Abstract
A survey of mercury (Hg) and selenium (Se) contents was performed in fish collected from lakes located in two National Parks of the northern patagonian Andean range. Two native species, catfish (Diplomystes viedmensis) and creole perch (Percichthys trucha), and three introduced species, brown trout (Salmo trutta), rainbow trout (Oncorhynchus mykiss), and brook trout (Salvelinus fontinalis), were caught from lakes Nahuel Huapi, Moreno, Traful, Espejo Chico, and Guillelmo belonging to Nahuel Huapi National Park and from lakes Futalaufquen and Rivadavia, Los Alerces National Park. In lake Moreno, fish diet items were analyzed and rainbow trout grown in a farm. Hg and Se were measured in muscle and liver tissues by instrumental neutron activation analysis. The average concentrations in muscle of Hg for all species, ages, and lakes are between 0.4 to 1.0 μg g−1 dry weight (DW) with a few fish, mainly native, exceeding the United States Environmental Protection Agency health advisory for freshwater fish limited consumption, and from 0.8 to 1.5 μg g−1 DW for Se. Average concentrations in liver of Hg in all species range from 0.4 to 0.9 μg g−1 DW. Brown trout, the top predator in these lakes, showed the lowest average Hg burden in both tissues. Se concentrations in the liver of brown and rainbow trout, up to 279 μg g−1 DW, are higher than those expected for nearly pristine lakes, exceeding 20 μg g−1 DW, the threshold concentration associated with Se toxicity. These species show lower Hg contents in muscle, suggesting a possible detoxification of Hg by a Se-rich diet. Creole perch and velvet catfish livers have lower Se concentrations, with a narrower span of values (2.3 to 8.5 μg g−1 and 3.3 to 5.5 μg g−1 DW respectively).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an element widely distributed in nature; it is nutritionally important as an essential trace element for some plants and animals but is harmful at slightly higher concentrations. The major natural source of environmental Se is the weathering of rocks. For example, in North America, Se tends to be present in large amounts in areas where the soils have been derived from Cretaceous rocks [1–3].
Although the sensitivity to Se and its compounds is extremely variable, a diet containing 0.05 to 0.1 μg g−1 of Se provides adequate protection to humans and to various species of fish and livestock against Se deficiency, whereas waterfowl has reportedly been adversely affected by diets containing 12 to 280 μg g−1 of Se. Laboratory and field studies with fish, mammals, and birds have led to agreement that elevated concentrations of Se in diet or water are associated with reproductive abnormalities, congenital malformations, and growth retardation [1].
In general, concern levels for Se in fish as diet for other fish are 3–7 μg g−1, and the toxic threshold is 7 μg g−1 fresh weight (FW) as whole-body. Lemly [4] reports 20–40 μg g−1 dry weight (DW) in viscera as associated with deformities in fish and reports 20 μg g−1 DW as a toxic threshold. Green sunfish from a lake in North Carolina receiving Se as fly ash wastes from a coal-fired power station had Se levels in liver up to 21.4 μg g−1 FW resulting in reproduction failure and population decline [5].
There is evidence that selenides of some heavy metals, such as mercury, arsenic, cadmium, and thallium are very insoluble and contribute to keep these metals biologically unavailable [6]. Moreover, Se has been introduced in aquatic systems to diminish Hg bioaccumulation [7]. Mercury (Hg) is considered one of the most toxic elements; although it is not essential for any metabolic process, it can be readily accumulated by biota [8–10]. Experiments performed in enclosures at the Hg contaminated English-Wabigoon river system showed that there was a reduction of Hg burden in fish proportional to the Se accumulation [11]. In another work, performed in 25 Norwegian lakes, Fjield and Rognerud [12] found that Se deposited from the atmosphere seemed to lower the bioavailability of Hg for brown trout, a top predator. A steady increase of Hg contents, from 0.02 to 0.61 μg g−1 FW, in muscle of large mouth bass from Rogers Quarry was observed after the elimination of selenium-rich discharges of fly ash to the quarry between 1990 to 1998 [13].
Laboratory tests showed that selenium-rich diets tend to reduce the retention of methylmercury (MeHg) in tissues of rainbow trout [14]. In this work, the researchers studied the effect of dietary Se on the retention of injected organic and inorganic mercury. Rainbow trout fed with a Se-supplemented diet (approximately 10 μg g−1) augmented the elimination of organic mercury from muscle, liver, kidney, bile, and erythrocytes, compared to those fed with a 1 μg g−1 Se diet. Elimination of inorganic Hg increased in muscle and kidney, with a rise of Se content in the trout livers from 1 to 26 μg g−1 during the exposure.
The cases of antagonistic character of Se and Hg mentioned above and others reported in the literature [15–17] show that it depends on the chemical form of both elements. Researchers have found that Se to Hg molar ratios in tissues of marine mammals are around 1:1, and although the correlation is not so evident in fish tissues, Se to Hg molar ratios are in general greater than 1. In addition, cases of synergistic enhancement of Se and Hg toxicity have been reported, as well as the situation that Hg and Se may be antagonistic to each other for adults and synergistic to the young in birds [15, 18–19].
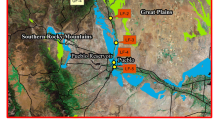
Historically, the Northern Patagonian Andean range was mostly protected from anthropogenic contamination due to the low population density and the access difficulty. Nahuel Huapi National Park, located on the Argentinian side of the northern Patagonian Andes, encloses a 7,850-km2 drainage basin with three major river systems and numerous lakes, including lake Nahuel Huapi (Fig. 1). The park encompasses from strictly controlled conservation areas to popular tourist sites. Three major urban settlements are located within the park; the largest of them is the city of Bariloche with circa 120,000 inhabitants.
Some areas surrounding the lakes are experiencing population growth, and the study of lacustrine sediment sequences, suspended particulate material, mussels, and lichens gave evidence of recent metal contamination [20–25]. At present, there are no relevant industrial or extensive agricultural activities in the surrounding area. Potential sources for contaminants, besides the volcanic activity of the area, could be found in human settlements around the lakes, although widespread regional and global contaminants can reach the area through atmospheric transport and subsequent deposition.
In the present work, we studied the contents of Se and total Hg in muscle and liver of five fish species of seven lakes within Nahuel Huapi and Los Alerces National Parks. The existence of wild and farmed rainbow trout, fed only with commercial pelletized food, at lake Moreno, arose the possibility of investigating the impact of different diets on the same species elemental contents. Therefore, farmed rainbow trout, commercial fish food, and the more significant food items of wild fish from lake Moreno were analyzed.
Study Area
The lakes chosen for this study belong to Nahuel Huapi and Los Alerces National Parks. Nahuel Huapi National Park (see Fig. 1) extends between the parallel 40°8′S on the northern border to the 41°36′S in the South, and from 71°2′W to 71°6′W, in the northern Patagonian Andean range. There is a strong vegetation gradient from dense temperate rain forest in the West to dry grasslands in the East. The West to East precipitation gradient is steep, from 3,000 mm y−1 in the West to 500 mm y−1 at the East. All the watersheds within the park are of glacial origin. Most of the lakes are ultraoligotrophic, monomictic, with thermal stratification in summer and complete mixing from autumn to spring [26]. The main human settlements in this Park are Bariloche, Dina Huapi, and Villa La Angostura on the South, East, and North margins of lake Nahuel Huapi, respectively, and Villa Traful in the South margin of lake Traful (Fig. 1). These towns are supported by tourism and some small scale cattle rising. On the Southern shore of lake Moreno East branch, there is a small 200 inhabitant settlement and a fish farm facility that raises 20 tons of rainbow trout per year in fish pens. The only potential source of Se anthropogenic contamination identified in the area is the use of Se compounds (sodium selenite) as a feeding supplement for the fish at the trout farm.
Los Alerces National Park (between 42°56′S and 43°12′S and 71°34′W and 72°07′W) located 150 km to the South has similar geological, climatological, and biological characteristics as Nahuel Huapi National Park. There are no permanent human settlements in this park. The lakes chosen are Futalaufquen and Rivadavia. Relevant data about these lakes are shown in Table 1.
Patagonian lakes are poor in fish species. The most widely distributed native fish species on Andean lakes are creole perch (Percichthys trucha), the small puyen (Galaxias maculatus), and the creole silverside (Odontesthes hatcherii) [27, 28]. The native catfishes (Diplomystes viedmensis and Diplomystes mesembranquinus) are present in lakes and rivers of the Atlantic drainage, including lakes and rivers of Nahuel Huapi National Park. Pacific and Atlantic salmonid species were introduced by the National Park Administration in early 1900 to enhance sport-fishing in the area [29]. Eggs of trout were imported from the Northern Hemisphere to supply fish farms in the area. Both native and introduced species that inhabit the water bodies of Patagonia are considered opportunistic predators of a wide trophic spectrum, being capable to adaptation to different diet types according to the availability of resources [30].
Two native and three introduced species were considered for the study: velvet catfish (D. viedmensis), creole perch (P. trucha), brook trout (Salvelinus fontinalis), rainbow trout (Oncorhynchus mykiss), and brown trout (Salmo trutta). Preliminary studies indicate that salmonids and creole perch have high mobility in the lakes, whereas velvet catfish, although it moves always along the bottom meanwhile it feeds, is not considered a great swimmer as compared to salmonids.
D. viedmensis feeds exclusively of benthic organisms in all studied environments. Creole perch feeds mostly on macrozoobenthic organisms and has fish as secondary prey. Rainbow trout is the species that shows a greater diet variation, with fish being the most important item in lakes Moreno and Nahuel Huapi, macrozoobenthic organisms and fish in lakes Espejo Chico and Traful, and macrozoobenthic and terrestrial organisms in lakes Rivadavia and Futalaufquen. Finally, brown trout behaves as an almost strict piscivorous fish on all lakes studied. Adult catfish is not a prey for any fish species. [31]
Fish were sampled from five sites of lake Nahuel Huapi: (Rincón Branch, BR; Huemul Branch, BH; López Bay, BL; Puerto Cisnes, PC; and Dina Huapi, DH), lakes Moreno East and West branches, Traful, Espejo Chico, and Guillelmo. Relevant data about these lakes are presented in Table 1.
Materials and Methods
Fish sampling was conducted following the methodology described elsewhere [29, 32]. Fish from lakes Nahuel Huapi, Traful, and Espejo Chico were sampled during the period of thermal stratification (that will be designated as “summer”) and during the period of thorough mixing (“winter”). Lake Guillelmo and the lakes of Los Alerces National Park were sampled only in summer. Rainbow trout, brook trout, and creole perch were sampled every season (summer, fall, winter, and spring) during 2 years from the West and East branches of lake Moreno. Rainbow trout were bought at the fish farm, as well as commercial pelletized fish food.
Fish were frozen after being caught. At the laboratory, before analysis, the individuals were thawed, and the whole liver, about 10 g of muscle tissue, stomach, and head were removed using titanium knives. Scales were extracted for age determination.
For each season and site, muscle tissue and the whole liver of the individuals of the each species differing in less than 10 mm length were used to create pooled samples. The individuals of the largest set of fish of each species caught at a same time in one site were analyzed separately to obtain information on intraspecific variability. Details of sample handling and conditioning are described in detail elsewhere [33]. Sample conditioning included freeze-drying and homogenization; handling was performed with Teflon® and titanium tools. Aliquots of about 120 to 150 mg of the dried material were sealed in Suprasil AN® quartz ampoules for analysis.
Se and Hg were determined by Instrumental Neutron Activation Analysis (INAA) in the RA-6 reactor at Bariloche. Corrections for interference due to impurities in the ampoules were performed, all being negligible. Se was determined by the 75Se(n,γ)75Se reaction. The mercury concentration measurements were done using the 279.2 keV gamma ray resulting from de decay of the 202Hg(n,γ)203Hg reaction product and the low energy lines (67.0, 68.8, and 77.9 keV) of 197gHg. The high contents of Se in several liver samples induced a significant interference in the 279.2 keV gamma ray peak, which prevented the use of this line for analytical purposes. In those cases, only the 196Hg(n,γ)197gHg reaction was used. Very good agreement between both reactions was observed when it was possible to obtain significant Hg concentrations from 203Hg measurements after the correction for the Se interference [33].
Reference materials NRCC-DORM-2 Dogfish Muscle, NRCC-DOLT-2 Dogfish Liver and NRCC-TORT-2 Lobster Hepatopancreas were analyzed together with the samples for analytical quality control, showing good agreement with the certified concentrations (Table 2).
Results and Discussion
Fish muscle and liver contents were measured for dry tissue. Dry to wet weight mass ratios for muscle ranged from 0.21 to 0.27 for brown trout, rainbow, brook trout, and creole perch and from 0.17 to 0.20 for velvet catfish. For liver, dry to wet weight ratios ranged from 0.21 to 0.54 for brown trout, 0.22 to 0.30 for rainbow and brook trout, 0.23 to 0.43 for creole perch, and 0.18 to 0.57 for velvet catfish.
The number of individual specimens caught in the nets varied with the season and the site.
Tables 3, 4, 5, 6, and 7 show the Hg and Se contents in muscle and liver of brown trout, rainbow trout, brook trout, creole perch, and velvet catfish. The number of specimens analyzed per site and their average lengths and ages are included in the tables. These tables also show the range of concentrations measured when individuals, instead of pooled samples, were analyzed for one site and the overall average for the species. Analytical uncertainties for Se determinations were about 11% in muscle samples and 9% in liver samples, and about 16% for Hg in muscle samples and 18% in liver samples. Figure 2a shows the plots of Hg contents in liver vs Hg contents in muscle, and Fig. 2b shows the plots of Se contents in liver vs Se contents in muscle for all the analyzed samples, indicating the lake of provenance. Table 8 shows the results of the analysis of diet items of wild fish from lake Moreno and commercial pelletized food for fish.
a Hg contents in liver tissue vs Hg contents in muscle for brown trout, rainbow trout, brook trout, creole perch, and velvet catfish from lakes Nahuel Huapi (N), Moreno (M), Traful (T), Espejo Chico (E), Guillelmo (G), Futalaufquen (F) and Rivadavia (R). Farmed rainbow trout from lake Moreno are included (O). b Se contents in liver tissue vs Se contents in muscle for the same fish species and sites. The least square linear fit of the set of data of each graph is shown. All data are shown in DW basis
The analysis of the data of the individuals of each species collected in one site showed that the standard deviation of the Se contents in muscle is similar or lower than the analytical uncertainty (which is around 11%), indicating that there are not significant differences in Se contents in muscle among individuals of the same species in one site (see Fig. 2). Se liver contents of salmonids and perch have much higher variability, reaching maximum to minimum value ratios of up to ten for brown and rainbow trout.
Selenium contents in brown and rainbow trout muscle samples range between 0.56 and 1.1 μg g−1 DW, for fish from lakes Moreno and Nahuel Huapi, whereas the samples of the same species of all the remaining lakes range between 1.2 and 1.7 μg g−1 DW, which are included within the expected range of values for non-contaminated lakes [1]. Se contents in creole perch muscle are higher but show a similar pattern concerning the lake of origin (see Fig. 2).
The differences in Se contents in muscle of each species in the different lakes cannot be related directly to the geographic setting of the lakes: lakes Futalaufquen and Rivadavia are located about 150 km to the South of lake Nahuel Huapi; Traful is located to the North without a direct connection to the Nahuel Huapi-Moreno lake system, and Espejo Chico is close to lake Nahuel Huapi (its waters discharge into lake Correntoso, which drains into Nahuel Huapi through a short river); and Guillelmo is about 40 km South of Nahuel Huapi, and it belongs to a basin that drains to the Pacific ocean. Furthermore, the results of a survey on heavy metal contents in soft tissues and digestive gland of a widespread patagonian mussel, Diplodon chilensis, performed in the four lakes of Nahuel Huapi National Park, showed that Se content in all sites was analytically equivalent [22].
Some authors ascribe differences in body burden to dilution effects with growth. We did not observe any correlation between Se with fish length or age. Regarding the length at a certain age, salmonids from lake Nahuel Huapi tend to attain larger sizes. It was possible to obtain the growth curves for rainbow trout of four lakes of Nahuel Huapi National Park (see Fig. 3). The larger fish sizes at lake Nahuel Huapi could explain for the lower Se concentrations. However, the growth curves for lakes Moreno and Traful are similar, whereas the Se concentrations are different.
No fish exceeded the Argentinian Se advisory (Código Alimentario Argentino and Res. SENASA N°533–10.05.94) for human adult consumption without restrictions (2.4 μg g−1 DW, assuming a dry weight to wet weight ratio of 0.25). The overall Se mean contents in muscle for each species is below the recommended value for consumption without restriction by children (0.3 μg g−1 FW, 1.2 μg g−1 DW, dry weight to wet weight ratio of 0.25), as well as fish from lakes Moreno and Nahuel Haupi. Fish from lakes Traful, Espejo Chico, Guillelmo, Rivadavia, and Futalaufquen are slightly above this guideline.
Conversely, Se concentrations in liver of brown and rainbow trout, up to 279 μg g−1 DW, greatly exceeded 20 μg g−1 DW, the concentration in liver associated with Se toxicity [4]. The values we measured are closer to the 21 μg g−1 FW level mentioned in [5], which could be an indication that salmonids from the lakes studied are having Se rich diets. Table 9 shows the selenium contents measured in this work compared to contents in salmonids, northern hemisphere perciformes, and catfishes, from Se contaminated and non-contaminated lakes. Se contents in muscle of the salmonids included in this study are similar or slightly above the values measured for salmonids of non-contaminated boreal lakes; however, they are clearly below the Se contents in muscle of fish from sites with Se rich waters. The same pattern is observed when perch is compared with other perciformes and velvet catfish to other catfishes of the world.
Although Se liver contents present a high degree of variability, there is a correlation between Se contents in liver and Se contents in muscle (ρ between 0.46 and 0.66, P between 0.0001 and 0.0050) for the whole set of data. The linear fits are shown in Fig. 2b. Because there were not significant differences in Se contents in fish muscle for each species and lake, these correlations indicate that Se contents in liver show the same geographic pattern as muscle. These fits give an average liver to muscle Se content ratio of 73 ± 23 for brown trout, 27 ± 8 for rainbow trout, 1.3 ± 0.4 for brook trout, 1.6 ± 0.4 for creole perch, and 1.4 ± 0.3 for velvet catfish.
Mercury contents do not exceed 1.0 μg g−1 FW, which is expected in biota from locations not directly affected by direct anthropic sources but higher than what is expected in remote lakes [6]. However, similar contents have been reported in trout from several wilderness lakes. The lower values measured in this study are similar or lower to those measured in low impacted lakes of the Northern hemisphere, whereas the higher values are about twice the maximum values measured in those lakes [12, 41–47].
For brown trout, rainbow trout, perch, and catfish, the longest specimens were, respectively, 25, 40, 34, and 67% longer than the smallest one; no correlation was found between Hg contents and length, except for the latter species (ρ = 0.66).
Bio-accumulation of Hg occurs in the species and lakes studied in this project. Although the ranges of Hg contents for each species overlap, the species considered as the top predator, brown trout, has lower Hg contents in muscle than the other species in contrast with the observations in other water bodies. Moreover, brown trout from lake Nahuel Huapi, where this species behaves exclusively as a piscivore, has the lowest Hg contents (see Fig. 2). Because organic Hg compounds, not total Hg, are the ones prone of being biomagnified in the trophic web, no conclusions can be drawn from the total mercury data alone regarding this phenomenon. A better knowledge of the trophic web structure of the lakes is needed to draw a conclusion on the low Hg burden of the species considered as the top predator.
Temperature is one of the more influential parameters in Hg bio-accumulation [48–50]. A rise in temperature could increase the accumulation of Hg in fish by increasing the metabolic rate. We analyzed the ratio between Hg contents in summer to contents in winter for each tissue at each sampling site. We found that Hg contents in both tissues were consistently equal or higher in summer than in winter of brown trout for all sites but not for the other species.
No significant correlation was found between Hg contents in liver vs muscle for a particular lake; however, when considering the whole data set, there is correlation between Hg contents in liver and Hg contents in muscle for each species (ρ between 0.55 and 0.89, P between <0.0001 and 0.0018). The linear fits are shown in Fig. 2a. These fits give an average liver to muscle Hg content ratio of 1.0 ± 0.1 for brown trout, 0.7 ± 0.2 for rainbow trout, 1.2 ± 0.3 for brook trout, 0.27 ± 0.06 for creole perch, and 1.8 ± 0.3 for velvet catfish. Salmonids have, in average, the same Hg concentration in liver as in muscle, whereas perch has four times more Hg contents in muscle than in liver, and catfishes have two times more Hg concentration in liver than in muscle.
Table 10 shows the ranges of concentrations measured in this work compared with Hg contents of fish found in the literature. Hg concentrations in the same species from other works are compared to present research when data is available; in other cases, species with similar habitat or diet are compared (creole perch is compared to other perciformes and velvet catfish with other carnivore catfishes). Mercury contents in fish tissues have a larger degree of variability than Se; however, the values reported in this work range from the lowest measured in other parts of the world. It is noteworthy to mention that Walleye, a perciforme from the North hemisphere, shows Hg liver to muscle content ratios smaller than 1, as is the case with the Patagonian creole perch. Catfish Hg contents in muscle are similar to other catfishes of the world living in water bodies without direct Hg inputs.
Considering that most Hg in muscle is in the organic form, few fish (creole perch, velvet catfish, and brook trout) exceeded Argentinian recommendation for methylmercury intake of freshwater fisheries consumption (0.5 μg g−1 FW, or approximately 2 μg g−1 DW) and the US Environmental Protection Agency’s health advisory for freshwater fish limited consumption.
Selenium and Mercury
There is evidence that selenites of Hg are insoluble and reduce Hg and Se bioavailability. Se and Hg contents in muscle were not correlated (ρ ranging from −0.36 to 0.46), as well as Se and Hg contents in livers from rainbow trout, brook trout, and creole perch (ρ < 0.3), and weakly correlated for livers of brown trout and catfish (ρ equal to 0.65 and 0.51, respectively). This lack of correlation has previously been observed in omnivorous and piscivorous fish [38].
Total Hg to Se molar ratios in the present work range from 0.04 to 0.4 for brown trout, 0.06 to 0.9 for rainbow trout, 0.03 to 1.2 for creole perch, and 0.07 to 0.4 for velvet catfish. Except for one unique muscle sample, Se always exceeded Hg, a fact which is consistent with the findings in other uncontaminated freshwater ecosystems [59–61]. In rivers contaminated by gold mining activities, Hg is typically in excess of Se [38].
Concentrations of Se in all food items from lake Moreno range between 0.21 and 3.2 μg g−1 DW, with only insect larvae above 3 μg g−1 DW, the aquatic ecological risk threshold for diet. Se contents in pelletized food lies between these limits. Both wild and farmed trout have similar Se contents in muscle, although Se content in wild trout liver is at least four times higher. Contents of Hg in food items was variable, from 0.08 to 1.4 μg g−1 DW, as well as Hg contents in different aliquots of pelletized food (ranging from 0.02 to 1 μg g−1 DW, see Table 8). The previous results do not allow to draw conclusions on Hg antagonism or synergism based on the two different diets of rainbow trout.
It should be noted that the species that have the lowest Hg contents in muscle, brown trout and rainbow trout, have the highest Se contents in liver, which could be an indication that these fish are protected from Hg by a Se-rich diet.
Conclusions
The following conclusions were drawn from this study:
-
Hg contents in liver and muscle tissue are higher than those expected for pristine lakes; however, the values are not unusual. The most remarkable result is that the species considered as the top predator does not have the highest contents of Hg in muscle.
-
The higher Hg contents in muscle from creole perch are in coincidence with the lower Se in liver. Evidence would indicate that brown trout and rainbow trout (with higher Se contents in liver) have Se-rich diets, which could be preventing Hg accumulation in muscle.
-
Selenium contents in liver of brown trout and rainbow trout are well above the toxicity threshold; however, all fish analyzed belonged to populations which did not appear stunted. More studies are necessary to assess the impact on the fish population.
-
Se contents in muscle of fish from lakes Moreno and Nahuel Huapi are about one half of the Se contents in muscle of fish from the other lakes. This geographical pattern, although less obvious, appears for Se contents in liver.
-
The use of Se compounds (sodium selenite) as a feeding supplement for the fish at the trout farm at lake Moreno East does not seem to affect the wild fish population.
References
Eisler R (1999) Selenium hazards to fish, wildlife, and invertebrates: a synoptic review, in Contaminant Hazard Reviews, Report 5, USGS/BRD/BSR-1999-0002
Presser T, Sylvester M, Low W (1994) Bioaccumulation of selenium from natural geological sources and its potential consequences. Environ Manage 18:423–436
Presser T (1994) The Kesterton effect. Environ Manage 18:437–454
Lemly AD (1998) Pathology of selenium poisoning in fish. In: Frankenberger WT, Engberg RR (eds) Environmental chemistry of selenium. Marcel Dekker, New York
Sorensen EMB, Cumbie PM, Bauer TL, Bell JS, Harlan CW (1984) Histopathological, hematological, condition-factor, and organ weight changes associated with selenium accumulation in fish from Belews Lake, North Carolina. Arch Environ Contam Toxicol 13:153–162
Eisler R (1999) Mercury hazards to fish, wildlife, and invertebrates: a synoptic review, in Contaminant Hazard Reviews, Report 10, USGS/BRD/BSR-1999-0002
Paulsson K, Lundbergh K (1991) Treatment of mercury contaminated fish by selenium addition. Water Air Soil Pollut 56:833–841
EPA (1997) Mercury report to congress. United States Environmental Protection Agency, EAP-425/R-97-003 and 005
UNEP (2002) Global mercury assessment. United Nations Environmental Program Report, Geneva
Sigel A, Sigel H (1997) Mercury and its effects on environment and biology. Marcel Dekker, New York
Rudd JW, Turner MA (1983) Selenium in lake enclosures: its geochemistry, bioaccumulation and ability to reduce mercury bioaccumulation. Can J Fish Aquat Sci 40:2228–2250
Fjield E, Rognerud S (1993) Use of path analysis to investigate mercury accumulation in brown trout (Salmo trutta) in Norway and the influence of environmental factors. Can J Fish Aquatic Sci 50:1158–1167
Southworth GR, Peterson MJ, Ryon MG (2000) Long-term increased bioaccumulation of mercury in largemouth bass follows reduction of waterborne selenium. Chemosphere 41:1101–1105
Bjerregaard P, Andersen D, Rankin JJ (1999) Retention of methylmercury in rainbow trout, Oncorhynchus mykiss: effect of dietary selenium. Aquatic Toxicol 45:171–180
Cuvin Aralar MA, Furness R (1991) Mercury and selenium interaction: a review. Ecotox Environ Saf 21:348–364
Chen YW, Belzile N, Gunn JM (2001) Antagonistic effect of Selenium on mercury assimilation by fish populations near Sudbury metal smelters. Limnol Oceanogr 46(7):1814–1818
Belzile N, Chen YW, Gunn JM, Tong J, Alarie Y, Delonchamp T, Lang CY (2006) The effect of selenium on mercury assimilation by freshwater organisms. Can J Fish Aquat Sci 63:1–10
Heinz GH, Hoffman DJ (1998) Methylmercury chloride and selenomethionine interactions on health and reproduction in mallards. Environ Toxicol Chem 17(2):139–145
Heinz GH, Hoffman DJ (1998) Effects of mercury and selenium on glutathione metabolism and oxidative stress in mallard ducks. Environ Toxicol Chem 17(2):161–166
Bubach DF, Arribére MA, Ribeiro Guevara S, Calvelo S (2001) Study on the feasibility of using transplanted Protousnea magellanica thalli as bioindicators of atmospheric contamination. J Radioanal Nuc Chem 250:63–68
Ribeiro Guevara S, Massaferro J, Villarosa G, Arribére MA, Rizzo AP (2002) Heavy metal contamination in sediments of lake Nahuel Huapi, Nahuel Huapi National Park, Northern Patagonia, Argentina. Water Air Soil Pollut 137:21–44
Ribeiro Guevara S, Bubach DF, Vigliano PH, Lippolt G, Arribére MA (2004) Heavy metals and other trace elements in native mussel Diplodon chilensis from Northern Patagonian lakes, Argentina. Biol Trace Elem Res 102(1–3):245
Ribeiro Guevara S, Bubach DF, Arribére MA (2004) Mercury in lichens of Nahuel Huapi National Park, Patagonia, Argentina. J Radioanal Nuc Chem 261(3):679–687
Ribeiro Guevara S, Arribére MA, Bubach DF, Vigliano P, Rizzo A, Alonso M, Sánchez R (2005) Silver contamination on abiotic and biotic compartments of lake Nahuel Huapi National Park lakes, Patagonia, Argentina. Sci Total Environ 336(1–3):119–134
Ribeiro Guevara S, Rizzo A, Sánchez RS, Arribére MA (2005) Heavy metal inputs in Northern Patagonia lakes from short sediment core analysis. J Radioanal Nuc Chem 265(3):481–493
Calcagno A, Fioritti MJ, Pedrozo F, Vigliano PH, López H, Rey C, Razquin ME, Quirós R (1995) Catálogo de lagos y embalses de la República Argentina. Ministerio de Economía y Obras y Servicios Públicos, Secretaría de Obras Públicas, Subsecretaría de Recursos Hídricos, Argentina
Pascual M, Macchi PJ, Urbanski J, Marcos F, Riva Rossi C, Novara M, Dell’ Arciprete P (2002) Evaluating potential effects of exotic freshwater fish from incomplete species presence–absence data. Biol Invasions 4:101–113
Vigliano PH, Alonso MF, Denegri MA, Garcia Asorey MI, Lippolt G, Macchi PJ, Milano D (2001) Estructura de las comunidades de peces de lagos y embalses patagónicos: estado actual del conocimiento y problemática. Proc. I Encuentro Binacional de Ecología, XX Reunión Argentina de Ecología and X Reunión de la Sociedad de Ecología de Chile, Bariloche, Argentina
Vigliano PH, Alonso MF (2007) Salmonid introductions in Patagonia Argentina: a mixed blessing. In: Bret TM (ed) Ecological and genetic implications of aquaculture. Springer, The Netherlands
Macchi PJ, Cussac VE, Alonso MF, Denegri MA (1999) Predation relationships between introduced salmonids and the native fish fauna in lakes and reservoirs in Northern Patagonia. Ecol Freshw Fish 8:227–236
Ribeiro Guevara S, Bubach DF, Macchi PJ, Vigliano PH, Arribére MA, Colombo JC (2006) Rb–Cs ratio as an indicator of fish diet in lakes of the Patagonia, Argentina. Biol Trace Elem Res 110:97–119
Vigliano PH, Macchi PJ, Denegri MA, Alonso MF, Milano D, Lippolt G, Padilla G (1999) Un diseño modificado y procedimiento de calado de redes agalleras para estudios cuali-cuantitativos de peces por estratos de profundidad en lagos araucanos. Natura Neotropicalis 30(1–2):1–11
Arribére MA, Ribeiro Guevara S, Bubach DF, Vigliano PH (2006) Trace elements as fingerprint of lake of provenance and of species of some native and exotic fish of northern Patagonian lakes. Biol Trace Elem Res 110:71–95
Ramirez Jr, P, Dickerson K (1997) Follow-up investigation of selenium and other trace elements in biota from the Riverton Reclamation Project, Fremont County, Wyoming, Contaminant Report Number: R6/709C/97
Kaiser II, Young P, Johnson JD (1979) Chronic exposure of trout to waters with naturally high selenium levels: effects on transfer RNA methylation. J Fish Res Board Canada 36:689–694
Evans MS, Muir D, Lockhart WL, Stern G, Ryan M, Roach P (2005) Persistent organic pollutants and metals in the freshwater biota of the Canadian Subarctic and Arctic: an overview. Sci Total Environ 351–352:94–147
Mauk RJ, Brown ML (2001) Selenium and mercury concentrations in brood-stock walleye collected from three sites on Lake Oahe. Arch Environ Contam Toxicol 40:257–263
Dorea JG, Moreira MB, East G, Barbosa AC (1998) Selenium and mercury concentrations in some fish species of the Madeira river, Amazon basin, Brazil. Biol Trace Elem Res 65:211–220
Stewart AR, Luoma SN, Schlekat CE, Doblin MA, Hieb KA (2004) Food web pathway determines how selenium affects aquatic ecosystems: a San Francisco Bay case study. Environ Sci Technol 38:4519–4526
Barwick M, Maher W (2003) Biotransference and biomagnification of selenium, copper, cadmium, zinc, arsenic and lead in a temperate seagrass ecosystem from Lake Macquarie Estuary, NSW, Australia. Mar Environ Res 56:471–502
Akielaszak JJ, Haines TA (1981) Mercury in the muscle tissue of fish from three northern Maine lakes. Bull Environ Contam Toxicol 27:201–208
Sloan R, Schofield C (1983) Mercury levels in brook trout (Salvelinus fontinalis) from selected acid and limed Adirondack lakes. Northeast Environ Sci 2:165–170
Vuorinen PJ, Rantio T, Witick A, Vuorinen M (1994) Organochlorines and heavy metals in sea trout (Salmo trutta) in the gulf of Bothnia off the coast of Finland. Aqua Fennica 24(1):29–35
Kim JP (1995) Methylmercury in rainbow trout (Oncorhynchus mykiss) from Lakes Okareka, Okaro, Rotomahana, Rotorua and Tarawera, North Island, New Zealand. Sci Total Environ 164:209–219
Kim JP, Burggraaf S (1999) Mercury bioaccumulation in rainbow trout (Oncorhynchus mykiss) and the trout food web in lakes Okarera, Okaro, Rotomahana and Rotorua, New Zealand. Water Air Soil Pollut 115:535–546
Rose J, Hutcheson MS, West CR, Pancorbo O, Hulme K, Cooperman A, DeCesare G, Isaac R, Screpetis A (1999) Fish mercury distribution in Massachusetts, USA lakes. Environ Toxicol Chem 18(7):1370–1379
Newman MC, Jagoe CH (1994) Ligands and the bioavailability of metals in the aquatic environments. In: Hamelink JM, Landrum PF, Bergman HL, Benson WH (eds) Bioavailability. CRC Press, Boca Raton, USA
Huckabee JW, Elwood JW, Hildebrand SG (1979) Accumulation of mercury in freshwater biota. In: Nriagu J (ed) The biogeochemistry of mercury in the environment. Elsevier, Amsterdam
Jobling M (1993) Bioenergetics: Feed intake and energy partitioning. In: Rankin JC, Jensen FB (eds) Fish ecophysiology. Chapman and Hall, USA
Bridges CR (1993) Ecophysiology of intertidal fish. In: Rankin JC, Jensen FB (eds) Fish ecophysiology. Chapman and Hall, USA
Neumann CM, Kauffman KW, Gilroy DJ (1997) Methylmercury in fish from Owyhee Reservoir in Southeast Oregon: scientific uncertainty and fish advisories. Sci Total Environ 204:205–214
Mason RP, Laporte JM, Andres S (2000) Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium and cadmium by freshwater invertebrates and fish. Arch Environ Contam Toxicol 38:283–297
Jenkins DW (1980) Biological monitoring of toxic trace metals. Vol. 2. Toxic trace metals in plants and animals of the world, Part 1. U.S. Environmental Protection Agency Rep. 600/3-80-090:30–138
Suns K, Hitchin G (1990) Interrelationships between mercury levels in yearling yellow perch, fish condition and water quality. Water Air Soil Pollut 650:255–265
Bloom NS (1992) On the chemical form of Hg in edible fish and marine invertebrate tissue. Can J Fish Aquatic Sci 49(5):1010–1017
Neumann RM, Ward SM (1999) Bioaccumulation and biomagnification of mercury in two warm water fish communities. J Freshw Ecol 14(4):487–497
Scheuhammer AM, Graham JE (1999) The bioaccumulation of mercury in aquatic organisms from two similar lakes with differing pH. Ecotoxicol 8:49–56
Holsbeek L, Das HK, Joiris CR (1997) Mercury speciation and accumulation in Bangladesh freshwater and anadromous fish. Sci Total Environ 198:201–210
Burger J, Cooper K, Gochfeld M (1992) Exposure assessment for heavy metal ingestion from a sport fish in Puerto Rico: estimating risk for local fishermen. J Toxicol Environ Health 36:355–365
Sindayigaya E, Van Cauwenbergh R, Robberecht H, Deelstra H (1994) Copper, zinc, manganese, iron, lead, cadmium, mercury and arsenic in fish from Lake Tanganika, Burundi. Sci Total Environ 144:103–115
Benemariya H, Robberecht H, Deelstra H (1994) Atomic absorption spectrometric determination of Zinc, Copper and Selenium in fish from Lake Tanganika, Burundi, Africa. Sci Total Environ 144:103–115
Acknowledgments
The authors wish to thank the RA-6 reactor operation staff for their assistance during the irradiations. This work was carried out within the Technical Cooperation Agreement ARG/7/006 with the International Atomic Energy Agency (IAEA)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arribére, M.A., Ribeiro Guevara, S., Bubach, D.F. et al. Selenium and Mercury in Native and Introduced Fish Species of Patagonian Lakes, Argentina. Biol Trace Elem Res 122, 42–63 (2008). https://doi.org/10.1007/s12011-007-8059-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-007-8059-6