acad Nutr Diet 112:1806–1809
Erejuwa OO, Gurtu S, Sulaiman SA et al (2010) Hypoglycemic and antioxidant effects of honey supplementation in streptozotocin-induced diabetic rats. Int J Vitam Nutr Res 80:74–82
Chepulis L, Starkey N (2008) The long-term effects of feeding honey compared with sucrose and a sugar-free diet on weight gain, lipid profiles, and DEXA measurements in rats. J Food Sci 73:H1–H7
Nemoseck T, Carmody EG, Furchner-Evanson A et al (2011) Honey promotes lower weight gain, adiposity, and triglycerides than sucrose in rats. Nutr Res 31:55–60
Bahrami M, Ataie-Jafari A, Hosseini S et al (2009) Effects of natural honey consumption in diabetic patients: an 8 week randomized clinical trial. Int J Food Sci Nutr 60:618–626
Abdulrhman M, El-Hefnawy MH, Aly RH et al (2013) Metabolic effects of honey in Type 1 diabetes mellitus: a randomized crossover pilot study. J Med Food 16:66–72
Yaghoobi N, Al-Waili N, Ghayour-Mobarhan M et al (2008) Natural honey and cardiovascular risk factors; effects on blood glucose, cholesterol, triacylglycerole, CRP, and body weight compared with sucrose. Sci World J 8:463–469
Deibert P, König D, Kloock B et al (2010) Glycaemic and insulinaemic properties of some German honey varieties. Eur J Clin Nutr 64:762–764
Cao H, Urban JF, Anderson RA (2007) Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch Biochem Biophys 459:214–222
Jeejeebhoy KN, Chu RC, Marliss EB et al (1977) Chromium deficiency, glucose intolerance and neuropathy reversed by chromium supplementation, in a patient receiving long-term total parenteral nutrition. Am J Clin Nutr 30:531–538
Sundaram B, Singhal K, Sandhir R (2012) Ameliorating effect of chromium administration on hepatic glucose metabolism in streptozotocin-induced experimental diabetes. BioFactors 38:59–68
Abdollahi M, Farshchi A, Nikfar S, Seyedifar M (2013) Effect of chromium on glucose and lipid profiles in patients with Type 2 diabetes; A meta-analysis review of randomized trials. J Pharm Pharm Sci 16:99–114
Suárez A, Pulido N, Casla A et al (1995) Impaired tyrosine–kinase activity of muscle insulin receptors from hypomagnesaemic rats. Diabetologia 38:1262–1270
Rodríguez-Morán M, Guerrero-Romero F (2011) Insulin secretion is decreased in non-diabetic individuals with hypomagnesaemia. Diabetes Metab Res 27:590–596
Baker W, Gutierrez-Williams G, White CM et al (2008) Effect of cinnamon on glucose control and lipid parameters. Diabetes Care 31:41–43
Letinsky D, Kelsberg G (2011) Is cinnamon safe and effective for treating lipid disorders? J Fam Pract 60:43–44
Ranasinghe P, Jayawardana R, Galappaththy P et al (2012) Efficacy and safety of ‘true’ cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: a systematic review and meta-analysis. Diabet Med 29:1480–1492
Akilen R, Tsiami A, Devendra D, Robinson N (2010) Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic Type 2 diabetic patients in the UK: a randomized placebo-controlled, double-blind clinical trial. Diabet Med 27:1159–1167
Ziegenfuss TN, Hofheins JE, Mendel RW et al (2006) Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. J Int Soc Sports Nutr 3:45–53
Anton SD, Morrison CD, Cefalu WT et al (2008) Effects of chromium picolinate on food intake and satiety. Diabetes Technol Ther 10:405–412
Hlebowicz J, Darwiche G, Björgell O, Almér L-O (2007) Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. Am J Clin Nutr 85:1552–1556
Akilen R, Pimlott Z, Tsiami A, Robinson N (2013) Effect of short-term administration of cinnamon on blood pressure in patients with prediabetes and Type 2 diabetes. Nutrition 29:1192–1196
Conflict of interest
The honey products and study funding were provided by Honeylab Ltd. The authors have no financial interest in this company, and the company had no influence on the reporting of results. The authors declare that they have no conflict of interest.
Ethical standard
This study was approved by the Ministry of Health Central Regional Ethics Committee (13/CEN/91) and was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12613000624785). The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants gave written informed consent prior to their inclusion in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Whitfield, P., Parry-Strong, A., Walsh, E. et al. The effect of a cinnamon-, chromium- and magnesium-formulated honey on glycaemic control, weight loss and lipid parameters in type 2 diabetes: an open-label cross-over randomised controlled trial. Eur J Nutr 55, 1123–1131 (2016). https://doi.org/10.1007/s00394-015-0926-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0926-x