Abstract
Marginal zinc deficiency (MZD), the subclinical stage of zinc deficiency, is common in industrialized societies. Serum zinc, the most common biomarker of zinc status, lacks sensitivity and specificity to diagnose this deficiency. Hair zinc, however, is sensitive and specific enough to detect MZD in children. Differences in hair zinc associated with age and sex have been reported. These differences have not been investigated thoroughly; therefore, interpretation of the results of hair analyses is difficult. This cross-sectional study was designed to examine the hair zinc status of a group of Vancouver preschoolers (24–71 months) and assess the age- and sex-based differences in their hair zinc. Hair samples were obtained (n = 719) and analyzed for zinc using inductively coupled plasma mass spectrometry. Our results indicated a mean hair zinc of 115 ± 43 μg/g with 17% below the low hair zinc cutoff (70 μg/g). Boys and girls had comparable mean hair zinc, while girls had a significantly higher occurrence of low hair zinc than boys (21% vs. 12%). Children <4 years of age had significantly lower mean hair zinc and higher rate of low hair zinc compared to children ≥4. Our study provides important reference values for the hair zinc of healthy North American preschoolers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of a low hair zinc level to indicate poor zinc nutriture was established in the 1960s when Prasad and co-workers reported the first cases of human zinc deficiency in young Middle Eastern men [1, 2]. One decade later, low hair zinc was found to be common among healthy low-income American children [3], and the existence of a subtle and mild zinc deficiency in these children was confirmed through a supplementation program [4]. Following the publication of these studies, other similar studies emerged [5, 6] which, combined, led to a better recognition of this subtle form of zinc deficiency. This deficiency had no specific clinical signs, and serum zinc, the most common biomarker of zinc status, was not able to detect it. Hair zinc, on the other hand, appeared able to do so. This deficiency, since it is marginal when seen against the full spectrum of zinc deficiency, has become known as marginal zinc deficiency (MZD).
During childhood, MZD may affect growth and development adversely [6–9]. Currently, there is little information available on the long-term effect of childhood zinc deficiency. However, it is recognized that the human brain continues to develop during the preschool period, and this growth can be adversely affected if adequate nutrients are not available. In addition, the available information indicates that some micronutrient deficiencies during infancy, such as iron, may have long-term consequences [10]. Therefore, it is crucial to err on the side of caution by detecting and rectifying childhood deficiencies as early as possible.
Hair zinc is commonly used in MZD studies of children, and its usefulness with children has been documented in many industrialized countries including Canada [6] and the USA [4]. Overall, it is believed that, in the absence of protein-energy malnutrition, hair zinc can be a good index of MZD in children [11].
Hair zinc is sensitive enough to detect the shortages at early stages. The uptake of zinc by the hair is quite slow and may be hindered preferentially if the body’s zinc supply is decreased. In these circumstances, the more important zinc-dependent organs and machineries continue to receive an adequate supply without any symptom of zinc deficiency becoming apparent, while the concentration of zinc in the hair is reduced [12]. It is this special physiology that confers sensitivity to hair zinc as a biomarker of marginal zinc deficiency.
In addition, once the metal is incorporated into the hair, it is no longer in equilibrium with the body and, therefore, is not susceptible to circadian variations or homeostatic regulations [13]. This feature confers specificity to hair zinc as a biomarker. On the practical side of the issue, also, the higher range of concentration of zinc in hair (parts per million compared to parts per billion in blood) facilitates the analyses greatly [11], while its convenience in sample collection and storage makes it a suitable method in studies of large populations, especially in children.
Despite these advantages, differences in hair zinc associated with age [3], sex [5, 6], and environmental exposure [14] have been documented. To date, there have not been adequately large studies conducted to characterize these differences. Therefore, one of the primary problems in interpreting the results of hair analyses is the lack of a normal range of concentration for different age groups and sexes.
In addition, in North America, the information regarding preschoolers’ zinc status is scarce. In the USA, the early works on childhood MZD [3, 4, 7] have not been followed by many further studies. In Canada, studies documenting the occurrence of MZD in preschoolers and its response to supplementation were last done nearly two decades ago [5, 6]. Therefore, we conducted this present study to fulfill these specific objectives: (1) to assess the hair zinc status and the extent of low hair zinc in a large sample of preschool children living in Vancouver, Canada and (2) to explore differences in preschoolers’ hair zinc content based on age and sex.
Methods and Materials
Study Design
This was designed as a citywide cross-sectional survey, representing all 23 social planning neighborhoods of the city of Vancouver. Childcare and preschool centers were planned as the sources for our recruitment. In studying data from a national census conducted in 2001, it was apparent that there was a wide variation in the socio-economic status (SES) both between and within individual neighborhoods. Accordingly, neighborhoods were broken down into smaller units referred to as dissemination areas (DA, the basic unit of Canada’s census geography composed of one or more neighboring blocks having a population of 400–700 people). The Geographic Information System of the Human Early Learning Partnership produced maps of each neighborhood with their DAs color-coded indicating the SES of that DA. The potential participating centers (childcare and preschool centers) were identified on the map of each neighborhood. Care was taken to have fair representation of each color (each SES) when selecting centers for participation within a neighborhood.
Centers were contacted by telephone and were asked about their willingness to participate in the survey. In total, 55 centers agreed to participate (55/68 = 81% response rate), while 13 refused to do so. With each refusal a new center, within the same neighborhood DA or within an other DA of that neighborhood with similar SES, was selected for contact. Following initial contact, survey information packages were mailed to the centers. Packages contained a letter from the principal investigator explaining the project and the institutions involved in it and a schematic presentation outlining the sequence of the survey events together with their allotted time intervals. Subsequently, meetings were held with participating centers, during which the survey and the survey team were introduced to the childcare professionals, the time line for the survey and the activities involved were discussed, and the initial letter of contact was given to the centers to be sent out to the parents in a week’s time. One to 2 weeks after the letter of initial contact, survey packages containing a subject information letter and a consent form and the survey questionnaire were sent to the children’s homes. During the next 2 weeks, completed survey packages were returned to the center. Following this, visits to the centers were scheduled during which anthropometric measurements and hair samples were obtained from the consenting children. The survey was planned in four waves, each starting and ending in a staggered manner such that the entire survey occurred over a period of 6 weeks, between March and June, 2006.
Inclusion Criteria
The inclusion criteria for our survey were: (1) residency in the city of Vancouver, (2) being healthy (not diagnosed with any major or chronic illness), and (3) being between 24–71 months old.
Biochemical Data
Following the protocol proposed by the International Atomic Energy Agency [15] and adopted by the Center of Disease Control, hair samples were cut from three to four locations at the back of the head and as close to the scalp as possible. Only the first 1–2 cm proximal to the scalp were taken, the remainder being discarded. Samples were each placed in a pre-labeled coin envelope and delivered to the analyzing laboratory every week. All samples were collected by the same individual.
In the laboratory, the samples were processed [16] and analyzed for zinc content by inductively coupled plasma mass spectrometry [17]. They were analyzed as single samples with a duplicate after every ten samples. The within-assay coefficient of variation (CV) for this method was 3.3% at a mean of 157.3 μg zinc/g hair, while between assays, CV was 6.0% at a mean of 157.7 μg zinc/g hair.
The cutoff of 70 μg/g, proposed by Hambidge [3] and defined as 3 SD below the adult mean, was used in this study to define low hair zinc. For exploring associations of hair zinc and growth parameters among preschoolers under 4 years of age, 30 μg/g was examined as an additional cutoff.
Anthropometrics
Trained research personnel measured the children’s height and weight at the childcare center. A measuring rod aligned and secured on any wall in the room that was flush with an uncarpeted floor was employed to measure the height as each child stood shoeless with back to the wall, feet together and looking straight ahead. Heights were measured to the nearest 0.1 cm. Body weight was measured to the nearest 0.1 kg using a lithium electronic scale (Taylor Precision Products, L.P, Model 7300) with the scale placed on a hard floor and children standing on it shoeless and wearing light indoor clothing only. All the readings were done in multiples until two consecutive numbers were identical or not different by more than 0.5 unit of measurement. When two consecutive readings were not identical, the average of the two readings was recorded.
Tables from the Center for Disease Control standards [18] were used to calculate the weight-for-age (WAZ), height-for-age (HAZ), and weight-for-height (WHZ) Z scores of all children.
Statistical Analyses
The data were analyzed using the Statistical Package for Social Science, SPSS, (version 13.0, 2005). Data summaries for continuous variables are reported as mean ± standard deviation (SD). Hair zinc levels were modeled both as a continuous and a binary (<70 or ≥70 μg/g) outcome.
The differences between hair zinc levels of different sexes and age groups were assessed using analyses of variance, ANOVA, followed by Tukey’s post hoc analyses, when applicable. When both the outcome variable and the explanatory variable were categorical, the differences were examined using a continuity corrected Chi-square contingency table.
To examine associations of age and sex with hair zinc levels, univariate regression analyses were carried out with hair zinc as the outcome variable.
Results
Study Population
Overall, out of 2,550 survey packages distributed to childcare centers, 772 were returned (response rate of 30%). Out of these, 719 (3% of the city preschoolers) had hair samples analyzed, out of which only 705 had data on age and sex available.
Descriptive and anthropometric characteristics of the survey children are shown in Table 1. Boys and girls were equally represented. More than half of the children had mothers with some post-secondary education. Over 40% of the families had a reported income level >$60,000/year.
Table 1 also presents the mean (±SD) of the HAZ, WAZ, and WHZ scores. These means did not indicate any growth retardation for our study population. About 50% of HAZ and 51% of WAZ scores of the study population were between −0.67 and 0.67, corresponding to the 25th and 75th percentiles. The rate of stunting (HAZ < −2) and malnutrition (WAZ < −2), 2% (n = 13) and 2.3% (n = 16), respectively, were not higher than the expected baseline for any normal distribution.
Hair Zinc Status
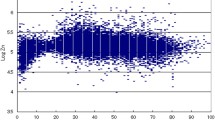
The hair zinc of this group of preschoolers was nearly normally distributed (Fig. 1 ) with mean hair zinc of 115 ± 43 μg/g, while 17% (n = 121) and 2% (n = 13) of children had hair zinc <70 and <30 μg/g, respectively.
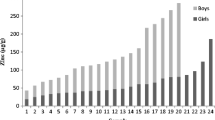
Mean hair zinc did not differ by sex (Table 2); however, girls were significantly more likely than boys to have low hair zinc (Fig. 2). In addition, both mean hair zinc and the prevalence of low hair zinc changed with age: mean hair zinc was significantly higher in children aged 4 and above than in those below the age of 4; and the prevalence of low hair zinc decreased significantly over the same time frame and appeared to plateau thereafter. The pattern of change in mean hair zinc with age was similar between boys and girls, as revealed by a non-significant sex-by-age interaction.
Comparison of the rate of low hair zinc in different age categories of boys (a) and girls (b) of the survey. Superscripts a and b are used to indicate statistically significant differences in the occurrence of low hair zinc between different age groups of a given sex. Superscripts x and y indicate statistically significant difference in the overall occurrence of low hair zinc between boys and girls. Differences in the occurrence of low hair zinc (<70 μg/g) were verified by adjusted chi-square analysis
Association of Hair Zinc with Growth Parameters
Regression analyses between anthropometric Z scores (height-for-age, weight-for-age, and weight-for-height) and hair zinc levels indicated that there were no significant associations, whether the data were analyzed for all children combined or separately by sex and age group (below or above 4 years of age). In all cases, R 2 values were <0.01. Table 3 summarizes mean values for HAZ, WAZ, and WHZ by age group and sex and for all ages and both sexes for those classified with low hair zinc and normal hair zinc. There were no differences in the indices of growth or nutrition between low hair zinc and normal hair zinc children or among younger (<4) children with normal and low hair zinc. Further, the lack of any significant differences in the parameters of growth and nutrition of children based on their hair zinc status persisted, even when the hair zinc status of the younger children was assigned using the cutoff of 30 μg/g (data not shown).
Discussion
Our study explored the hair zinc status of a large sample of Vancouver preschoolers. The study findings indicated that the average hair zinc in this population was comparable to previous reports for Canadian [5, 6], American [19] and other industrialized societies’ [20] children. The rate of low hair zinc (17%) in our study was also comparable with 16% reported for a group of preschoolers from Eastern Canada [5]. Age-based differences in the hair zinc level and age- and sex-based differences in the occurrence of low hair zinc were apparent. In our study, we did not observe any associations between hair zinc and indices of growth (HAZ) or nutrition (WAZ and WHZ). As a population, the average (±SD) of the anthropometric Z scores were within the normal range.
Among our study participants, we observed that the two sexes had comparable mean hair zinc, while girls had a significantly higher occurrence of low hair zinc. We could not explain this finding given that the majority of evidence in the zinc literature suggests male children are more vulnerable to low hair zinc [5, 20] and show a better response to zinc supplementation [4, 7, 8, 21, 22]. The literature speculates on a higher physiological need in males as the underlying reason for these observed differences. However, in light of our finding and that of studies such as Zachiwieja and co-workers’ [23] that have shown higher hair zinc for boys in some, not all, regions of Poland combined with other works [24] that have shown a comparable hair zinc values for boys and girls, it is reasonable to speculate that there may be additional factors besides physiology at work here; factors that, unlike the physiology of male humans (which is the same for male children from different regions), differ for male children of different regions/studies. One such factor could be sex-based differences in food intake that have been documented in some Canadian studies [5, 25, 26] as well as in studies of other industrial societies [27–29]. This area remains as one in need of further investigation.
Further exploration of the data confirmed an increase in hair zinc as the age of the children increased. The zinc literature indicates a change in hair zinc concentration with age [3, 20, 30–32]. While there seems to be consensus that hair zinc decreases during infancy and increases post-infancy, information on the nadir and zenith of this change is inconsistent. A result somewhat similar to ours has been reported in the cross-sectional study of Hambidge and co-workers [3], which showed that the hair zinc for neonates (n = 25) was closely comparable with that of young adults (n = 88) but that these levels were lower and remained low for children in the category of 3 months to 4 years of age (n = 93) and then rose for the age category of 4–17 years olds (n = 132). However, due to the age clusters used in this study, it is not possible to draw any clear conclusions on the hair zinc levels of older and younger preschoolers. Others [24, 30, 31] indicate a different age for this rise in hair zinc. Overall, these studies either did not look at the preschool years individually [3, 30, 31] or had only a small sample size for each age segment [24]. Our study is the first sufficiently large study of this age group that has been able to sample an adequate number of children from each age cluster (≥2 to <6 years old) and has explored their differences. This large sample size confers a high reliability on our results which indicates a sharp increase in hair zinc of children upon completion of year 4.
This age-based difference in hair zinc deserves further investigation. If the lower hair zinc during early childhood has no functional/clinical ramification, it may be indicative of normal physiological processes. If so, it may challenge the usefulness of the commonly used hair zinc cutoff (<70 μg/g) as a biomarker of MZD among younger preschoolers and necessitate investigation and validation of a more suitable cutoff for this age group. Hambidge and co-workers [3] documented associations between hair zinc and some clinical signs of zinc deficiency such as anorexia and poor growth among apparently healthy younger children (<4 years). However, these associations were evident only for those children who had a hair zinc level below 30 μg/g and were not apparent in children whose hair zinc was 30–70 μg/g. This observation clearly undermines the appropriateness of using the cutoff of 70 μg/g for younger children. However, since then the researchers studying preschoolers have continued to analyze the combined data and to use the same cutoff for both younger and older preschoolers [4, 19, 20, 31].
In our study, we did not observe any differences in the indices of growth or nutrition between low hair zinc and normal hair zinc children or between younger low hair zinc children and their counterparts with normal hair zinc. This lack of significant difference persisted even when the hair zinc status of the younger children was classified using 30 μg/g as the cutoff. However, this lack of association could also be due to the small sample size (n = 13) of younger children with hair zinc <30 μg/g.
In summary, our study provides important reference values for hair zinc for healthy children of North America and perhaps for children of industrialized societies in general. It presents evidence for age-based changes in hair zinc during the preschool years and indicates this area as one in need of further investigation. Lastly, this study presents some information on the hair zinc status and the prevalence of low hair zinc in an apparently healthy population of Canadian preschoolers two decades after the last Canadian study on this age group was published.
Limitations of our study should be kept in mind when generalizing its findings. One such limitation arises from our sampling method, which may have conferred “selection bias” to the findings. In the city of Vancouver, only 15% of the families with preschoolers use the preschool and childcare centers (personal communication with West Coast Family Services). By using these services as a major source of recruitment, we may have excluded children of stay-at-home parents who are not the consumers of these services and who could differ systematically from children who attend childcare.
Another factor that may have introduced some bias to our study is the selection bias due to non-response. The overall response rate of our survey was 30%, with 70% of the parents receiving our survey package choosing not to respond. Non-response in survey research can pose a threat to the generalizability of results if respondents and non-respondents differ systematically [33]. We did not have any way of comparing the characteristics of non-respondents with respondents. However, in general, it has been shown that non-respondents have a lower socioeconomic status [34–36], worse health status [37, 38], and better self-reported health [35] than respondents. One possible implication of this error on the findings of our study would be under-estimation of the prevalence of low hair zinc due to the participation of the healthier fraction of the population in our survey.
Finally, it is important to keep in mind that this was a cross-sectional survey with a limitation inherent in all cross-sectional studies. All the relationships observed were those of association. As a result, we did not and cannot establish the existence of any causal relationship or lack of it.
References
Prasad AS, Halstead JA, Nadimi M (1961) Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. AJM 31:220–223
Prasad AS, Miale FZ, Sandsted HH, Schulert AR, Darby WJ (1963) Biochemical studies on dwarfism, hypogonadism, and anemia. Arch Intern Med 111:407–428
Hambidge KM, Hambidge C, Jacobs M, Baum JD (1972) Low levels of zinc in hair, anorexia, poor growth, and hypogeusia in children. Pediatr Res 6:868–874
Walravens PA, Krebs NF, Hambidge KM (1983) Linear growth of low income preschool children receiving a zinc supplement. Am J Clin Nutr 38:195–201
Smit Vanderkooy PD, Gibson RS (1987) Food consumption patterns of Canadian preschool children in relation to zinc and growth status. Am J Clin Nutr 45:609–616
Gibson RS, Vanderkooy PD, MacDonald AC, Goldman A, Ryan BA, Berry MA (1989) Growth-limiting, mild zinc-deficiency syndrome in some southern Ontario boys with low height percentiles. Am J Clin Nutr 49:1266–1273
Hambidge KM, Chavez MN, Brown RM, Walravens PA (1979) Zinc nutritional status of young middle-income children and effects of consuming zinc-fortified breakfast cereals. Am J Clin Nutr 32:2532–2539
Walravens PA, Hambidge KM (1976) Growth of infants fed zinc supplemented formula. Am J Clin Nutr 29:1114–1121
Ploysangam A, Falciglia GA, Brehm BJ (1997) Effect of marginal zinc deficiency on human growth and development. J Trop Pediatr 43:192–198
Yehuda S, Yehuda M (2006) Long lasting effects of infancy iron deficiency—preliminary results. J Neural Transm 71:197–200
Gibson RS (1989) Assessment of trace element status in humans. Prog Food Nutr Sci 13:67–111
Hambidge KM (1980) Hair analysis. Pediatr Clin North Am 27:855–860
Assarian GS, Oberleas D (1977) Effect of washing procedures on trace-element content of hair. Clin Chem 23:1771–1772
Sky-Peck HH (1990) Distribution of trace elements in human hair. Clin Physiol Biochem 8:70–80
Deppisch LM, Centeno JA, Gemmel DJ, Torres NL (1999) Andrew Jackson’s exposure to mercury and lead: poisoned president? JAMA 282:569–571
Puchyr RF, Bass DA, Gajewski R, Calvin M, Marquardt W, Urek K, Druvan ME, Quig D (1998) Preparation of hair for measurement of elements by inductively coupled plasma-mass spectrometry (ICP-MS). Biol Tr Elem Res 62:167–182
Clesceri LS (1998) Standard Methods for Examination of Water and Wastewater. 20th Edition
Center for Disease Control (2000) CDC growth charts. Retrieved December 7, 2004 from http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.htm
Hambidge KM, Walravens PA, Webster J, White S, Anthony M, Roth ML (1976) Zinc nutrition of preschool children in the Denver Head Start program. Am J Clin Nutr 29:734–738
Lombeck I, Wilhelm L, Hafner D, Roloff K, Ohnesorge KL (1988) Hair zinc of young children from rural and urban areas in North Rhine-Westphalia, Federal Republic of Germany. Eur J Pediatr 147:179–183
Heinersdorff N, Tylor TG (1979) Concentration of zinc in the hair of school children. Arch Dis Child 54:958–960
Sazawal S, Bentley M, Black RE, Dhingra P, George S, Bhan MK (1996) Effect of zinc supplementation on observed activity in low socioeconomic Indian preschool children. Pediatrics 98:1132–1137
Zachwieja Z, Chlopicka J, Schlege-Zawadzka M, Zagrodzk P, Wypchlo J Krosniak M (1995) Evaluation of zinc content in children’s hair. Biol Trace Elem Res 47:141–145
Sakai T, Warishi M, Nishiyama K (2000) Changes in trace element concentrations in hair of growing children. Biol Trace Elem Res 77:43–51
Nakano T, Fediuk K, Kassi N, Egeland GM (2005) Dietary nutrients and anthropometry of Dene/Metis and Yukon children. Int J Circumpolar Health 64:147–156
Kuhnlein HV, Receveur O, Soueida R, Berti PR (2007) Unique patterns of dietary adequacy in three cultures of Canadian Arctic indigenous peoples. Public Health Nutr 5:1–12
Ganjii V, Hampl JS, Betts NM (2003) Race-, gender- and age-specific differences in dietary micronutrient intakes of US children. Int J Food Sci Nutr 54:485–490
Glynn L, Emmett P, Rogers I (2005) ALSPAC Study Team. Food and nutrient intakes of a population sample of 7-year-old children in the south-west of England in 1999/2000—what difference does gender make? J Hum Nutr Diet 18:7–19
Touvier M, Lioret S, Vanrullen I, Bocle JC, Boutron-Ruault MC, Berta JL, Volatier JL (2006) Vitamin and mineral inadequacy in the French population: estimation and application for the optimization of food fortification. Int J Vitam Nutr Res 76:343–351
Klevay LM (1970) Hair as a biopsy material. I. Assessment of zinc nutrition. Nutrition 23:284–289
Van Wouwe JP (1995) Clinical and laboratory assessment of zinc deficiency in Dutch children. A review. Biol Trace Elem Res 49:211–225
Meng Z (1998) Age- and sex-related differences in zinc and lead levels in human hair. Biol Trace Elem Res 61:79–87
Barriball KL, While AE (1999) Non-response in survey research: a methodological discussion and development of an explanatory model. J Adv Nurs 30:677–686
Groves RM, Couper MP (1992) Correlates of non response in personal visit surveys. Am Stat Assoc Proc Sect Survey Res Meth 13:102–111
Mishra SI, Dooley D, Catalano R, Serxner S (1993) Telephone health surveys: potential bias from noncompletion. Am J Public Health 83:94–99
Goyder J, Warriner K, Miller S (2002) Evaluating Socioeconomic Status (SES) bias in survey nonresponse. J Stat 18:1–11
Jackson R, Chambless LE, Yang K (1996) Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. J Clin Epidemiol 49:1441–1446
Shahar E, Folsom AR, Jackson R (1996) The effect of non-response on prevalence estimates for a referent population: insights from a population-based cohort study. Ann Epidemiol 6:498–506
Acknowledgments
The authors would like to thank the West Coast Child Care Resource Centre for granting us the access to the childcare and preschool centers under their jurisdiction and to the personnel of these centers for their strong support throughout this study. We also like to extend our heartfelt gratitude to our survey volunteers for their dedication and hard work in the process of data collection. This study was supported by a grant from the Human Early Learning Program (HELP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaghri, Z., Barr, S., Wong, H. et al. Age-Based Differences in Hair Zinc of Vancouver Preschoolers. Biol Trace Elem Res 126 (Suppl 1), 21–30 (2008). https://doi.org/10.1007/s12011-008-8215-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-008-8215-7