Abstract
Cadmium (Cd) is a highly toxic element, which may cause toxicity to most organs in the body. Zinc (Zn) and magnesium (Mg) are essential minerals with probable benefits on Cd harmful effects. Finding an efficient and non-pathological treatment against Cd toxicity seems promising. Fifty adult rats were divided into ten experimental groups of five rats each. The Cd group was treated with 1 mg Cd/kg and the control group received 0.5 cm3 normal saline. The other eight groups received Zn (0.5 and 1.5 mg/kg) and Mg (0.5 and 1.5 mg/kg) either alone or in combination with 1 mg Cd/kg through IP injection for 3 weeks. Testis malondialdehyde (MDA), sperm parameters, and testis histopathology were investigated. Cd reduced sperm parameters and increased testis MDA. Moreover, Cd exposure caused a significant histological damage in testis of male rats. However, Zn or Mg treatment prevented and reversed Cd toxic alterations in testis. These findings suggest that co-administration of Zn or Mg could improve cadmium testicular toxicity in male Wistar rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the World Health Organization (WHO), infertility is “a disease of the reproductive system defined by the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse” [1]. Infertility affects 15% of all couples of reproductive age. It is believed that about 20–30% of infertility is related to male factors including decreased semen quality [2]. Many factors such as physiological, genetic, environmental, and social factors contribute to reproductive health. Among the environmental factors, air pollution, chemicals, radiation, and heavy metals can be pointed out. Moreover, lifestyle factors such as exercise, diet, smoking, and alcohol have crucial effects on reproductive health [3]. Additionally, these factors have an effect on spermatogenesis, sperm parameters, sperm chromatin integrity, DNA modification, and hormones regulation [4]. Semen quality is a valuable parameter to assess male reproductive health. Semen analysis may be useful in both clinical and research settings, for investigating male fertility status as well as monitoring spermatogenesis during and following male fertility regulation [1, 5].
Cadmium (Cd) is a widespread environmental pollutant. Cd exposure may occur through drinking water, food, mining, manufacturing, and smoking [6]. Cd induced toxicity to various organs (e.g., liver, kidney) and many studies have shown that testis is extremely sensitive to Cd damages [7]. In vivo and in vitro studies have indicated that Cd exposure causes blood-testis barrier (BTB) disruption, germ cell loss, testicular edema, hemorrhage, necrosis, and sterilized testicular cells in mammalians [6]. Moreover, the relationships between parameters of semen quality and Cd exposure have been illustrated in many studies [8, 9]. The precise mechanism of Cd testicular toxicity is not well understood. Cd probably disrupts the BTB via specific signal transduction pathways and signaling molecules, such as p38 mitogen-activated protein kinase [6, 10]. Furthermore, Cd plays a role in oxidative stress, since this metal can alter the antioxidant defense system and generate free radicals that change cellular membrane structure through lipid peroxidation process [11]. Attempts have been made to minimize the severity of Cd toxicity via enhanced sequestration (chelation) and elimination using different agents. However, in case of chronic and low Cd exposure, chelation therapy has not produced acceptable results. Therefore, developing a safer and more affordable therapeutic approach for Cd testicular toxicity seems inevitable. As a result, there is an interest to determine a natural treatment for Cd poisoning. Experimental evidence has confirmed the positive effects of different supplements such as vitamins and antioxidant to Cd damage on various tissues [12,13,14]. Furthermore, it has been reported that treatment with Mg or Zn during Cd exposure prevented the harmful effects of Cd [15].
Zn is an essential trace element that has a major role in cell proliferation, reproduction, homeostasis, immune system, and antioxidant defense system [16]. Specifically, Zn is an essential element in spermatogenesis and male fertility. Zn supplement improves semen quality and pregnancy rate, and significantly increases the sperm volume, sperm motility, and percentage of normal sperm morphology [17]. Zn interferes with Cd at different levels of absorption, distribution, and excretion level. Zn could have protective effects against Cd toxicity in various organs [15]. A possible mechanism of Zn protection is metallothionein (Mt) stimulation [18, 19]. Moreover, Zn plays a very important role in antioxidant defense system via antioxidative enzymes’ gene expression [20]. Mg, a mineral element, acts as a cofactor in many enzymatic reactions in the body. Spermatogenesis and sperm motility are increased as a result of Mg therapy [21]. According to research finding, Mg supplement reduces Cd levels in peripheral blood, organ Cd accumulation, and Cd-related lipid peroxidation (LPO) [22, 23]. Moreover, Mg protective roles against Cd toxicity may be due to the antagonistic properties of Mg or the stimulatory effect of Mg in producing de novo glutathione (GSH) [24].
It is therefore indispensable to determine a possible protective role of Mg and Zn on Cd testicular toxicity. In that regard, this study will aim to find novel therapeutic agent or preventive approaches against Cd testicular toxicity in male Wistar rats.
Method and Material
Animals
Fifty adult (10-week-old) male Wistar rats, weighing 200 ± 50 g, were housed in conventional conditions at a temperature of 25 ± 1 °C, with a relative humidity of 50 ± 10% and a 12/12-h light/dark cycle. The study was approved by Isfahan University of Medical Science Experimental Animals Local Ethics Committee. A commercial balanced diet and tap water ad libitum were provided throughout the experimental period. All animal experiments were performed in accordance with the National Institute of Health Guideline for the Care and Use of Laboratory Animals (NIH Publications No. 80-23), revised 1996 [25].
Experimental Design
Cadmium chloride (CdCl2•2 1/2H2O), zinc chloride (ZnCl2), and magnesium chloride (MgCl2.2H2O) were purchased from Acros Organics (New Jersey, USA). The rats were randomly divided into ten experimental groups (five rats per group) as follows: control group, one group treated with Cd alone, two groups received Zn alone (0.5 and 1.5 mg/kg), two groups received Mg alone (0.5 and 1.5 mg/kg), and four groups received Zn (0.5 and 1.5 mg/kg) or Mg (0.5 and 1.5 mg/kg) during Cd exposure for 21 days. CdCl2 was interaperitoneally injected at a dose of 1 mg/kg/day for 21 days. Control rats received only physiological saline (0.9%). Cd, Zn, and Mg doses were chosen based on available literature data [24, 26]. At the end of exposure duration, animals were sacrificed by cervical dislocation and rats’ testes were removed. The epididymis was separated, trimmed free of fat, placed on a paper towel to remove any liquid, and then weighed. The left testis weighed and was used for histological studies. The right testis was kept in − 80 °C until assayed.
Sperm Parameters
The caudal epididymis was used for sperm analysis. Briefly, cauda epididymis was disrupted in Petri dishes containing Ham’s F10 medium, and left for 30 min at 36 °C for sperm release into the media [27,28,29]. Sperm analysis was performed based on WHO criteria [30].
Sperm Count
Sperm counts were performed manually using an improved Neubauer hemocytometer. The slides were then examined at magnifications of × 40 under optical microscope. The sperm concentration was expressed as × 106 ml−1.
Sperm Morphology
One drop of sperm suspension was used for preparing smears on a clean, air-dried slide. Then, smears were fixed in 95% ethanol and stained with Papanicolaou. Two hundred sperm cells were examined at × 100 magnification. Any disorders in the morphology and structure of either head or tail or both were considered as abnormal.
Sperm Motility
The percentage of motile sperm cells was assessed at × 40 magnification of objective lens under light microscope. At least 200 spermatozoa were counted and the percentage of motile sperm cells (progressive or non-progressive) was counted.
Sperm Viability
In order to study the sperm viability, supravital staining method was used. A drop of sperm suspension was placed on a spot plate and mixed with one drop of 1% aqueous eosin Y solution. After 15 s, two drops of 10% aqueous nigrosin solution were added and thoroughly mixed. A drop of this mixture was transferred to a clean glass slide. A thin smear was made and then air-dried. The smears were examined with a 100 oil magnification. Live sperm cells appear white and dead sperms were stained dark pink. Two hundred sperms were counted for each sample and viability percentages were calculated.
Tissues Homogenization
Frozen tissue samples were quickly weighed and homogenized in phosphate buffer (100 mM) containing EDTA (1 mM; pH 7.4; 1:10 w/v) and then centrifuged (12,000 × rpm, 30 min, 4 °C). The supernatant was used for biochemical analysis. All procedures were performed at 4 °C.
Biochemical Analysis
Measurement of Testis Protein Content
The protein content in rat testis was determined by protein measurement kit from Tali Gene Pars Company (Isfahan, Iran). The principle of the Tali-Lowry Protein Assay Kit is based on Lowry method.
Measurement of MDA Levels
Trichloroacetic (TCA) acid, thiobarbituric acid (TBA), and butylated hydroxy toluene (BHT) were purchased from Sigma–Aldrich chemical company (St. Louis, MO, USA). Testis malondialdehyde (MDA) levels were assayed as an index of lipid peroxidation (LPO) by the method of Esterbauer and Cheeseman [31]. In this assay, The MDA in the sample is reacted with TBA at high temperature (95 °C) to generate the 1:2 MDA-TBA adducts. The MDA-TBA adduct can be easily quantified calorimetrically (532 nm). The MDA results were expressed as nanomoles of malondialdehyde formation per milligram of protein (nmol/mg protein).
Histopathologic Analysis
Left testes’ tissues were fixed in 10% neutral formalin solution. The tissue samples were embedded in paraffin wax and sectioned at a thickness of 7 μm. The sections were stained with hematoxylin and eosin according to [32].The tissues were graded as +: mild, ++: moderate, +++: severe changes depending on the extent of observed changes ([33] and [34]).
Statistical Analysis
Statistical analysis of data was achieved by using SPSS statistics software 15. All data were expressed as mean ± standard deviation (SD) for five results. The groups were compared using one-way analysis of variance (ANOVA), followed by post hoc Fisher’s least significant difference (LSD) test. P values less than 0.05 were considered significant.
Results
Body and Testis Weight
Cd exposure exerted a significant reduction in body and testis weight in comparison to the control group. However, co-administration of 1.5/0.5 mg/kg Mg or 1.5 mg/kg Zn for 3 weeks restored body weight as compared to Cd group. Moreover, co-administration of Zn (1.5/0.5 mg/kg) or 1.5 mg/kg Mg during chronic Cd intoxication slightly improved testis weight as compared to Cd group. In addition, Mg (0.5/1.5 mg/kg) and (0.5/1.5 mg/kg) Zn treated group had higher body and testis weight in comparison to the Cd group (Table 1).
Sperm Parameters
Exposure to 1 mg/kg Cd caused a significant decrease in sperm count, sperm morphology, sperm motility, and sperm viability (Table 2 and 3). Three categories (progressive, non-progressive, and immotile) were considered for sperm motility indexing. As with the control group, Cd treatment significantly decreased the progressive and immotile sperms in rats. These toxic effects of Cd were prevented as a consequence of Zn and Mg co-administration (Table 2 and 3). Overall, administration of Zn at two dosages alone or in combination with Cd revealed a significant improvement in sperm quality compared with that of the Cd group (Table 2). As shown in Tables 2 and 3, Zn and Mg alone have no effect on sperm parameters compared to that of the controls. Mg co-administration in Cd treated rats significantly reduced Cd alterations on sperm quality mostly at higher doses, as compared to the animals exposed to Cd alone (Tables 3).
Measurement of MDA Levels
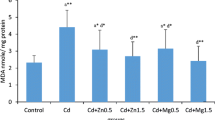
The presented results in Figs. 1 and 2 show a statistically significant increase of testis MDA levels in Cd-exposed rats compared to those of the control animals. Treatment with Zn and Mg at the both two dosages alone resulted in a significant (p < 0.001) improvement in MDA levels in rats treated with the Cd. Although, MDA levels were higher than those of the control group in the group that received Cd + Zn (0.5 mg/kg Zn) and Cd + Mg (0.5 mg/kg Mg), there was a significant reduction in MDA concentrations in these two groups in comparison with the Cd treated rats. In addition, co-administration of Zn and Mg (1.5 mg/kg doses reversed Cd-induced testis MDA levels.
Concentrations of malondialdehyde (MDA) in testis tissue of control and rats exposed for 21 days to cadmium (Cd), Zn and cadmium + zinc (Cd + Zn). Data are mean ± SD for five experiment. Statistically significant differences (SPSS, one-way ANOVA) are indicated by avs. control, bvs. 1 mg/kg Cd. *p < 0.05, ǂp < 0.001
Concentrations of malondialdehyde (MDA) in testis tissue of control and rats exposed for 21 days to cadmium (Cd), Mg and cadmium + magnesium (Cd + Mg). Data are mean ± SD for five experiments. Statistically significant differences (SPSS, one-way ANOVA) are indicated by avs. control, bvs. 1 mg/kg Cd. *p < 0.05, ǂp < 0.001
Histopathologic Analysis
In the control rats, the seminiferous tubules and interstitial connective tissue were structurally normal and contained normal spermatogenic cells layer and spermatozoa (Fig. 3a).
However, Cd treatment caused degenerative changes in the seminiferous tubule with loss of spermatogenesis. Moreover, testis section in Cd group showed severe necrosis of seminiferous tubules and an absence of spermatogenic cells (Fig. 3b).
Zn and Mg alone did not induce any significant histological change in testes (Fig. 4a–d). However, the spermatogenic cells, spermatozoa, and spermatid were observed in the seminiferous tubules of 0.5 mg/kg Mg (Fig. 4a) group. Testis of the rats, treated with Cd + Mg (0.5 and 1.5 mg/kg), showed mild to moderated necrosis of seminiferous tubules in comparison those of the control group (Fig. 5a). In addition, a mild necrosis was observed in the seminiferous tubules of the rats in the Cd + Zn (at both dosages) groups (Fig. 6a, b). Nonetheless, the severity of the necrosis was lower in these groups when compared with the Cd group.
Discussion
The present study was undertaken in order to investigate the protective roles of zinc and magnesium against cadmium testicular toxicity.
The body and testis weight were decreased after 3 weeks of Cd exposure, which is in agreement with the findings of other studies. Earlier results have shown that cadmium could inhibit growth and protein synthesis [12, 35]. There is a positive correlation between the weight of testis and number of germ cells [36]. Moreover, Schlappack et al. demonstrated that testicular weight loss is a result of damage to the differentiated spermatogenic cells [37]. The results of the present histopathological study confirmed and elucidated these findings. The histopathological investigations provided more detailed data on the mechanisms of Cd testicular toxicity. This heavy metal caused degenerative changes in the seminiferous tubule with loss of spermatogenesis. Actually, the pathological damage to seminiferous epithelium may cause the deterioration of sertoli and germ cells, which in turn results in impaired spermatogenesis [38, 39]. There are several of hypotheses, which precisely explain the mechanism of cadmium testicular toxicity. The first mechanism, which can be partly explained the toxic effect of Cd is oxidative stress alteration, antioxidant enzymes activity reduction, reactive oxygen species (ROS) production, and lipid peroxidation [40]. Accordingly, the current study revealed that MDA level was increased in the testis as an index of lipid peroxidation. Despite the fact that Cd is not able to generate ROS directly, its toxicity leads to superoxide anion (O−2), hydroxyl radicals (OH−), and hydrogen peroxide (H2O2) production [41, 42]. Oxidative stress and lipid peroxidation appears to have a major role in Cd-induced testicular toxicity. Since human spermatozoa contain high concentrations of polyunsaturated fatty acids (PUFA) within their plasma membrane, they are predominantly susceptible to peroxidative damage and possess a significant ability to generate superoxide anion and hydrogen peroxide. Therefore, susceptibility of spermatozoa to oxidative stress and ROS results in sperm quality reduction as a consequence of Cd toxicity. As it could be expected, Cd exposure decreased sperm parameters including sperm count, motility, viability, and morphology in the present study. On the other hand, ROS production in the testis may lead to further injury to vital components of the cell, including DNA, RNA, and proteins, which cause low and abnormal sperm [12, 41, 43]. According to the second mechanism, Cd activates a number of signal transduction pathways such as MAPK, PI3K/c-Src/FAK, and c-JNK signaling pathway, which, in turn, disrupts blood-testis barrier (BTB) and cell junction in the seminiferous epithelium [6, 13]. In addition, cadmium induces a cascade of inflammatory reactions with increased production of pro-inflammatory cytokines, particularly TNFa that generates ROS from the tissues, which in turn causes lipid peroxidation and leads to further testicular tissue injuries [11]. These findings support the results of the other reports on the ability of Cd to seriously alter toxicity of the testis and reproductive tract in male rats [12, 41, 43].
There is a host of evidence on the benefits of Mg and Zn co-treatment on the prevention of the harmful effects of Cd [15].
In the current study, the treatment of the Zn alone and along with Cd improved the testes and body weight, sperm parameters, and histopathological alterations caused by Cd. Moreover, results showed that the co-supply of Zn recovered the MDA accumulation in Cd-exposed animals to control values. However, higher doses of Zn were more effective on Cd alterations. Zn is a vital trace element with numerous benefits for male reproductive system function, including testicular development, testosterone production, and sperm maturation [44]. In case of Zinc deficiency, male hypogonadism, reduction of sperm fertilization capacity, and partial development of sex characteristics are observed in humans [20]. A meta-analysis study has shown that there is a significant positive correlation between seminal plasma Zn concentrations and male fertility. In the human reproductive system, Zn contributes to a wide range of functions, from the ultra-structural stabilization of chromatin compaction, to mitochondria-dependent process modulations, including cell respiration and programmed cell death [17]. The observed therapeutic potency of Zn might be due to several contributing factors, primarily including its antioxidant potential and its participation in the antioxidant defense system. Zinc is a recognized antioxidant factor that plays a crucial role in free radical scavenging enzymes such as Cu/Zn-SOD. Moreover, Zn is known as a defender agent for thiols and sulfhydryl groups, inhibitor of NADPH oxidase and free radical generation by preventing interaction between chemical groups with iron [16, 45]. Furthermore, Al badr et al. showed that zinc deficiency enhances expression of T-helper1-type cytokines TNF-α and IFN-γ, which in turn increases ROS and lipid peroxidation generation [46]. Cd likely uptake into Sertoli and/or germ cell probably occurs through Cd2+/Zn2+ transporters (ZIP8) that mediates the transport of one or more essential metals such as Zn2+ or Mn2+/ HCO3. Apparently, ZIP8-mediated Cd2+ uptake is most highly inhibited by Zn2+ [6, 10]. Zn induces synthesis of metallothionein (MT) in the liver and increases Cd concentration in hepatic tissues but then decreases it in the testis. Additionally, in case of zinc deficiency, more active cadmium is released from the MT and is accumulated in the reproductive tissue [45, 46].
Results obtained from this study have shown that Mg administrations prevented Cd-induced adverse effects on sperm parameters, testis’ histopathology, and MDA levels, since the values obtained for these parameters in Cd + Mg1.5 mg/kg group were within the values of controls. To the best of our knowledge, this is the first study to provide data on the potential protective effects of Mg on Cd-induced toxicity in testis. These positive effects of Mg treatment can be explained by the fact that Mg induces de novo synthesis of GSH that is an important cellular antioxidant and could decreases lipid peroxidation production [24]. Additionally, magnesium deficiency enhances oxidative stress by increasing production of free radicals and decreasing antioxidant defense system [24]. The observed decrease in the level of MDA further supports the beneficial effects of Mg in antioxidant activity via lipid peroxidation prevention in rats’ testis. It has been shown that serum and seminal plasma Mg levels in the sub fertile male group were significantly higher than those in the fertile male group [47, 48]. Wong et al. showed that there is a positive correlation between Mg levels in seminal plasma and sperm concentration. Even though the effects of Mg on sperm quality is not yet clear, it could be due to the role of Mg in biological processes such as glycolysis, protein synthesis, and reproduction [13]. In addition, Mg supplementation could reduce Cd accumulation in different organs. The more prominent beneficial effect of Mg against Cd toxicity is interactions between Mg and Cd at different stages of their metabolism, i.e., absorption, distribution, and excretion from the body. Mg can prevent Cd absorption from GIT through competition for similar transporter channels including Mag T1 and Mag T2. Consequently, an increase in the intake of Mg can prevent Cd transport and eventually decrease Cd absorption through GIT ([23, 24, 49]; and [15]).
In conclusion, the results of the current study have revealed that Cd exposure affects sperm and oxidative stress parameters and causes histopathological damages to the testis. In contrast, Zn and Mg co-treatment can prevent Cd toxic effects on testis. Noteworthy, further studies are necessary to explain other possible protective effects of Zn and Mg in subjects exposed to Cd and to establish the most effective dose of these elements. The protective effects of Zn and Mg may be accompanied by their antagonistic action on cadmium toxicity and antioxidant property. Therefore, the results of the present study may leads to the discovery of preventive as well as therapeutic agents against Cd damages on testicular function. However, further studies are necessary to explain other underlying mechanism of Zn and Mg protective roles in male infertility in populations that are environmentally exposed to Cd, especially smokers and industrial workers.
References
Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, Vanderpoel S (2009) International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 92(5):1520–1524. https://doi.org/10.1016/j.fertnstert.2009.09.009
Feng HL (2003) Molecular biology of male infertility. Arch Androl 49:19–27
Sharma R, Biedenharn KR, Fedor JM, Agarwal A (2013) Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol 11(1):66. https://doi.org/10.1186/1477-7827-11-66
Wong EW, Cheng CY (2011) Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol Sci 32(5):290–299. https://doi.org/10.1016/j.tips.2011.01.001
Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, Paneth N, Wirth JJ (2008) Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect 116(11):1473–1479. https://doi.org/10.1289/ehp.11490
Siu ER, Mruk DD, Porto CS, Cheng CY (2009) Cadmium-induced testicular injury. Toxicol Appl Pharmacol 238(3):240–249. https://doi.org/10.1016/j.taap.2009.01.028
Laskey JW, Rehnberg GL, Laws SC, Hein JF (1984) Reproductive effects of low acute doses of cadmium chloride in adult male rats. Toxicol Appl Pharmacol 73(2):250–255. https://doi.org/10.1016/0041-008X(84)90330-2
Amara S, Abdelmelek H, Garrel C, Guiraud P, Douki T, Ravanat JL et al (2008) Preventive effect of zinc against cadmium-induced oxidative stress in the rat testis. J Reprod Dev 54(2):129–134. https://doi.org/10.1262/jrd.18110
Benoff S, Hauser R, Marmar JL, Hurley IR, Napolitano B, Centola GM (2009) Cadmium concentrations in blood and seminal plasma: correlations with sperm number and motility in three male populations (infertility patients, artificial insemination donors, and unselected volunteers). Mol Med 15(7-8):248–262. https://doi.org/10.2119/molmed.2008.00104
He L, Wang B, Hay EB, Nebert DW (2009) Discovery of ZIP transporters that participate in cadmium damage to testis and kidney. Toxicol Appl Pharmacol 238(3):250–257. https://doi.org/10.1016/j.taap.2009.02.017
Al-Azemi M, Omu FE, Kehinde EO, Anim JT, Oriowo MA, Omu AE (2010) Lithium protects against toxic effects of cadmium in the rat testes. J Assist Reprod Genet 27(8):469–476. https://doi.org/10.1007/s10815-010-9426-3
El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH (2004) Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and beta-carotene. Food Chem Toxicol 42(10):1563–1571. https://doi.org/10.1016/j.fct.2004.05.001
Wong WY, Flik G, Groenen PM, Swinkels DW, Thomas CM, Copius-Peereboom JH et al (2001) The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod Toxicol 15(2):131–136. https://doi.org/10.1016/S0890-6238(01)00113-7
Wu SH, Oldfield JE, Whanger PD, Weswig PH (1973) Effect of selenium, vitamin E, and antioxidants on testicular function in rats. Biol Reprod 8:625–629
Matovic V, Bulat ZP, Djukic-cosic D, Soldatovic D (2010) Zinc, copper, or magnesium supplementation against cadmium toxicity. Nova Science Publishers, UK
Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME (2012) Zinc and human health: an update. Arch Toxicol 86(4):521–534. https://doi.org/10.1007/s00204-011-0775-1
Zhao J, Dong X, Hu X, Long Z, Wang L, Liu Q, Sun B, Wang Q, Wu Q, Li L (2016) Zinc levels in seminal plasma and their correlation with male infertility: a systematic review and meta-analysis. Sci Rep 6(1):22386. https://doi.org/10.1038/srep22386
Bonda E, Wlostowski T, Krasowska A (2004) Testicular toxicity induced by dietary cadmium is associated with decreased testicular zinc and increased hepatic and renal metallothionein and zinc in the bank vole (Clethrionomys glareolus). Biometals 17(6):615–624. https://doi.org/10.1007/s10534-004-1226-8
Rafique M, Pervez S, Tahir F (2010) Protective effect of zinc over lead toxicity on testes. J Coll Physicians Surg Pak 20:377–381
Ahmadi S, Bashiri R, Ghadiri-Anari A, Nadjarzadeh A (2016) Antioxidant supplements and semen parameters: an evidence based review. Int J Reprod Biomed 14:729–736
Theophanides T, Anastssopoulou J (1997) Magnesium: current status and new developments. Springer, Netherlands. https://doi.org/10.1007/978-94-009-0057-8
Jacobs RM, Jones AO, Fox MR, Lener J (1983) Effects of dietary zinc, manganese, and copper on tissue accumulation of cadmium by Japanese quail. Proc Soc Exp Biol Med 172:34–38
Noel L, Huynh-Delerme C, Guerin T, Huet H, Fremy JM, Kolf-Clauw M (2006) Cadmium accumulation and interactions with zinc, copper, and manganese, analysed by ICP-MS in a long-term Caco-2 TC7 cell model. Biometals 19(5):473–481. https://doi.org/10.1007/s10534-005-5147-y
Matovic V, Plamenac Bulat Z, Djukic-Cosic D, Soldatovic D (2010) Antagonism between cadmium and magnesium: a possible role of magnesium in therapy of cadmium intoxication. Magnes Res 23(1):19–26. https://doi.org/10.1684/mrh.2010.0196
National Research Council (US) (2011) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 8th edn, Washington (DC), National Academies Press (US)
Jacquillet G, Barbier O, Cougnon M, Tauc M, Namorado MC, Martin D, Reyes JL, Poujeol P (2006) Zinc protects renal function during cadmium intoxication in the rat. Am J Physiol Renal Physiol 290(1):F127–F137. https://doi.org/10.1152/ajprenal.00366.2004
Ben KL, Hamilton MS, Alexander NJ (1988) Vasectomy-induced autoimmunity: monoclonal antibodies affect sperm function and in vitro fertilization. J Reprod Immunol 13(1):73–84. https://doi.org/10.1016/0165-0378(88)90050-2
Golshan Iranpour F, Rezazadeh Valojerdi M (2013) The epididymal sperm viability, motility and DNA integrity in dead mice maintained at 4-6oC. Iran J Reprod Med 11(3):195–200
Mdhluli MC, van der Horst G (2002) The effect of oleanolic acid on sperm motion characteristics and fertility of male Wistar rats. Lab Anim 36(4):432–437. https://doi.org/10.1258/002367702320389107
WHO Organization (2010). Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Intraction New York: Cambridge University Press
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421. https://doi.org/10.1016/0076-6879(90)86134-H
Drury RA, Wallington EA (1980) Carleton’s histological techniques. Oxford University Press, London
Adil M, Kandhare AD, Dalvi G, Ghosh P, Venkata S, Raygude KS, Bodhankar SL (2016) Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Ren Fail 38(6):996–1006. https://doi.org/10.3109/0886022X.2016.1165120
Shackelford C, Long G, Wolf J, Okerberg C, Herbert R (2002) Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol 30(1):93–96. https://doi.org/10.1080/01926230252824761
Waalkes MP, Diwan BA (1999) Cadmium-induced inhibition of the growth and metastasis of human lung carcinoma xenografts: role of apoptosis. Carcinogenesis 20(1):65–70. https://doi.org/10.1093/carcin/20.1.65
Hikim AP, Amador AG, Klemcke HG, Bartke A, Russell LD (1989) Correlative morphology and endocrinology of Sertoli cells in hamster testes in active and inactive states of spermatogenesis. Endocrinology 125(4):1829–1843. https://doi.org/10.1210/endo-125-4-1829
Schlappack OK, Delic JI, Harwood JR, Stanley JA (1988) Protection from radiation-induced damage to spermatogenesis in the androgen pretreated rat. Radiother Oncol 12(3):219–224. https://doi.org/10.1016/0167-8140(88)90264-2
De Souza Predes F, Diamante MA, Dolder H (2010) Testis response to low doses of cadmium in Wistar rats. Int J Exp Pathol 91:125–131
Martin LJ, Chen H, Liao X, Allayee H, Shih DM, Lee GS, Hovland DN Jr, Robbins WA, Carnes K, Hess RA, Lusis AJ, Collins MD (2007) FK506, a calcineurin inhibitor, prevents cadmium-induced testicular toxicity in mice. Toxicol Sci 100(2):474–485. https://doi.org/10.1093/toxsci/kfm229
Koriem KM, Fathi GE, Salem HA, Akram NH, Gamil SA (2013) Protective role of pectin against cadmium-induced testicular toxicity and oxidative stress in rats. Toxicol Mech Methods 23(4):263–272. https://doi.org/10.3109/15376516.2012.748857
Ola-Mudathir KF, Suru SM, Fafunso MA, Obioha UE, Faremi TY (2008) Protective roles of onion and garlic extracts on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem Toxicol 46(12):3604–3611. https://doi.org/10.1016/j.fct.2008.09.004
Ozdamar AS, Soylu AG, Culha M, Ozden M, Gokalp A (2004) Testicular oxidative stress. Effects of experimental varicocele in adolescent rats. Urol Int 73(4):343–347. https://doi.org/10.1159/000081596
Oliveira H, Spano M, Santos C, Pereira Mde L (2009) Adverse effects of cadmium exposure on mouse sperm. Reprod Toxicol 28(4):550–555. https://doi.org/10.1016/j.reprotox.2009.08.001
Bepari M, Maity P, Das M, Choudhury SM (2014) Zinc and αlpha-lipoic acid alleviate cypermethrin induced reproductiv toxicity in mature male Wistar rats. Pharm Sci 4:9–14
Sankako MK, Garcia PC, Piffer RC, Dallaqua B, Damasceno DC, Pereira OC (2012) Possible mechanism by which zinc protects the testicular function of rats exposed to cigarette smoke. Pharmacol Rep 64(6):1537–1546. https://doi.org/10.1016/S1734-1140(12)70951-9
Al-Bader A, Omu AE, Dashti H (1999) Chronic cadmium toxicity to sperm of heavy cigarette smokers: immunomodulation by zinc. Arch Androl 43(2):135–140. https://doi.org/10.1080/014850199262643
Papadimas J, Bontis J, Ikkos D, Mantalenakis S (1983) Seminal plasma zinc and magnesium in infertile men. Arch Androl 10(3):261–268. https://doi.org/10.3109/01485018308987576
Sorensen MB, Bergdahl IA, Hjollund NH, Bonde JP, Stoltenberg M, Ernst E (1999) Zinc, magnesium and calcium in human seminal fluid: relations to other semen parameters and fertility. Mol Hum Reprod 5(4):331–337. https://doi.org/10.1093/molehr/5.4.331
Grosicki A, Małagocki P, Kycko A, Monkiewicz J, Korol W (2015) Magnesium supplements affect selected cadmium toxic actions and uptake of repeated doses of cadmium. Bull Vet Inst Pulawy 59:12–30
Author information
Authors and Affiliations
Contributions
All authors participated in the writing of this paper.
Corresponding author
Ethics declarations
The study was approved by Isfahan University of Medical Science Experimental Animals Local Ethics Committee
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Babaknejad, N., Bahrami, S., Moshtaghie, A.A. et al. Cadmium Testicular Toxicity in Male Wistar Rats: Protective Roles of Zinc and Magnesium. Biol Trace Elem Res 185, 106–115 (2018). https://doi.org/10.1007/s12011-017-1218-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1218-5