Abstract
Autism is a neurodevelopmental disorder of childhood with poorly understood etiology and pathology. This pilot study aims to evaluate the levels of antioxidant enzymes, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), and levels of malondialdehyde (MDA), a marker of lipid peroxidation, in Egyptian autistic children. Autism is a neurodevelopmental disorder of childhood with poorly understood etiology and pathology. The present study included 20 children with autism diagnosed by DSM-IV-TR criteria and Childhood Autism Rating Scale. Controls included 25 age-matched healthy children. Cases were referred to Outpatient Clinic of Children with Special Needs Department, National Research Center, Cairo, Egypt. We compared levels of SOD, GSH-Px, and MDA in children with autism and controls. In children less than 6 years of age, levels of SOD, and GSH-Px were significantly lower in autistic children compared with their controls, while MDA was significantly higher among patients than controls. In children older than 6 years, there was no significant difference in any of these values between cases and controls. We concluded that children with autism are more vulnerable to oxidative stress in the form of increased lipid peroxidation and deficient antioxidant defense mechanism especially at younger children. We highlight that autistic children might benefit from antioxidants supplementation coupled with polyunsaturated fatty acids. Moreover, early assessment of antioxidant status would have better prognosis as it may decrease the oxidative stress before inducing more irreversible brain damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism is a neurodevelopmental disorder that is usually diagnosed before the age of 3 years. It is characterized by behavioral impairment and communication deficits [1]. Autism affects males more than females, occurring at a ratio of 4:1 [2]. Autism is considered a multi-factorial disorder that is influenced by genetic, immunological, and environmental factors, including oxidative stress [3]. The rate of autism was 3.4 per 1,000 for children aged 3 to 10 years in the 1990s while, recently, the prevalence estimates are in the range of 6.5 to 6.6 per 1,000 [4]. The increase in the rate of autism as revealed by epidemiological studies and government reports implicates the importance of external or environmental factors that might be changing [5].
Abnormal genes of oxidative stress pathways and increased oxidative stress have been reported in autism spectrum disorders [6].
Oxidative stress has been implicated in the pathogenesis of diverse disease states. There may be a common pathogenic mechanism underlying many major psychiatric disorders because the brain has comparatively greater vulnerability to oxidative damage [7]. Limited studies on oxidative stress in autism have been reported [8]. They suggest that oxidative stress may play a role in the development and clinical manifestations of autism. Both central and peripheral markers of oxidative stress have been reported in these studies. Peripheral markers have included lipid peroxidation levels. Increases in these markers correlated with loss of previously acquired language skills in autism [9]. Furthermore, metabolic markers of oxidative stress have been identified including abnormal levels of metabolites signifying impaired methylation and increased oxidative stress in autism [10]. In addition, alterations in the levels of detoxifying agents (such as glutathione), and antioxidants involved in the defense system against reactive oxygen species have been reported in autism [11].
The aim of this study was to evaluate levels of antioxidant enzymes, superoxide dismutase and glutathione peroxidase, and to assess lipid peroxidation in autistic children which may be of great importance in the management of this disorder.
Subjects and Methods
The present study included 20 patients with autism (18 males and two females) aged 3–10 years. Clinical diagnosis was based on the criteria for autistic disorder as defined in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) [12] and the Childhood Autism Rating Scale (CARS) [13]. All patients were referred to Outpatient Clinic of Children with Special Needs Department, National Research Center, Cairo, Egypt. Exclusion criteria included all subjects with other causes of mental subnormality and delayed language and all patients receiving antioxidants. Caregiver consent was obtained for all the studied cases. A comparable 25 children (23 males and two females) were selected and enrolled in the study as a normal control group.
All patients were subjected to:
-
1.
Detailed history taking including three generation pedigree analysis, pregnancy history, peri-natal history, developmental history, similarly affected family members, and age of onset of presenting manifestation.
-
2.
Thorough clinical examination including all body systems with special emphasis on the neurological system. Weight, height, and head circumference were measured and compared with age- and sex-matched controls. All measurements followed the recommendations of the International Biological Program (IBP) [14].
-
3.
Application of the criteria for autism defined in the DSM-IV-TR [12].
-
4.
Diagnostic interviews using Childhood Autism Rating Scale (CARS) [13] were conducted.
-
5.
Biochemical tests:
-
(a)
Blood sampling: Venous blood sample were obtained from all patients and controls and collected into 5-ml vacutainer tubes containing potassium EDTA and mixed by gentle inversion. The samples were placed on ice immediately. Each sample was centrifuged and processed on the same day in order to avoid oxidation and changes in enzyme activity during storage.
-
(b)
Assay of Superoxide Dismutase (SOD): An amount of 0.5 ml of whole blood was centrifuged for 10 min at 3,000 rpm, then aspirated of the plasma. Then the erythrocytes were washed four times with 3 ml of 0.9% Nacl solution, centrifuged for 10 min at 3,000 rpm after each wash. The washed centrifuged erythrocytes were increased to 2.0 ml with cold redistilled water then mixed and left to stand at 4°C for 15 min. The lysate was diluted with 0.01 mol/l phosphate buffer pH 7.0, so that the percent inhibition fell between 30% and 60%. SOD was assayed by spectrophotometer using kit from Randox Laboratories Ltd. (Ardmore, Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QY). Wavelength used was 505 nm; at temperature 37°C. Semi-micro procedure was used where 0.05 ml of diluted sample was added to the reagent kit and mixed together. Absorbance was read initially then read again after 30 s and the timer was started simultaneously. Final absorbance was read after 3 min [15].
-
(c)
Assay of Glutathione Peroxidase (GSH-Px): GSH-Px was assayed by spectrophotometer using kit from Randox Laboratories Ltd. (Ardmore, Diamond Road, Crumlin, Co. Antrim, UK, BT29 4QY). Wavelength used was 340 nm; at temperature 37°C. Semi-micro procedure was used where 0.02 ml of diluted sample was added to reagent kit and mixed together. The absorbance of the sample and reagent blank was read initially then after 1 min and the timer was started simultaneously. Then the reagent blank value was subtracted from that of the sample [16].
-
(d)
Lipid peroxidation in plasma: We measured plasma MDA as an indicator of lipid peroxidation status in all autistic children and control subjects according to the method described by Chauhan et al. [17]. MDA reacts with thiobarbituric acid, and forms a colored complex with a maximum absorbance at 532 nm [18].
-
(a)
Statistical Analysis
Distribution of the two groups according to age was analyzed with one sample Kolmogrov–Smimov test. As both groups showed normal distribution, parametric statistic methods were used. Categorical variables were compared by Pearson’s Chi-square test. Independent t test, one-way ANOVA and least significant difference as post hoc test were performed to compare continuous variables. Bivariate comparisons were examined using Pearson’s and Spearman’s correlation coefficients for parametric and non parametric variables, respectively. P value ≤0.05 was considered significant.
Results
Among the studied subjects, there was no significant age difference between cases with autism and controls (4.7 ± 2.41 and 6.0 ± 2.33, respectively).
According to CARS, seven cases (35% of the studied cases) were classified as mild autism (CARS score, 30–33), six cases (30% of the studied cases) as moderate autism (CARS score, 34–37) and seven cases (35% of the studied cases) as severe autism (CARS score, >37). Moreover, there was no significant correlation between the degree of severity of autism and neither age of the patients, nor parental ages at time of birth of the affected children (Table 1).
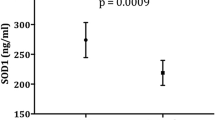
The mean, erythrocyte, levels of both SOD and GSH-Px were significantly lower in autistic children compared with their controls. On the other hand, the mean plasma levels of MDA were significantly higher among patients than controls (Table 2).
In children younger than age 6 years, the levels of SOD and GSH-Px were significantly lower in autistic children compared to their controls. In children 6 years or older there was no significant difference in SOD or GSH-Px levels in cases versus controls (Tables 3 and 4). On the other hand, Spearman correlation coefficient revealed no significant relation to age which could be due to small sample size.
Discussion
The present study aimed at evaluating the oxidative stress among Egyptian autistic children by measuring the levels of antioxidant enzymes; SOD and GSH-Px and assessment of lipid peroxidation by measuring MDA. We included 20 children with autism diagnosed by DSM-IV-TR criteria and CARS. Controls included 25 age-matched healthy children.
Levels of SOD and GSH-Px were significantly lower in autistic children compared to their controls, while MDA was significantly higher among patients than controls denoting the increased vulnerability to oxidative stress among Egyptian autistic children.
Our study showed that there was no correlation between the degree of severity of autism and the age of the patients denoting that the severity of the condition appears not to be related to age. Coplan and Jawad [19] reported that that there was no significant relationship between CARS score and age. However, their data could not establish whether the decline in CARS over time reflects adequate treatment or a natural decrease of the severity over time. On the other hand, another study that was done to examine the predictive validity of symptom severity with age reported that the pattern of autistic symptoms varied over time and the variability in scores and symptom severity increased with age [20].
The results of the present study regarding the antioxidant enzymes SOD and GSH-Px denotes impaired antioxidant defense mechanisms which lead to harmful effects of oxygen radicals that could have an important role in the etiology of autism. Our results are consistent with the study conducted by Yorbik et al. [21], who measured the levels of the two enzymes in the erythrocytes of a group of 45 autistic children aged between 4 and 12 years, and 41 controls of the same age. They reported a significant decrease in erythrocyte SOD level in autistic children denoting that one of the very important defense mechanisms against oxidative stress has been impaired. Erythrocyte and plasma GSH-Px levels were, also, significantly lower in the autistic group, which might have lead to inadequate removal of H2O2 and an increase in the production of hydroxyl radicals that are highly reactive. They concluded that, the decreased activity of the two enzymes might be due to their lesser production or greater consumption in a trial to protect the body against the increased oxidative stress.
MDA is an end product of peroxidation of polyunsaturated fatty acids and related esters, and is a marker of lipid peroxidation. The measurement of MDA is the most widely used method for assaying lipid peroxidation. Our results denoted that oxidative stress is increased in our sample of Egyptian autistic children which is similar to those reported by Chauhan et al. [22].
The oxidative stress in autism may be caused by an imbalance between the generation of ROS and the defense mechanism against ROS by antioxidants. Oxidative damage is counteracted by the body’s antioxidant systems, which convert oxygen radicals into harmless byproducts [23, 24].
Furthermore, alterations of the advanced glycation end products (AGEs)—their putative receptor RAGE axis in autism were studied by Boso et al. [25]. They reported significantly reduced peripheral level of endogenous secretory RAGE coupled with elevated proinflammatory ligand S100A9 which pointed to a definite dysfunction of the AGEs/RAGE axis in autism that could play a role in the pathophysiology of this disorder through promotion of neuroinflammation, oxidative stress and neuronal degeneration. Recently, high mobility group box 1 (HMGB1) which is a ubiquitous protein that functions as an activator for inducing the immune response was assessed in patients with autistic disorder and it was found to be elevated in patients with autism and authors concluded that increased HMGB1 might be a biological correlate of the impaired reciprocal social interactions in this neurodevelopmental disorder [26].
The two important antioxidant enzymes combating ROS are the SOD and GSH-Px. Low SOD can contribute to superoxide-mediated DNA damage and thereby affect neuronal growth and migration. Another possible explanation that may contribute for the decreased levels of the two enzymes is the nutritional status, as some of the antioxidant nutrient levels affect the status of the antioxidant enzymes. For example, adequate amounts of superoxide dismutase cannot be produced unless the body receives an adequate and balanced intake of copper and zinc. Copper deficiency reduces the level of superoxide dismutase [27]. Zinc deficient diet decreases superoxide dismutase, glutathione peroxidase, total glutathione, and vitamin E [28]. Reduced levels of antioxidant vitamins and minerals that have been identified in various autistic cohorts could exacerbate the toxic effect of oxidative stress, as a consequence of depletion of antioxidant reserves.
In contrast to our results, Söğüt et al. [8] reported a statistically significant increase in the GSH-Px activity without a concomitant change in SOD activity in autistic children compared to their controls. They reported that this increase in the level of GSH-Px might reflect a preceding cellular oxidative stress thus, serving as a compensatory mechanism. GSH-Px may also use lipid hydroperoxides as substrates, so its level might be increased together with increased lipid peroxidation. On the other hand increased H2O2 may explain the increased levels of GSH-Px. Since H2O2 is a neutral and highly lipophilic molecule, it can easily pass through tissue membranes with excessive amount in the CNS and pass through membranes into the plasma leading to an increase in GSH-Px activity. Normal SOD levels in autistic patients may possibly lead to an increase in the other antioxidant enzyme; GSH-Px.
In our study, comparison of the levels of SOD and GSH-Px between cases and controls according to age showed that autistic children below 6 years had significantly lower levels than controls, while cases ≥6 years had no significant difference. This may be explained by the fact that, infants and younger children are more vulnerable than older ones to oxidative stress due to their naturally low glutathione levels [29]. However, we could not find a significant relation between age of affected children and either of the two enzymes. This could be attributed to the limitation of our study being a pilot one with a small sample size.
In conclusion, our study lends further support to the concept that an increased vulnerability to oxidative stress may contribute to the development of manifestation of autism. We highlight that autistic children could benefit from antioxidants supplementation coupled with polyunsaturated fatty acids. In children at risk, blood tests should be obtained to assess antioxidant status as early as possible, as this would allow protective measures to be taken prior to irreversible brain damage by oxygen radicals.
References
Bölte S, Poustka F (2002) The relation between general cognitive level and adaptive behavior domains in individuals with autism with and without co-morbid mental retardation. Child Psychiatry Hum Dev 33(2):165–172
Blaxill M, Redwood L, Bernard S (2004) Thimerosal and autism? A plausible hypothesis that should be dismissed. Med Hypotheses 62:788–794
Sung YJ, Dawson G, Munson J, Estes A, Schellenberg GD, Wijsman EM (2005) Genetic investigation of quantitative traits related to autism: use of multivariate polygenic models with ascertainment adjustment. Am J Hum Genet 76:68–81
Fombonne E, Zakarian R, Bennett A, Meng L, McLean-Heywood D (2006) Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with immunizations. Pediatrics 118(1):e139–e150
Kern JK, Jones AM (2006) Evidence of toxicity, oxidative stress, and neuronal insult in autism. J Toxicol Environ Health B Crit Rev 9(6):485–499
Ming X, Johnson WG, Stenroos ES, Mars A, Lambert GH, Buyske S (2010) Genetic variant of glutathione peroxidase 1 in autism. Brain Dev 32(2):105–109
Ng F, Berk M, Dean O, Bush AI (2009) Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 11(6):851–876
Söğüt S, Zoroğlu SS, Ozyurt H, Yilmaz HR, Ozuğurlu F, Sivasli E, Yetkin O, Yanik M, Tutkun H, Savaş HA, Tarakçioğlu M, Akyol O (2003) Changes in nitric oxide levels and antioxidant enzyme activities may have a role in the pathophysiological mechanisms involved in autism. Clin Chim Acta 331(1–2):111–117
Perry G, Nunomura A, Harris S, Smith M, Salomon R (2005) Is Autism a disease of oxidative stress. Oxidative stress in autism symposium, New York State, Institute for basic research in Developmental Disabilities. p. 15
James SJ, Cutler P, Melnyk S, Hernigan S, Janak L, Gaylor DW, Neubrander JA (2004) Metabolic biomarkers of increased oxidative stress and methylation capacity in children with autism. Am J Clin Nutr 80(6):1611–1617
Chauhan A, Chauhan V (2006) Oxidative stress in autism. Pathophysiology 13(3):171–181
American Psychiatric Association (APA) (2000) Diagnostic and statistical manual of mental disorders, 4th edn. Text revision, Washington, DC
Schopler E, Reichler RJ, DeVellis RF, Daly K (1980) Toward objective classification of childhood autism: childhood autism rating scale (CARS). J Autism Dev Disord 10(1):91–103
Tanner JM, Hiernaux J, Jarman S (1969) Growth and physique studies. In: Weiner JS, Lourie JA (eds) Human biology: a guide to field methods, I.B.P. handbook No.9. Blackwell Scientific Publications, Oxford and Edinburgh, pp 1–29
Arthur JR, Boyne R (1985) Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sci 36(16):1569–1575
Anderson PH, Berret S, Patterson DS (1978) Glutathione peroxidase activity in erythrocytes and muscle of cattle and sheep and its relationship to selenium. J Comp Pathol 88(2):181–189
Chauhan VPS, Tsiouris JA, Chauhan A, Sheikh AM, Brown WT, Vaughan M (2002) Increased oxidative stress and decreased activities of Ca2+/Mg2+—ATPase and Na+/K +—ATPase in the red blood cells of the hibernating black bear. Life Sci 71:153–161
Jain SK (1989) Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J Biol Chem 264(35):21340–21345
Coplan J, Jawad A (2005) Modeling clinical outcome of children with autistic spectrum disorders. Pediatrics 116(1):117–122
Charman T, Taylor E, Drew A, Cockerill H, Brown JA, Baird G (2005) Outcome at 7 years of children diagnosed with autism at age 2: predictive validity of assessments conducted at 2 and 3 years of age and pattern of symptom change over time. J Child Psychol Psychiatry 46(5):500–513
Yorbik O, Sayal A, Akay C, Akbiyik DI, Sohmen T (2002) Investigations of antioxidant enzymes in children with autistic disorder. Prostaglandins Leukot Essent Fatty Acids 67(5):341–343
Chauhan A, Chauhan V, Brown WT, Cohen I (2004) Oxidative stress in autism: increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin—the antioxidant proteins. Life Sci 75(21):2539–2549
Klein JA, Ackerman SL (2003) Oxidative stress, cell cycle, and neurodegeneration. J Clin Invest 111(6):785–793
Chauhan A, Chauhan V, Cohen IL, Brown WT (2005) Increased lipid peroxidation and membrane rigidity in autism: relationship with behavior abnormalities, oxidative stress. In autism symposium, Institute for Basic Research in Developmental Disabilities Staten Island, New York
Boso M, Emanuele E, Minoretti P, Arra M, Politi P, Ucelli di Nemi S, Barale F (2006) Alterations of circulating endogenous secretory RAGE and S100A9 levels indicating dysfunction of the AGE-RAGE axis in autism. Neurosci Lett 410(3):169–173
Emanuele E, Boso M, Brondino N, Pietra S, Barale F, Ucelli di Nemi S, Politi P (2010) Increased serum levels of high mobility group box 1 protein in patients with autistic disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 34:681–683
Klevay LM (2003) Advances in cardiovascular-copper research. In: Schrauzer GN, (ed) First international bio-minerals symposium: trace elements in nutrition, health and disease, Institute Rosell, Montreal, Canada
Powell SR (2000) The antioxidant properties of zinc. J Nutr 130:1447–1454
Erden-Inal M, Sunal E, Kanbak G (2002) Age related changes in the glutathione redox system. Cell Biochem Funct 20(1):61–66
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meguid, N.A., Dardir, A.A., Abdel-Raouf, E.R. et al. Evaluation of Oxidative Stress in Autism: Defective Antioxidant Enzymes and Increased Lipid Peroxidation. Biol Trace Elem Res 143, 58–65 (2011). https://doi.org/10.1007/s12011-010-8840-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-010-8840-9