Abstract
Nonenzymatic glycation of long-lived proteins has been implicated in several complications related to age and diabetes. Dicarbonyl compounds such as methylglyoxal (MGO) have been identified as the predominant source for the formation of advanced glycation end-products (AGEs) in various tissues. We investigated the effect of 13 micronutrients on MGO-mediated in vitro glycation of bovine serum albumin (BSA), as formation of AGEs and protein carbonyls. BSA (10 mg/ml) was incubated at 37°C with 100 mM MGO for 24 hours, in presence of ascorbic acid, Trolox (water-soluble α-tocopherol analog), β-carotene, retinol, riboflavin, thiamin, folic acid, niacin, pyridoxine, zinc, iron, manganese, and selenium. Fluorescence was measured at the wavelength pair of 370 and 440 nm as an index of the formation of AGEs and spectra were recorded for promising interactions at λex = 280 nm and λex = 370 nm. Within four standard antiglycating agents, aminoguanidine showed highest inhibitory response for BSA glycation followed by quercetin, gallic acid, and tannic acid. Promising antiglycation potential was seen for Trolox, riboflavin, Zn, and Mn as evidenced by decrease in the formation of AGEs and protein carbonyls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a complex noncommunicable disease characterized by elevated level of glucose (hyperglycemia) in the blood and urine. Presently, the affliction rate of patients with NIDDM is alarmingly high. One of the consequences of hyperglycemia is the excessive nonenzymatic glycation of proteins also known as the Maillard reaction resulting in formation of the advanced glycation end-products (AGEs). Glycation and AGE modifications result in pathological changes to the protein, contributing to diabetic complications. Due to their potential noxious effects, alimentary AGEs are also called glycotoxins.
Methylglyoxal (MGO) is known to be formed nonenzymically by amine-catalyzed sugar fragmentation reactions and by spontaneous decomposition of triosephosphate intermediates in glycolysis. It is also a product of the metabolism of acetol, an intermediate in the catabolism of both threonine and the ketone body acetone [1]. MGO and glyoxal (GO) have been identified as major sources for AGE formation in various tissues. It has been reported that MGO binds and modifies a number of proteins, including bovine serum albumin (BSA), RNase A, collagen, lysozyme, and lens crystallins [2–4]. MGO is known to induce formation of heterogeneous AGE and protein cross-linking. Current research into the AGE pathway is proceeding along various fronts. Several potential drug candidates as AGE inhibitors have been reported recently. Aminoguanidine is the first drug extensively studied both in vitro and in vivo [5].
Micronutrients play a central role in metabolism and in the maintenance of tissue function, but effects in preventing or treating disease which is not due to micronutrient deficiency cannot be expected from increasing the intake. There is a highly integrated system to control the flux of micronutrients in illness, and this demonstrates just how important the body perceives the micronutrients to be. An adequate intake therefore is necessary to sustain metabolism and tissue function [6]. The health beneficial properties of essential micronutrients for prevention of protein glycation have been extensively studied only for pyridoxamine and ascorbic acid [6, 7]. Despite the large amount of information available on the pathological significance of AGE protein, relatively little is known about the effect of micronutrients on AGE formation as well as the dicarbonyl modifications of proteins. This study was therefore undertaken to screen nine vitamins, viz. ascorbic acid, α-tocopherol, β-carotene, retinol, riboflavin, thiamin, folic acid, niacin, and pyridoxine, and four trace metals, viz. zinc, iron, manganese and selenium, for their in vitro effect on MGO-mediated glycation using bovine serum albumin (BSA) as model protein.
Results and Discussion
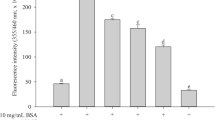
As a response of BSA to standard glycation inhibitors upon incubation of MGO with BSA, a significant AGE fluorescence was produced at the wavelength pair of 330 and 440 nm, indicative of albumin glycation (Fig. 1). Quercetin, aminoguanidine, gallic acid, and tannic acid (as aqueous extracts at the concentration at 1% w/v) were chosen as reference antiglycating agents for the validation of experimental protocol. ANOVA indicated significant decrease of glycation in presence of all the four compounds as compared to MGO control (F = 31.55, p < 0.001). Multiple comparison test indicated aminoguanidine to be the most promising inhibitor for BSA glycation followed by quercetin, gallic acid, and tannic acid (Fig. 1).
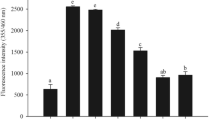
Figure 2 shows the percent change in AGE fluorescence for BSA in the presence of different vitamins. Except folic acid, all the vitamins showed significant inhibitory effects on glycation of BSA. Further, in this protein model, Trolox followed by riboflavin showed prominent inhibitory response. ANOVA indicated significant difference in glycation in the presence of all the vitamins (F = 10.26, p < 0.001). Multiple comparison tests and computation of critical difference indicated significant decrease of glycation for riboflavin and Trolox as compared to MGO control. The ANOVA for BSA glycation in the presence of the trace metals indicated significant difference in glycation in the presence of zinc, manganese, iron, and selenium (F = 4.29, p < 0.05). Within trace metal subgroup, zinc and manganese showed significant inhibitory activity (Fig. 3). Fluorescence spectra were also recorded for MGO-mediated glycation of BSA in the presence of micronutrients showing distinct response (Fig. 4a, b). The spectra for Trolox, zinc, and manganese confirm the results about the inhibitory behavior in concurrence with the AGE measurements taken at fixed wavelengths.
It was noteworthy that manganese, Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, a water-soluble derivative of vitamin E), and riboflavin inhibited glycation at a very low concentrations as compared to zinc. In light of these results, a dose–response experiment was further undertaken for the selected micronutrients. Accordingly, zinc (5, 10, and 15 ppm, parts per million = milligrams per liter), manganese (0.03, 0.06, and 1 ppm), Trolox (0.1, 0.2, and 0.3 ppm), and riboflavin (0.3, 0.6, and 1 ppm) were assessed to confirm their inhibitory response to glycation. The AGE fluorescence of the four micronutrients with BSA can be seen in Fig. 5.
Formation of Protein Carbonyls
Protein carbonyls are considered to be more sensitive index for determination of the extent of protein oxidation. Hence, we assessed the levels of protein carbonyls for the glycated samples with candidate micronutrients. It was observed that though all the screened micronutrients elicited decreased levels of carbonyls as compared to positive control, Trolox, riboflavin, zinc, and manganese in particular showed demonstrable decrease. Figure 6 shows the responses seen for decrease in formation of carbonyls due to these promising micronutrients.
Protein glycoxidation is of great interest in geriatric research because protein modifications are recognized as markers of and contributors to the aging process. Since aging is associated with impaired handling of blood sugar and increased oxidative stress, the cellular constituents of older adults are at elevated risk for glycation/glycoxidation. This risk becomes especially relevant in tissues with a slow rate of regeneration, such as the brain, blood vessels, and the heart. Nε-(carboxymethyl) lysine (CML) is the most abundant AGE in proteins in the body and appears to be a useful biomarker of aging. It is generally accepted that the Maillard reaction in vivo contributes to the pathogenesis of diabetes, uremia, and aging; questions arise concerning the intake of AGEs via daily food and their possible pathophysiological role [4, 5].
Albumin is present in the circulation at a relatively high concentration (35–50 g L–1), and it is prone to glycation in vivo. It is the predominant protein adducted by CML in the circulation [8, 9]. MGO, a reactive intermediate in the auto-oxidation of reducing sugars, can readily glycate proteins [10], and it is also a physiological metabolite of the glyoxalase system [11]. Moreover, the nature of AGE structures and extent of modifications depend largely on the glycating reagents and conditions employed for the in vitro glycation reaction [4].
In foodstuffs, MGO is formed during cooking and prolonged storage. Food processing, heating in particular, has a significant accelerating lipoxidation products. Heat helps create tasteful flavors. A significant proportion (~10%) of ingested AGEs is absorbed with food. In this context, only limited information concerning digestion, resorption, and elimination of dietary glycation compounds can be found in the literature [6]. There is apparently a direct correlation between circulating AGE levels and those consumed [11]. In one of the reported study, median concentration of MGO was increased five- to six-fold in blood samples of patients with insulin-dependent diabetes mellitus and two- to three-fold in patients with non-insulin-dependent diabetic mellitus, relative to control values [12]. Fasting and metabolic disorders and/or deironcts in MGO detoxification processes cause accumulation of this reactive dicarbonyl in vivo. In addition, the intake of low doses of MGO over a prolonged period of time can cause degenerative changes in different tissues [13, 14]. MGO was hence chosen as glycating agent for the present experiments.
Glycated albumin is the commonly used prototype protein-AGE adduct that can be prepared in vitro by incubating albumin with glucose-6-phosphate [10, 11]. For screening large number of candidate antiglycating factors, it was essential to have a rapid glycation inducer. Using albumin as a model protein, we have demonstrated antiglycation potential in Trolox, riboflavin, zinc, and manganese. These findings not only give a new dimension to the biological functions of these micronutrients but also suggest a natural alternative to drugs used for the treatment of diabetes and its complications.
CML is the most abundant AGE in proteins in the body and appears to be a useful biomarker of aging. Antioxidants can inhibit metal-mediated auto-oxidation of glucose leading to the formation of AGEs [15]. There are several mechanisms that can contribute to this activity. While flavonoids and other phenolic compounds possess free-radical scavenging activity, certain flavonoids such as quercetin and kaempherol are capable of complexing metal ions directly and individual phenolics have distinct inhibitory properties on protein glycation, a result that has been correlated with antioxidant properties [16, 17]. Pyridoxamine and thiamine pyrophosphate have been reported as novel inhibitors of AGE formation through BSA glycation [18, 19]. Pyridoxamine is hypothesized to trap intermediates in the formation of Amadori products released from glycated proteins, possibly preventing the breakdown of glycated proteins by disrupting the catalysis of this process through disruptive interactions with the metal ions crucial to the redox reaction [20]. One research study found that pyridoxamine specifically reacts with the carbonyl group in Amadori products, but inhibition of post-Amadori reactions is due in much greater part to the metal chelation effects of pyridoxamine. However, there are no systematic studies on majority of the micronutrients selected for the present study. Much more research is needed to characterize better markers of micronutrient status in terms of antiglycating effects apart from radical scavenging potential.
Several in vitro and in vivo studies have shown that the action of ROS on proteins results in the formation of carbonyl groups. Carbonyl groups (aldehyde and ketone) can be introduced into proteins by a variety of oxidation reactions [21]. Evaluation of carbonyl group content in plasma proteins provides a significant clue to the magnitude of oxidative stress under disease conditions such as diabetes [22]. Elevated levels of protein carbonyl group content in plasma proteins are also reported in various diseases such as Alzheimer’s disease, Parkinson disease, diabetes mellitus, rheumatoid arthritis, muscular dystrophy, cataractogenesis, renal tumor, uremia, bronchopulmonary dysplasia, and amylotrophic lateral sclerosis. The assessment of carbonyl proteins is therefore a valuable biomarker of the protein redox status in the experimental protein and was used to assess the extent of protein oxidation. All of the micronutrients studied are naturally occurring in the body and several of them such as ascorbate are known to be lowered in the tissues of subjects with diabetes [23, 24]. In our study, the decrease in carbonyls has been shown by zinc and manganese. This may be due to the fact that trace metals such as iron and copper act as prooxidants while zinc with a single oxidation state acts as antioxidant. The inhibitory action of Trolox on carbonyl formation might also be due to its action as a potent radical scavenger. Behavior of ascorbic acid in glycation may also be dependent on its redox state [25]. These results need to be further confirmed through animal and human studies.
Although the study was intended for understanding the role of micronutrients in glycation of human serum albumin (HSA), BSA and HSA have 90% homology. Therefore, study of BSA glycation using MGO can give important leads for future studies in this direction. As compared to positive control (BSA + MGO), the presence of zinc and manganese overall significantly reduced the AGE fluorescence, indicating that the extent of glycation was lowered. Additional dose–response experiments to assess the carbonyl formation were hence carried out for the four promising micronutrients. As AGE measurement is nonspecific, the carbonyl estimations by DNPH assay are generally used to resolve ambiguities. This assay indicated that samples incubated with zinc and manganese reduced the protein carbonyls significantly (p < 0.01). Although there is no direct evidence of the role of zinc in albumin glycation, a decrease in glycosylated hemoglobin by zinc supplementation in type 2 diabetic patients has been reported [26]. The role of Zinc as a putative antiglycating agent in long-term glucose-induced glycation of BSA has also been recently reported by our group [27]. The results from the present experiments are suggestive, and further studies are, however, warranted in this regard.
Conclusions
Trolox, riboflavin, zinc, and manganese elicited promising antiglycation potential as evidenced by decrease in the formation of AGEs and protein carbonyls. The protective action of these micronutrients could be attributed to their antioxidant and free-radical scavenging activity. Nevertheless, this fact and the results of the present study point to the necessity of good nutrition in diabetes and to the possibility of inexpensive, relatively non-toxic therapies for the prevention and treatment of diabetic complications like cataract.
References
Eble A, Thorpe S, Baynes J (1983) Nonenzymatic glycation and glucose-dependent cross linking of protein. J Biol Chem 258:9406–9412
Chellan P, Nagaraj R (1999) Protein crosslinking by the Maillard reaction: dicarbonyl-derived imidazolium crosslinks in aging and diabetes. Arch Biochem Biophys 368:98–104
Beranek M, Novakova D et al (2006) Glycation and advanced glycation end-products in laboratory experiments in vivo and in vitro. Acta Med 49(1):35–39
Stadtman E (1990) Metal catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic Biol Med 9:315–25
Samuel R, Yernini K, Scott S et al (1999) Novel inhibitors of advanced glycation end products. Biochem Biophys Res Commun 262:651–656
Shenkin A (2006) The key role of micronutrients. Clin Nutr 25:1–13
Sing R, Barden A, Mori T et al (2001) Advanced glycation end products: a review. Diabetologia 44:129–146
Westwood M, McLellan A et al (1994) Receptor-mediated endocytic uptake of methylglyoxal-modified serum albumin. J Biol Chem 269:32293–32298
Yasujiro M, Kazunari Y, Sachico E, Akira H (1995) Protein glycation inhibitors from Thyme (Thymus vulgaris). Biosci Biotechnol Biochem 59:2018–21
Uchida K, Kanematsu M, Sakai K et al (1998) Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci USA 95:4882–4887
Bouma B, Kroon-Batenburg MJL, Ya-Ping W (2003) Glycation induces formation of amyloid cross-β structure in albumin. J Biol Chem 278(43):41810–41819
Nagaraj R, Sarkar P, Mally A et al (2002) Effect of pyridoxamine on chemical modification of proteins by carbonyls in diabetic rats: characterization of a major product from the reaction of pyridoxamine and methylglyoxal. Arch Biochem Biophys 402:110–119
Melpomeni P, Jaime U, Helen V (2003) Glucose, advanced glycation end products, and diabetes complications: what is new and what works. Clin diabetes 21:4–7
Rebecca P, Dearlove, Phillip G et al (2008) Inhibition of protein glycation by extracts of culinary herbs and spices. J Med Food 11(2):275–281
Wu C, Yen G (2005) Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J Agric Food Chem 53:3167–3173
Leopoldini M, Russo N, Chiodo S, Toscano M (2006) Iron chelation by the powerful antioxidant flavonoid quercetin. J Agric Food Chem 54:6343–6351
Metz TO, Alderson NL, Thorpe SR, Baynes JW (2003) Pyridoxamine as a multifunctional pharmaceutical: targeting pathogenic glycation and oxidative damage. Cell Mol Life Sci 62(15):1671–81
Voziyan P, Hudson B (2005) Pyridoxamine: the many virtues of a maillard reaction inhibitor. Ann NY Aca Sci 1043:807–16
Giannoukakis N (2005) Pyridoxamine (BioStratum). Curr Opin Investig Drugs 6(4):410–418
Stadtman E, Levine R (2000) Protein oxidation. Ann NY Aca Sci 889:191–208
Ashley Booth A, Raja Khalifah G et al (1997) In vitro kinetic studies of formation of antigenic advanced glycation end products. J Biol Chem 272:5430–5437
Stankova L, Riddle M, Larned J et al (1984) Plasma ascorbate concentrations and blood cell dehydroascorbate transport in patients with diabetes mellitus. Metabolism 33:347–353
Tarwadi K, Agte V (2004) Linkages of antioxidant, micronutrient and socio-economic status with the degree of oxidative stress and lens opacity in Indian cataract patients. Nutrition 20(3):261–267
Agte V, Nagmote R, Tarwadi K (2004) Comparative in vitro uptake of zinc by erythrocytes of normal vs type 2 diabetic individuals and the associated factors. Diabet Nutr Metab 17:343–349
Vinson A, Howard T (1996) Inhibition of protein glycation and AGEs by ascorbic acid and other vitamins and nutrients. Nutr Biochem 7:659–663
Al-Maroof R, Al-Sharbatti S (2006) Serum zinc levels in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetics. Saudi Med J 27:344–350
Tupe R, Agte V (2009) An in vitro study for antiglycation role of zinc along with ascorbic acid and folic acid in BSA glycation. Br J Nutr 103:370–377
Acknowledgements
The authors wish to thank Dr. Rashmi Tupe for her help in glycation experiments. The work presented in this paper is a part of the project funded by the Department of Science and Technology (SR/WOS-A/LS-67/2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tarwadi, K.V., Agte, V.V. Effect of Micronutrients on Methylglyoxal-Mediated In Vitro Glycation of Albumin. Biol Trace Elem Res 143, 717–725 (2011). https://doi.org/10.1007/s12011-010-8915-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-010-8915-7