Abstract
The present study was conducted to investigate whether the combined treatment with Se and Zn offers more beneficial effects than that provided by either of them alone in reversing Cd-induced oxidative stress in the kidney of rat. For this purpose, 30 adult male Wistar albino rats, equally divided into control and four treated groups, received either 200 ppm Cd (as CdCl2), 200 ppm Cd + 500 ppm Zn (as ZnCl2), 200 ppm Cd + 0.1 ppm Se (as Na2SeO3), or 200 ppm Cd + 500 ppm Zn + 0.1 ppm Se in their drinking water for 35 days. The results showed that Cd treatment decreased significantly the catalase (CAT) and glutathione peroxidase (GSH-Px) activities, whereas the superoxide dismutase (SOD) activity and the renal levels of lipid peroxidation (as malondialdehyde, MDA) were increased compared to control rats. The treatment of Cd-exposed rats with Se alone had no significant effect on the Cd-induced increase in the MDA concentrations but increased significantly the CAT activities and reversed Cd-induced increase in SOD activity. It also partially prevented Cd-induced decrease in GSH-Px activity. The treatment of Cd-exposed animals with Zn alone increased significantly the CAT activity and partially protected against Cd-induced increase in the MDA concentrations, whereas it had no significant effect on the Cd-induced increase in SOD activity and decrease in GSH-Px activity. The combined treatment of Cd-exposed animals with Se and Zn was more effective than that with either of them alone in reversing Cd-induced decrease in CAT and GSH-Px activities and Cd-induced increase in MDA concentrations. Results demonstrated beneficial effects of combined Se and Zn treatment in Cd-induced oxidative stress in kidney and suggest that Se and Zn can have a synergistic role against Cd toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd), a potent toxic metal, is very harmful to the environment and to humans because of its in vivo accumulation in liver, kidney, and other tissues. Kidney is one of the most critical organs for the toxicity of Cd. Studies on mammals showed that Cd induces multiple renal injury, mainly tubular dysfunction, marked reduction of renal energy metabolism, altered essential mineral composition, and many transmembrane transport abnormalities [1]. Following absorption, Cd is bound to the metallothionein and is filtered through the glomerulus into the urinary space where it is endocytosed by the proximal tubule cells and degraded by the lysosomes, resulting in the release of Cd. The intracellular release of Cd causes depletion in the levels of reduced glutathione, an alteration in the activity of antioxidant enzymes, as well as a change in the structure of the cellular membrane through a process of lipid peroxidation [2].
It is well known that many of the toxic effects of Cd result from interactions with essential elements such as zinc (Zn) and selenium (Se). These interactions can take place at different stages of the absorption, distribution, and excretion of trace elements, and they can affect the biological functions of such elements as well [3]. Se is a vital trace element which, in mammals, exerts its most important function via selenium-dependant glutathione peroxidase (GSH-Px). Se deficiency is usually associated with increased lipid peroxidation which alters the integrity of cell membranes and, consequently, affects cell functions [4]. Zn acts as an antioxidant, since it is involved in cell membrane stabilization, copper/zinc superoxide dismutase (Cu/Zn SOD) structure, and metallothionein induction. Zn deficiency can be a compromised oxidant defense system, which in turn can be reflected by evidence of cellular or tissue oxidative damage [5].
The treatment with Zn [6–8] or Se [9, 10] during Cd exposure has been demonstrated to have protective effects on Cd-induced toxicity in various organs and tissues. Recent studies have indicated that the treatment with Se [2] or Zn [11] protects renal tissues against the toxicity of Cd. However, the co-effect of the two trace elements on the toxicity caused by Cd is not yet well studied. Indeed, we have found only one published work in this context [12] which assessed the protective effect of Se and Zn on Cd-induced oxidative stress in the kidney of the rat; besides, Cd was administrated using the intraperitoneal route. This led us to wonder about the simultaneous administration of Cd, Zn, and Se by oral route, as it is the main mode of exposure to Cd in humans and animals. Therefore, it was considered of interest to investigate whether the combined treatment with Se and Zn offers more beneficial effects than that provided by either of them alone in reversing Cd-induced oxidative stress in the kidney of rat orally exposed to Cd.
Materials and Methods
Animals
Thirty 8-week-old male Wistar rats were subjected to a 2-week acclimatization period. The animals were housed in individual stainless steel cages at 23 ± 1°C and exposed to 12-h light–dark cycle. They had access to a standard rodent laboratory diet (SICO, Sfax, Tunisia; Table 1) and drinking water ad libitum. The animals were housed according to the EEC 609/86 Directives regulating the welfare of experimental animals.
Experimental Design
The experiment was conducted over a period of 5 weeks. After a period of adaptation, the animals, at the age of 10 weeks and 216 ± 13 g initial body weight, were divided into five experimental groups of six animals each: the control group was not treated with Cd. Four experimental groups received either 200 ppm Cd (as CdCl2), 200 ppm Cd + 500 ppm Zn (as ZnCl2), 200 ppm Cd + 0.1 ppm Se (as Na2SeO3), or 200 ppm Cd + 500 ppm Zn + 0.1 ppm Se in their drinking water. Cd, Zn, and Se doses and manner of administration were chosen on the basis of available literature data [6, 13]. To evaluate the daily intake of Cd, Se, and Zn in each of the experimental groups and express them as parts per million per kilogram body weight, the 24-h consumption of drinking water and body weight were monitored during the whole experiment. Drinking water consumption and daily Cd, Se, and Zn were investigated according to the method described by Brzoska and Moniuszko-Jakoniuk [14]. Detailed data on Cd, Zn and Se intakes have been presented in our previous study [15]. Daily Cd intakes were within the same ranges of values independently of whether this metal was administered alone or in combination with Zn, with Se, or with Zn and Se. Also, Zn intakes in the rats supplemented 500 ppm Zn were within the same ranges of values independently of whether this element was administered alone with Cd or in combination with Se and Cd. The same refers to Se intakes. Thus, the comparison of Cd, Zn and Se intakes in the treated groups confirms that the used experimental model is appropriate to investigate effects of the two elements on Cd toxicity.
Samples and Measurements
On day 35, the animals were killed by decapitation under anesthesia and the kidneys were removed, washed with cold saline, and immediately frozen at −80°C. Kidneys were sliced and homogenized in cold sodium phosphate buffer (pH 7.4) containing 1 mM EDTA. The homogenates were then centrifuged at 4,000 rpm for 15 min at 4°C. The supernatants were separated and used for enzyme assays and protein determination.
SOD activity was determined using pyrogallol as a substrate by the method of Marklund and Marklund [16]. This method is based on pyrogallol oxidation by the superoxide anion (O2 −) and its dismutation by SOD. One unit (U) of total SOD is defined as the amount of enzyme required to inhibit the rate of pyrogallol auto-oxidation by 50%. Catalase (CAT) activity was determined using the method described by Beers and Sizers [17] by measuring hydrogen peroxide decomposition at 240 nm. CAT units (U/mg protein) were determined as micromoles of H2O2 consumed per second per milligram protein. GSH-Px activity was assayed by the subsequent oxidation of NADPH at 240 nm with t-buthyl-hydroperoxide as substrate [18]. GPx units (U/mg protein) were defined as micromoles of NADPH oxidized per second per milligram protein. Lipid peroxidation was estimated by measuring thiobarbituric acid reactive substances and was expressed in terms of malondialdehyde (MDA) content according to the method of Yagi [19]. The concentration of total protein was estimated according to the Biuret method [20] using serum albumin as standard.
Statistical Analysis
All the data were expressed as mean ± SE. Differences among the experimental groups were assessed by one-way analysis of variance followed by protected least significant difference Fisher’s test. Values were considered statistically significant when p < 0.05.
Results
CAT Activities
As shown in Fig. 1, exposure to Cd induced a significant (p < 0.01) decrease in CAT activity (3.05 ± 0.21 U/mg protein) compared with control rats (4.15 ± 0.30 U/mg protein). When rats were concomitantly exposed to Cd and Zn, activity of CAT (5.39 ± 0.31 U/mg protein) was significantly higher than in both Cd-exposed (p < 0.0001) and control (p < 0.01) animals. Se supply alone or combined with Zn was effective in normalizing CAT activities. In Cd+Se group, CAT activity (3.93 ± 0.24 U/mg protein) was significantly lower (p < 0.05) than that in Cd+Se+Zn group (4.85 ± 0.28 U/mg protein); thus, co-treatment with Se and Zn was more effective than that with Se alone in reversing Cd-induced decrease in CAT activity.
Activities of catalase (CAT) in kidney tissues of control (C) and rats exposed for 35 days to cadmium (Cd), cadmium+selenium (Cd+Se), cadmium+zinc (Cd+Zn), and cadmium+selenium+zinc (Cd+Se+Zn). Means ± SE from six animals in each group. Significance from C: a1 p < 0.01. Significance from Cd: b p < 0.05, b3 p < 0.0001. Significance from Cd+Se+Zn: c p < 0.05. Significance from Zn: d2 p < 0.001
SOD Activities
The data presented in Fig. 2 showed that the exposure to Cd led to a significant increase (p < 0.001) in SOD activity (1.65 ± 0.09 U/mg protein) compared to the control (1.19 ± 0.10 U/mg protein). Zn supply had no significant effect on Cd-induced increase in the SOD activity, whereas Se supply alone (1.28 ± 0.04 U/mg protein) or in combination with Zn (1.32 ± 0.07 U/mg protein) entirely reversed this change.
Activities of superoxide dismutase (SOD) in kidney tissues of control (C) and rats exposed for 35 days to cadmium (Cd), cadmium+selenium (Cd+Se), cadmium+zinc (Cd+Zn), and cadmium+selenium+zinc (Cd+Se+Zn). Means ± SE from six animals in each group. Significance from C: a2 p < 0.001. Significance from Cd: b1 p < 0.01. Significance from Cd+Se+Zn: c1 p < 0.01. Significance from Zn: d2 p < 0.001
GSH-Px Activities
The GSH-Px activity was significantly decreased (p < 0.001) in Cd-exposed rats (4.61 ± 0.57 U/mg protein) as compared to control rats (7.57 ± 0.69 U/mg protein; Fig. 3). The treatment of Cd-exposed rats with Zn alone had no effect on the Cd-induced decrease in the GSH-Px activity, whereas Se supply alone partially reversed this change. In fact, no significant difference in GSH-Px activity was found between Cd+Se group (6.10 ± 0.20 U/mg protein) and both control (p = 0.060) and Cd-exposed rats (p = 0.056). With the combined Se and Zn treatment (7.93 ± 0.63 U/mg protein), we noticed a complete prevention from the Cd-induced decrease in GSH-Px activity.
Activities of glutathione peroxidase (GSH-Px) in kidney tissues of control (C) and rats exposed for 35 days to cadmium (Cd), cadmium+selenium (Cd+Se), cadmium+zinc (Cd+Zn), and cadmium+selenium+zinc (Cd+Se+Zn). Means ± SE from six animals in each group. Significance from C: a2 p < 0.001, a3 p < 0.0001. Significance from Cd: b3 p < 0.0001. Significance from Cd+Se+Zn: c p < 0.05, c3 p < 0.0001. Significance from Zn: d p < 0.05
MDA Concentrations
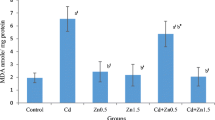
There was a significant (p < 0.0001) increase in MDA concentrations in kidney tissues after Cd treatment for 35 consecutive days (0.83 ± 0.06 μmol/mg protein) compared to the control group (0.51 ± 0.03 μmol/mg protein; Fig. 4). The treatment of Cd-exposed rats with Se had no significant effect on the Cd-induced increase in the MDA concentrations. However, Zn supply at the exposure to Cd only partially reversed this change. In fact, MDA concentrations in the Cd+Zn group (0.67 ± 0.07 μmol/mg protein) was lower than in the Cd-exposed group (p < 0.05) but still significantly higher than in the control group (p < 0.05). Interestingly, our results showed that co-treatment with Se and Zn (0.58 ± 0.03 μmol/mg protein) was more effective than that with Zn alone in reversing Cd-induced increase in the MDA concentrations.
Concentrations of malondialdehyde (MDA) in kidney tissues of control (C) and rats exposed for 35 days to cadmium (Cd), cadmium+selenium (Cd+Se), cadmium+zinc (Cd+Zn), and cadmium+selenium+zinc (Cd+Se+Zn). Means ± SE from six animals in each group. Significance from C: a p < 0.05, a1 p < 0.01, a3 p < 0.0001. Significance from Cd: b p < 0.05, b1 p < 0.01
Discussion
Through this study, we investigated the potential benefit of combined treatment with Zn and Se in reversing Cd-induced renal oxidative stress compared to Se or Zn treatment alone in rats orally exposed to Cd.
In agreement with several reports [2, 21, 22], our results indicated a significant decrease in the activity of GSH-Px in the kidney tissues of Cd-treated rats. The reduction in activity of GSH-Px might be due to depletion of Se by Cd [23] or due to the formation of a chemical complex between Cd and Se at the active site of GSH-Px [24]. It is well known that the presence of Cd in the organism decreases the level of iron (Fe) in blood, kidney, and liver [25]. Since CAT contains Fe in its active center, the decreased activity of the enzyme in the kidney of the rats exposed to Cd might be a result of Fe deficiency. The increased SOD activity in kidney, in response to Cd toxicity, observed in our study may be a defensive mechanism towards free radical damage to tissues. It is known that Cd induces the formation of superoxide anion radicals in several tissues and it is reasonable to expect an increased activity of SOD [26–28]. Consistent with our finding, Jurczuk et al. [25] have also reported a significant increase in SOD activity in the kidney of orally Cd-treated rats.
The present study demonstrated that the lipid peroxidation, reflected in MDA concentration, was significantly elevated in kidney tissues of rats treated with Cd compared to control group. The enhanced lipid peroxidation might result from the reduction in the renal activities of CAT and GSH-Px observed in these animals, since these antioxidant enzymes protect from this process via elimination of reactive oxygen species (ROS). There are several possible sources of ROS following exposure to Cd [29]. Phagocytic cells may be an important source of ROS in response to Cd ions [30]. In addition, Cd could displace Fe from its binding sites, leading to Fe redistribution and the generation of ROS via Fenton chemistry, which could then give rise to lipid peroxidation [31]. The mechanism of Cd-induced lipid peroxidation is still not fully clarified. It is multidirectional and may involve a decrease in the level of glutathione and the total pool of sulphydryl groups as well as change in the activities of antioxidant enzymes. These can induce the pro-oxidative state in the biological systems and, in turn, lead to peroxidation of polyunsaturated fatty acid [25, 32].
Several lines of evidence indicate that the treatment with Se [2, 9, 10] or Zn [6–8, 11] during Cd exposure prevented or decreased the harmful effects of Cd on the antioxidant system in different tissues. Consistent with these reports, the current study has shown that the treatment of Cd-exposed rats with Se alone increased significantly the CAT activity and reversed Cd-induced increase in SOD activity. It also partially prevented Cd-induced decrease in GSH-Px activity, but did not reverse Cd-induced increase in MDA concentrations. With Zn alone, we have noticed a significant increase in CAT activity and a partial protection against Cd-induced increase in the MDA concentrations.
Large numbers of enzymatic activities, such as those of antioxidant enzymes, are influenced by Cd [33], and the mechanisms of these effects have been hypothesized to be due to either displacement of a beneficial metal from the active site or the binding to the active site in the enzyme itself [34]. On the other hand, the activity of the antioxidant enzymes depends on a sufficient supply of the trace elements, such as Se and Zn [35].
Previous studies demonstrated that co-administration of Se restricted the uptake and distribution of Cd in some tissues, such as rat kidney and liver [36, 37], and caused an increase in Cd excretion via feces [38]. It has been found that Se may bind directly with Cd, forming a low-molecular-weight, stable, and biologically inactive Cd–selenide complex [39], and as a result, it may ameliorate the toxic effects of Cd including oxidative damage in the kidney. Se is a well-known antioxidant, which has a protective effect on Cd metabolism and complex responses of glutathione-dependent enzymes [37, 40]. Increased GSH-Px activity caused by Se treatment could be due to increased incorporation of selenocysteine which is essential for the enzyme activity [41].
The protective effect of Zn in condition of Cd exposure has been known for many years. Interactions at the stage of intestinal absorption of both metals to induce metallothionein which sequesters Cd have been suggested as the most likely mechanisms [3]. As it can be seen from the data of the present work, concomitant treatment with Cd and Zn partially reversed the Cd-induced increase in MDA concentrations in kidney tissues. This confirms our previous results indicating that Zn supply in conditions of exposure to Cd can partially protect against Cd-induced oxidative stress [7]. Zn can act as an antioxidant since it is an essential component of Cu/Zn SOD. Zn can also indirectly function as an antioxidant by inducing the synthesis of metallothionein, a thiol-rich protein which can act by binding metals with pro-oxidant activity such as Cd and by providing thiol groups which can scavenge hydroxyl radicals and singlet oxygen [42]. Zn is also involved in cell membrane stabilization and has the ability to interact with essential elements such as copper (Cu) and Fe, decreasing their content in tissues and retarding the oxidative processes [43].
Interestingly, the results of the present investigation clearly indicated that the combined treatment with Se and Zn during Cd exposure was more impressive, both in terms of recovery in antioxidant enzyme activities and reduction in MDA concentration in renal tissues, than that with Se or Zn alone. Consistent with our findings, Xiao et al. [12] have also reported that the restorative effect of Zn and Se co-treatment in Cd-induced oxidative impair in the kidney is better than that of Se or Zn. Our recent published results obtained in the same experiment show that this treatment was also more effective than that with Se or Zn alone in reversing Cd-induced thyroid dysfunction [15]. With Se and Zn treatment, we have also noticed a partial prevention from the Cd-induced changes in renal structure [44]. In the same way, Faure et al. [45] have demonstrated that association of Zn, Se, and vitamin E is more efficient than Zn alone to improve the antioxidant defense system in an animal model of insulin resistance.
Based on our results and the aforementioned reports, we suggest that Se and Zn can have a synergistic role against Cd toxicity. Indirect evidence for this mechanism was indicated in a chemical study conducted by Feroci et al. [46] on the interactions between different Se compounds and Zn, Cd, and mercury. These authors have reported that Zn and Cd display weak interactions in the presence of Se compounds. At the present stage of our study, we are unable to explain the mechanisms involved in this synergistic role; however, we can suggest that the antioxidant properties of Zn and Se and their ability to reduce Cd accumulation in the organism can be, at least in part, involved in their synergistic role against Cd toxicity.
References
World Health Organisation (WHO) Environmental Health Criteria, 134 Cadmium. IPCS, Geneva 1992.
El-Sharaky AS, Newairy AA, Badreldeen MM, Eweda SM, Sheweita SA (2007) Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology 235:185–193
Brzóska MM, Moniuszko-Jakoniuk J (2001) Interactions between cadmium and zinc in the organism. Food Chem Toxicol 39:967–980
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208
Oteiza PI, Adonaylo VN, Keen CL (1999) Cadmium-induced testes oxidative damage in rats can be influenced by dietary zinc intake. Toxicology 137:13–22
Chowdhury BA, Friel JK, Chandra RK (1987) Cadmium-induced immunopathology is prevented by zinc administration in mice. J Nutr 117:1788–1794
Jemai H, Messaoudi I, Chaouch A, Kerkeni A (2007) Protective effect of zinc supplementation on blood antioxidant defense system in rats exposed to cadmium. J Trace Elem Med Biol 21:269–273
Brzóska MM, Galażyn-Sidorczuk M, Rogalska J, Roszczenko A, Jurczuk M, Majewska K, Moniuszko-Jakoniuk J (2008) Beneficial effect of zinc supplementation on biomechanical properties of femoral distal end and femoral diaphysis of male rats chronically exposed to cadmium. Chem Biol Interact 171:312–324
Jamba L, Nehru B, Bansal MP (2000) Effect of selenium supplementation on the influence of cadmium on glutathione and glutathione peroxidase system in mouse liver. J Trace Elem Exp Med 13:299–304
Newairy AA, El-Sharaky AS, Badreldeen MM, Eweda SM, Sheweita SA (2007) The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology 242:23–30
Jacquillet G, Barbier O, Cougnon M, Tauc M, Namorado MC, Martin D, Reyes JL, Poujeol P (2006) Zinc protects renal function during cadmium intoxication in the rat. Am J Physiol Renal Physiol 290:127–137
Xiao P, Jia XD, Zhong WJ, Jin XP, Nordberg G (2002) Restorative effects of zinc and selenium on cadmium-induced kidney oxidative damage in rats. Biomed Environ Sci 5:67–74
Stajn A, Zikic RV, Ognjanovic B, Saicic ZS, Pavlovic SZ, Kostic MM, Petrovic VM (1997) Effect of cadmium and selenium on the antioxidant defense system in rat kidneys. Comp Biochem Physiol 117:167–172
Brzóska MM, Moniuszko-Jakoniuk J (2005) Bone metabolism of male rats chronically exposed to cadmium. Toxicol Appl Pharmacol 207:195–211
Hammouda F, Messaoudi I, El Hani J, Baati T, Saïd K, Kerkeni A (2008) Reversal of cadmium-induced thyroid dysfunction by selenium, zinc, or their combination in rat. Biol Trace Elem Res 126:194–203. doi:10.1007/s12011-008-8194-8
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Beers B, Sizer W (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–139
Gunzler WA, Kremers H, Flohe L (1974) An improved coupled test procedure for glutathione peroxidase in blood. Z Klin Chem Klin Biochem 12:444–448
Yagi K (1976) A simple fluorimetric assay for lipoperoxide in blood plasma. Biochem Res 15:212–216
Gornall AG, Bardawill CS, David MM (1949) Mise au point d’une méthode quantitative pour doser les protéines avec le Biuret. J Biol Chem 177:751–766
Sidhu M, Mridula S, Mandeep B, Yohesh C, Awasthi M, Ravindra N (1993) Effect of chronic cadmium exposure on glutathione S-transferase and glutathione peroxidase activities in Rhesus monkey: the role of selenium. Toxicology 83:203–213
Bragadin M, Scutari G, Folda A, Bindoli A, Rigobello M (2004) Effect of metal complexes on thioredoxin reductase and the regulation of mitochondrial permeability conditions. Ann NY Acad Sci 1030:348–354
Lazarus M, Orct T, Blanusa M, Kostial K, Pirsljin J, Beer-Ljubic B (2006) Effect of selenium pre-treatment on cadmium content and enzymatic antioxidants in tissues of suckling rat. Toxicol Lett 164:S1–S191
Gambhir J, Nath R (1992) Effect of cadmium on tissue glutathione and glutathione peroxidase in rats. Influence of selenium supplementation. Indian J Exp Biol 30:597–601
Jurczuk M, Brzoska MM, Moniuszko-Jakoniuk J, Galazyn-Sidorczuk M, Kulikowska-Karpinska E (2004) Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food Chem Toxicol 42:429–438
Sarkar S, Yadav P, Bhatnagar D (1997) Cadmium-induced lipid peroxidation and the antioxidant system in rat erythrocytes: the role of antioxidants. J Trace Elem Med Biol 11:8–13
Ogjanovic B, Pavlovic SZ, Maletic SD, Zikic RV, Stajn AS, Radojicic RM, Saicic ZS, Kostic MM, Petrovic VM (2003) Protective influence of vitamin E on antioxidant defense system in the blood of rats treated with cadmium. Physiol Res 52:563–570
Tandon SK, Singh S, Prasad S, Khandekar K, Dwivedi VK, Chatterjee M, Mathur N (2003) Reversal of cadmium induced oxidative stress by chelating agent, antioxidant or their combination in rat. Toxicol Lett 2003:145:211–217
Wang Y, Fang J, Leonard SS, Rao, KMK (2004) Cadmium inhibits electron transfer chain and induced reactive oxygen species. Free Radic Biol Med 36:1434–1443
Stohs SJ, Bagachi C (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–336
Casalino E, Cesare S, Clemente L (1997) Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement in lipid peroxidation. Arch Biochem Biophys 346:171–179
Bagchi D, Bagchi M, Hassoun EA, Stohs SJ (1996) Cadmium induced excretion of urinary lipid metabolites, DNA damage, glutathione depletion and hepatic lipid peroxidation in Sprague–Dawley rats. Biol Trace Elem Res 52:143–53
Prasadarao PV (1983) Effect of intraperitoneal cadmium administration on mitochondrial enzymes in rat tissues. Toxicology 27:81–87
Stillman MJ, Zelazowski AJ (1988) Domain specificity in metal binding to metallothionein. A circular dichroism and magnetic circular dichroism study of cadmium and zinc binding at temperature extremes. J Biol Chem 263:6128–6133
Rükgauer M, Neugebauer RJ, Plecko T (2001) The relation between selenium, zinc and copper concentration and the trace element dependent antioxidative status. J Trace Elem Med Biol 15:73–78
Badiello R, Feroci G, Fini A (1996) Interaction between trace elements: selenium and cadmium ions. J Trace Elem Med Biol 10:156–162
Rana SV, Verma S (1996) Protective effects of GSH, vitamin E and selenium on lipid peroxidation in cadmium-fed rats. Biol Trace Elem Res 51:161–168
He R, Bao KG (1994) Effects of selenium and DTPA on cadmium excretion in rats. Chung Hua Yu Fang I Hsueh Tsa Chih 28:97–99
Ohta H, Seki Y, Yoshikawa H (1995) Interactive effects of selenium on chronic cadmium toxicity in rats. ACES 8:97–104
Gan L, Liu Q, Xu HB, Zhu YS, Yang XL (2002) Effects of selenium overexposure on glutathione peroxidase and thioredoxin reductase gene expressions and activities. Biol Trace Elem Res 89:165–175
Berggren MM, Mangin JF, Gasdaka JR, Powis G (1999) Effect of selenium on rat thioredoxin reductase activity: increase by supranutritional selenium and decrease by selenium deficiency. Biochem Pharmacol 57:187–93
Sato M, Bremner I (1993) Oxygen free radicals and metallothionein. Free Radic Biol Med 14:325–337
Girotti AW, Thomas JP, Jordan JE (1985) Inhibitory effect of zinc (II) on free radical lipid peroxidation in erythrocyte membranes. Free Radic Biol Med 1:395–401
El-Heni J, Messaoudi I, Hammouda F, Kerkeni A (2008) Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver and kidney of the rat: Histology and Cd accumulation. Food Chem Toxicol 46:3522–3527
Faure P, Barclay D, Joyeux-Faure M, Halimi S (2007) Comparison of the effects of zinc alone and zinc associated with selenium and vitamin E on insulin sensitivity and oxidative stress in high-fructose-fed rats. J Trace Elem Med Biol 21:113–9
Feroci G, Badiello R, Fini A (2005) Interactions between different selenium compounds and zinc, cadmium and mercury. J Trace Elem Med Biol 18:227–234
Author information
Authors and Affiliations
Corresponding author
Additional information
I. Messaoudi and J. El Heni have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Messaoudi, I., El Heni, J., Hammouda, F. et al. Protective Effects of Selenium, Zinc, or Their Combination on Cadmium-Induced Oxidative Stress in Rat Kidney. Biol Trace Elem Res 130, 152–161 (2009). https://doi.org/10.1007/s12011-009-8324-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-009-8324-y