Abstract
Green unicellular alga Acutodesmus obliquus (Turpin) Hegewald et Hanagata (SAG strain no. 276-6) (Chlorophyceae) was used for determination of phytotoxicity of lead (Pb) at the range of concentrations 0.01–500 μM during 7 days of culture. The accumulation of Pb in algal cells was found to be increased in a concentration- and duration-dependent manner. The highest Pb uptake value was obtained in response to 500 μM Pb on the seventh day of cultivation. The decrease in the number and the size of cells and the contents of selected primary metabolites (photosynthetic pigments, monosaccharides, and proteins) in A. obliquus cells were observed under Pb stress. Heavy metal stimulated also formation of reactive oxygen species (hydrogen peroxide) and oxidative damage as evidenced by increased lipid peroxidation. On the other hand, the deleterious effects of Pb resulting from the cellular oxidative state can be alleviated by enzymatic (superoxide dismutase, catalase, ascorbate peroxidase, and glutathione reductase) and non-enzymatic (ascorbate, glutathione) antioxidant systems. These results suggest that A. obliquus is a promising bioindicator of heavy metal toxicity in aquatic environment, and it has been identified as good scavenger of Pb from aqueous solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contamination of aquatic environment by heavy metals has drawn the attention of a large number of researchers and environmentalists in the last decades. Environmental level of lead (Pb) has been rising over this period as a result of various human activities. Elevated contents of heavy metals in water bodies elicit deleterious impact on microalgae, which are the primary producers at the base of the aquatic food chain (Zhou et al. 2011; Rajamani et al. 2014). They are one of the first groups to be affected by heavy metal contamination, and therefore, they provide important information for predicting the environmental impact of pollution (Yang et al. 2014; Polonini et al. 2015). Microalgae can sequester metal ions because of the presence of cell wall, which is composed of a fiber-like structure and amorphous embedding matrix of various polysaccharides (Hu et al. 2001; Peña-Castro et al. 2004; Javanbakht et al. 2014). For example, heavy metals have been effectively removed using green microalgae, such as Chlorella vulgaris, Chlorella kesslerii, Scenedesmus quadricauda, and Scenedesmus incrassatules (Gin et al. 2002; Wilde et al. 2006; Lourie et al. 2010; Bajguz 2011). In this respect, study of the biosorption of Pb by Acutodesmus obliquus (Chlorophyceae) as a crucial component of natural phytocenoses seems to be extremely important.
Previous studies indicate that Pb can cause adverse effects on the growth and the content of primary metabolites in algal cultures (Bajguz 2011; Piotrowska-Niczyporuk et al. 2012). Moreover, Pb is a strongly phytotoxic metal generating reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) that can react with lipids, proteins, photosynthetic pigments, and nucleic acids leading to cell death (Piotrowska et al. 2010; Bajguz 2011; Piotrowska-Niczyporuk et al. 2012). It is generally accepted that the overproduction of ROS is a universal higher plant and algal response to abiotic stress factors, including heavy metals (Tripathi and Gaur 2006; Tripathi et al. 2006; Gill and Tuteja 2010). For example, the short-time influence of three pollutants: cadmium (Cd), anthracene, and/or chloridazone on algae in three Scenedesmus (Scenedesmus microspina, Scenedesmus subspicatus, and Scenedesmus obliquus) species may result from triggering the oxidative stress by ROS production, which can simultaneously act as a signal for the activation of stress response and defense pathways (Tukaj and Pokora 2006). Therefore, the effect of Pb on the level of oxidative stress expressed as H2O2 content and lipid peroxidation in A. obliquus culture was studied.
The cellular mechanisms, such as extracellular detoxification, reduced uptake, efflux, sequestration by phytochelatins, etc., have been proposed to explain algal tolerance to the presence of heavy metals in aquatic environment (Tripathi and Gaur 2006; Tripathi et al. 2006). Algae contain also several enzymatic (e.g., superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), glutathione reductase (GR)) and non-enzymatic (e.g., glutathione, ascorbate) antioxidant defense systems protecting cells from oxidative damage (Bajguz 2011; Piotrowska-Niczyporuk et al. 2012; Polonini et al. 2015), which generally results at elevated levels of metals. SOD constitutes the primary line of defense in this system, as it dismutates superoxide radicals to H2O2. Degradation of H2O2 to water and oxygen is mainly carried out by CAT and also by APX via the glutathione/ascorbate cycle (Radotic et al. 2000). GR catalyzes the reduction of glutathione disulfide to the sulfhydryl form glutathione, which is a critical molecule in resisting oxidative stress and maintaining the reducing environment of the cell (Verma and Dubey 2003; Gill and Tuteja 2010). The changes in enzyme and non-enzymatic system have been the focus of several algae stress studies (Pinto et al. 2003; Tripathi and Gaur 2006; Bajguz 2011; Piotrowska-Niczyporuk et al. 2012). For example, Nagalakshmi and Prasad (2001) observed increases in APX, SOD, and glutathione peroxidase (GPX) activities in Scenedesmus bijugatus exposed to different copper (Cu) concentrations (0–100 μM). Moreover, they also observed a progressive depletion of glutathione content in the cells with increasing concentrations of Cu. The alteration of the equilibrium between synthesis and utilization of glutathione was attributed to its antioxidant role or its use as precursor in the synthesis of phytochelatins (Nagalakshmi and Prasad 2001). Filamentous brown seaweed Ectocarpus siliculosus, the model organism for the algal class Phaeophyceae also exhibited oxidative stress, with increases in hydrogen peroxide and lipid peroxidation, and decreased concentrations of photosynthetic pigments in response to Cu. Moreover, the activation of enzymatic (SOD, CAT, APX) and non-enzymatic (ascorbate, glutathione, phenolics) antioxidants was observed in brown algae treated with Cu (Sáez et al. 2015). The increase in SOD activity in S. microspina, S. subspicatus, and S. obliquus was observed in response to three pollutants (Cd, anthracene, and/or chloridazone) (Tukaj and Pokora 2006). For that reason, the involvement of antioxidant response including enzymatic (SOD, CAT, APX, GR) and non-enzymatic (ascorbate, glutathione) antioxidants in the biochemical detoxification of H2O2 generated in response to Pb was examined in details in A. obliquus culture. The aim of this study was also to evaluate the effect of Pb on the growth expressed as cell number and volume, the content of proteins, photosynthetic pigments, and monosaccharides with special references to metal bioaccumulation during 7 days of algal culture.
We tested the hypothesis that A. obliquus cells are able to sequester Pb ions from aquatic environment, and accumulation of this metal depends on its concentration in culture medium. To monitor cellular responses to heavy metal at the range of concentrations 0.01–500 μM, the growth rate, cell size, and the contents of primary metabolites such as photosynthetic pigments, proteins, and monosaccharides as well as the level of oxidative stress and antioxidant response were followed. Here, we tested also the hypothesis that the toxic effect of Pb enhances with increase of its concentration and that low concentrations of Pb may induce cellular defense against oxidative stress in algal cultures involved in the activation of various enzymatic and non-enzymatic antioxidants.
Material and methods
Algal material and growth conditions
The wild-type A. obliquus 276-6 (formerly named: Scenedesmus obliquus (Turpin) Kützing, SAG strain no. 276-6) was purchased from the SAG Culture of Algae Collection (Germany). The homogenous population of young synchronous algal cells was collected by centrifugation (1000×g, 10 min) and used for subsequent experiments. Complete synchronization has been obtained by a regular change of light and dark periods according to the method of Pirson and Lorenzen (1966). Synchronization of the culture was controlled by studying cell division and the diagrams of cell size distribution every day at the beginning of light period using microscope. Growth of cultures was initiated by introduction of inoculums containing about 1.5 × 106 (about 22 mL) algal cells when the pre-cultures reached the exponential growth phase. The culture mineral medium used was the pure mineral autoclaved bold basal medium (BBM). The pH of the medium was adjusted to 7.0 with 1 M NaOH prior to autoclaving. A. obliquus cells were cultured in an Erlenmeyer flasks (250 mL) containing 100-mL medium. The axenic cultures were cultivated for 7 days under controlled sterile conditions at 25 ± 0.5 °C. Illumination was supplied during a 16-h photoperiod (8-h dark period) by a bank of fluorescent lights yielding a photon flux 50 μmol m−2 s−1 at the surface of the tubes. Using air pumps, cell suspension was bubbled by atmospheric air at 1 L min−1 to provide necessary CO2.
In the experiment, the effect of Pb at the range of concentrations 0.01–500 μM on the metal biosorption, growth, and biochemical response was analyzed on the first, third, fifth, and seventh day of cultivation. Heavy metal concentrations used in this work are chosen appropriately from the lowest algal exposure concentration (0.01 μM) to moderate levels of Pb. The preliminary experiment showed that the 96 h LC50 of Pb for A. obliquus was approximately 10 μM. An exposure concentration which is lower than LC50 is considered as sub-lethal concentration. In algal toxicity test, the LC50 value is affected by the initial cellular density, culture medium, temperature condition, etc., and even a small alteration in those conditions may give rise to a different LC50 value, even if the same algae and metal are used. Therefore, we choose as 0.01, 0.1, and 1 μM Pb as sub-lethal concentrations of heavy metal as well as 10, 100, and 500 μM Pb as toxic concentrations to algal growth. Higher concentrations of Pb induced cell death during the first 12 h, and lower than 0.01 μM Pb did not exert statistically significant influence on algal growth (data not shown). In order to that, appropriate amount of its nitrate form PbNO3)2 (Sigma-Aldrich Co., USA) was dissolved in distilled water and then added in right concentrations to Erlenmeyer flasks with BBM medium (100 mL).
Determination of lead in A. obliquus cells
To determine the concentration of heavy metal in algal cells, cultures of A. obliquus were centrifuged for 10 min at 10,000×g (Bajguz 2011; Piotrowska-Niczyporuk et al. 2012). The algal suspension was dried at 105 °C for 12 h and mineralized in 200 μL of 65 % nitric acid. Concentrations of Pb in prepared samples were determined by flame atomic absorption spectrometry (AAS) using a Solaar M6 (Thermo Electron Corporation, UK) spectrometer with deuterium background correction system. The absorbance of Pb was measured in air-acetylene flame with 0.5 nm spectral bandpass at wavelength λ = 217.0 nm. Heavy metal level in algal cells was determined on the first, third, fifth, and seventh day of culture.
Determination of cell number, cell size, proteins, monosaccharides, and photosynthetic pigments
The number of A. obliquus cells was determined by direct counting of cells in the growth medium using a Bürker chamber using the standard procedure (Andersen 2005). To verify the volume of A. obliquus cells (μm3), we employed light microscope (Leica, Germany) and MultiScanBase v.14 (Computer Scanning System CSS) for imaging analysis. The content of protein in algal cells was determined following the Bradford (1976) method, using bovine serum albumin as the standard. The monosaccharide content was estimated according to the Somogyi (1954) method. Wellburn (1994) method was used for the determination of the content of photosynthetic pigments (chlorophyll a and b as well as total carotenoids) in A. obliquus. All parameters were analyzed on the first, third, fifth, and seventh day of culture.
Stress marker determination
One of the major consequences of the Pb treatment is the enhanced production of ROS, such as H2O2, which can damage cell membranes and induce lipid peroxidation, protein denaturation, and DNA mutation (Aliexieva et al. 2001). Heavy metals disrupt cellular membranes resulting in the conversion of unsaturated fatty acids into small hydrocarbon fragments such as malondialdehyde (MDA). MDA is a cytotoxic product of lipid peroxidation and an indicator of free radical production and consequent tissue damage. Thus, cell membrane stability has widely been utilized to study effects of stress on plants (Tripathi and Gaur 2006; Tripathi et al. 2006; Gill and Tuteja 2010). The amount of total MDA was determined using Heath and Packer (1968) method. Algal cells were harvested by centrifugation at 10,000×g for 10 min, and the resulting pellet was treated with 0.25 % (w/v) thiobarbituric acid in 10 % (w/v) trichloroacetic acid (TCA). After heating at 95 °C for 30 min, the mixture was cooled and centrifuged. The absorbance of the supernatant at 532 nm was recorded and corrected for unspecific turbidity by subtracting the value at 600 nm. The level of H2O2 in A. obliquus cells was measured spectrophotometrically at 390 nm by reaction with KI. The results were calculated using a standard curve prepared with fresh H2O2 solutions (Aliexieva et al. 2001). Stress markers were determined on the first, third, fifth, and seventh day of algal culture.
Determination of antioxidants
For extraction of total ascorbate, A. obliquus cells were harvested by filtration and quickly homogenized in liquid N2 and 5 % (w/v) TCA (Kampfenkel et al. 1995). The homogenate was centrifuged for 5 min at 15,600×g (4 °C), and the supernatant was assayed for the ascorbate content in a reaction mixture with 10 mM dithiothreitol, 0.2 M phosphate buffer (pH 7.4), 0.5 % N-ethylmaleimide, 10 % TCA, 42 % H3PO4, 4 % 2,2′-dipyridyl, and 3 % FeCl3.
Determination of total glutathione (oxidized and reduced) in A. obliquus cells was essentially as described by de Kok et al. (1986). Glutathione was extracted from algal cells in extracting buffer (2 % sulfosalicylic acid, 1 mM Na2EDTA, and 0.15 % ascorbate) and homogenized. The homogenate was centrifuged at 12,000×g for 5 min. An aliquot of supernatant was then used for the measurement of the glutathione content by glutathione assay kit (Sigma Chemical Co. USA; product number CS0260-1KT). The samples were first deproteinized with the 5 % 5-sulfosalicylic acid solution. Glutathione content of the sample was then assayed using a kinetic assay in which catalytic amounts of glutathione cause a continuous reduction of 5,5′-dithiobis-(2-nitrobenzoic) acid (DTNB) to 2-nitro-5-thiobenzoate (TNB). The oxidized glutathione formed was recycled by GR and NADPH. The product, TNB, was assayed colorimetrically at 412 nm. Non-enzymatic antioxidants were determined on the first, third, fifth, and seventh day of algal culture.
Determination of the antioxidant enzymes’ activities
The antioxidant enzymes were extracted in 50 mM phosphate buffer, pH 7.0, containing 1 mM EDTA, 0.05 % (v/v) Triton X-100, 2 % (w/v) polyvinylpyrrolidone, and 1 mM ascorbic acid. Superoxide dismutase (SOD) (EC 1.15.1.1) activity of A. obliquus was determined by measuring the inhibition of photochemical reduction of nitroblue tetrazolium (NBT) at 560 nm as suggested by Beauchamp and Fridovich (1971). One unit of SOD (per milligram protein) was defined as the amount causing 50 % inhibition of the photochemical reduction of NBT. Catalase (CAT) (EC 1.11.1.6) activity was estimated by recording the decrease in absorbance of H2O2 at 240 nm (Aebi 1984). One unit of CAT activity (U) was assumed as the amount of enzyme that decomposes 1 μmol of H2O2 per milligram of soluble protein per minute at 30 °C. The method given by Nakano and Asada (1981) was followed for determining ascorbate peroxidase (APX) (EC 1.11.1.1) activity of A. obliquus. The enzyme activity (U) was calculated as the amount of the enzyme that oxidizes 1 μmol of ascorbate consumed per milligram of soluble protein per minute at 30 °C. Glutathione reductase (GR) (EC 1.8.1.7) activity was determined from the rate of NADPH oxidation as measured by the decrease of absorbance at 340 nm (extinction coefficient 6.2 mmol L−1 cm−1), following the procedure of Schaedle and Bassham (1977). Reaction mixture was composed of 100 mmol L−1 potassium phosphate (pH 7.8), 2 mmol L−1 EDTA, 0.2 mmol L−1 NADPH, 0.5 mmol L−1 glutathione disulfide (Sigma, USA), and the appropriate volume of enzyme extract. The reaction was initiated by the addition of NADPH at 25 °C. The activity of activities of antioxidant enzymes in A. obliquus cells were analyzed on the first, third, fifth, and seventh day of culture.
Replication and statistical analysis
Each treatment consisted of four replicates, and each experiment was carried out at least twice at different times. The data were analyzed by one-way analysis of variance (ANOVA), and the means were separated using Duncan’s multiple-range test (IBM SPSS Statistics 21, USA). The level of significance in all comparisons was p < 0.05.
Results
Heavy metal uptake and algal growth
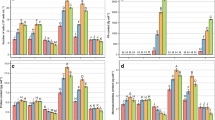
Green unicellular alga A. obliquus accumulated Pb in a concentration- and exposure-dependent manner (Table 1). Algae treated with the highest concentration of Pb (500 μM) contained the highest amounts of this heavy metal (2557.3 fg cel−1) after 7 days of culture. As shown in Fig. 1a, the number of algal cells decreased proportionally with increasing intracellular Pb accumulation. The most phytotoxic effect of examined heavy metal on A. obliquus growth was observed at concentration 500 μM on the seventh day of cultivation. In these conditions, the number of cells decreased by 67 % in relation to control. In the presence of the lowest concentration of Pb, only a slight inhibition of algal growth occurred. For example, Pb at concentration of 0.01 μM did not affect statistically significant effect on algal growth, except fifth day of cultivation when cell number decreased by 10 % in relation to control. When treated with Pb especially at 1–500 μM, the whole cell volume decreased by 5–18 % compared to untreated cells (Fig. 2) on the seventh day of cultivation. Pb at lower concentrations was not characterized by statistical significant effect on the size of algal cells, except cultures exposed to 1 μM Pb on the fifth and seventh day of experiment, when cell volume has been reduced by 6–7 %.
The number of cells (a) and the contents of proteins (b), chlorophyll a (c), chlorophyll b (d), carotenoids (e), and monosaccharides (f) in Acutodesmus obliquus cultures under the influence of Pb at range of concentrations 0.01–500 μM on the first, third, fifth, and seventh day of culture. Data are the means of four independent experiments ± SD. Treatment with at least one letter the same are not significantly different according to Duncan’s test
The effect of Pb at range of concentrations 0.01–500 μM on the cell size expressed as cell volume (μm3) of Acutodesmus obliquus on the first, third, fifth, and seventh day of culture. Data are the means of four independent experiments ± SD. Treatment with at least one letter the same are not significantly different according to Duncan’s test
The effect of Pb on protein, photosynthetic pigments, and monosaccharide content in A. obliquus cells
The protein content (Fig. 1b) in A. obliquus cells decreased proportionally with the increase in Pb concentration in the medium at all exposure periods. The maximum decline in this biochemical parameter was recorded as 61 % on the seventh day of cultivation under the influence of 500 μM Pb. On the other hand, Pb at 0.01 μM provoked slight (3 %) but statistically insignificant increase in protein level on the fifth day of culture.
A. obliquus cultures exposed to Pb displayed chlorosis because significant loss in the content of both chlorophylls (chlorophyll a and b) was observed during 7 days of culture (Fig. 1c–d). Therefore, the maximum decrease by 27 and 37 % in chlorophyll a and b, respectively, was obtained after application of 500 μM Pb on the seventh day of cultivation. Heavy metal at lower concentrations did little harm to these chloroplast pigments in A. obliquus cells.
On the other hand, carotenoids were less sensitive than chlorophylls toward Pb presence in the medium probably protecting the photosynthetic apparatus against toxic effect of abiotic stress on algal cells (Fig. 1e). The external supply of 500 μM Pb induced 43 % decrease in their content on the seventh day of experiment. By contrast, this heavy metal at lower concentrations (0.01–0.1 μM) were characterized by stimulating effect by 6–12 % on the carotenoid content compared to the control on the fifth day of algal cultivation.
Pb inhibited the monosaccharide accumulation in a concentration- and time-dependent manner (Fig. 1f). Therefore, the significant decrease by 70 % in the monosaccharide level on the seventh day of A. obliquus cultivation was obtained under the highest concentration (500 μM) of Pb. The application of Pb at 0.01 μM did not exhibit toxic effect on sugar level in algal cells during the whole period of cultivation.
The effect of Pb on stress markers in A. obliquus cells
Pb enhanced lipid peroxidation, measured as total MDA content, which is the phytotoxic product of lipid peroxidation. MDA level in A. obliquus cells increased gradually proportionally to increased concentration of Pb in the medium (Fig. 3a). The highest stimulation by 65 % in MDA level was recorded under the influence of 500 μM Pb on the seventh day of A. obliquus culture compared to control. The application of Pb ions at the lowest concentration of 0.01 μM provoked weaker response leading to 7–12 % increase in MDA accumulation during the whole period of experiment.
The effect of Pb at range of concentrations 0.01–500 μM on lipid peroxidation, expressed as MDA content (a) and hydrogen peroxide (b) level in Acutodesmus obliquus cells on the first, third, fifth, and seventh day of culture. Data are the means of four independent experiments ± SD. Treatment with at least one letter the same are not significantly different according to Duncan’s test
Compared to unstressed algal cells, the significant increase in the content of H2O2 was observed in the presence of Pb in the culture (Fig. 3b). H2O2 production was proportional with external heavy metal concentration. For example, the increase in H2O2 level by 73 % was observed in algal cells treated with 500 μM Pb on the seventh day of culture in relation to the control. Heavy metal even at the lowest concentration (0.01 μM Pb) provoked oxidative stress, because 0.01 μM Pb induced 4–11 % increase in H2O2 level during 7 days of cultivation.
The effect of Pb on non-enzymatic antioxidants in A. obliquus cells
The highest enhancement in the ascorbate content by 25 % was observed in A. obliquus cells treated with 0.1 μM Pb on the seventh day of cultivation in relation to control. By contrast, Pb at higher concentration range of 10–500 μM possessed toxic effect on the ascorbate level. Pb, especially at 500 μM, induced the significant decrease by 50 % in the content of this antioxidant on the seventh day of experiment (Fig. 4a). Similarly, Pb at lower concentration range of 0.01–0.1 μM stimulated (9–38 %) the glutathione level upon long-term exposure compared to control. However, the application of Pb at 500 μM resulted in the highest decline in glutathione level by 70 % compared to the control on the seventh day of experiment (Fig. 4b). Heavy metal at the concentration of 1 μM did not possess statistically significant effect on the level of both studied antioxidants.
The effect of Pb at range of concentrations 0.01–500 μM on the ascorbate (a) and glutathione (b) content in Acutodesmus obliquus cells on the first, third, fifth, and seventh day of culture. Data are the means of four independent experiments ± SD. Treatment with at least one letter the same are not significantly different according to Duncan’s test
The effect of Pb on antioxidant enzymes in A. obliquus cells
Heavy metal affected also the activity of antioxidant enzymes involved in antioxidant defense (Fig. 5a–d). Pb at small concentration range of 0.01–1 μM stimulated the activity of antioxidant enzymes responsible for ROS removing in A. obliquus cells. Moreover, it was found that the presence of 0.1 μM Pb in the nutrient solution resulted in the highest activities of SOD, CAT, APX, and GR activity in A. obliquus culture. The SOD activity increased by 11 % on the third day of cultivation in algal cells treated with 0.1 μM Pb. Heavy metal at concentration of 0.1 μM elevated CAT activity by 38 % on the seventh day of experiment in relation to control. Enhancement of APX activity by 32 % in comparison with control was observed in algal cells exposed to 0.1 μM Pb on the fifth day of cultivation. GR activity was the most stimulated by 95 % on the seventh day of experiment in response to 0.1 μM Pb. However, Pb at higher concentration range of 10–500 μM inhibited all studied antioxidant enzymes’ activities involved in scavenging of ROS. For example, the activity of SOD, CAT, APX, and GR decreased by 33, 74, 75, and 88 %, respectively, in A. obliquus culture treated with 500 μM Pb.
The effect of Pb at range of concentrations 0.01–500 μM on the activity of superoxide dismutase (a), catalase (b), ascorbate peroxidase (c), and glutathione reductase (d) activity in Acutodesmus obliquus cells on the first, third, fifth, and seventh day of culture. Data are the means of four independent experiments ± SD. Treatment with at least one letter the same are not significantly different according to Duncan’s test
Discussion
Microalgae, the primary producers at the base of the aquatic food chain, are the first target affected by heavy metal pollution. Pb is a non-essential and very toxic heavy metal for many living organisms, accumulates in algae (Bajguz 2011). In fact, microalgae could be used to clean up contaminated water and waste streams by removing metals or solubilizing them in order to facilitate their extraction (Andrade et al. 2005; Yoshida et al. 2006). Therefore, we utilized a strain of A. obliquus, an unicellular chlorophyte, as a model system for growth and biochemical studies including heavy metal accumulation.
The intracellular accumulation of Pb by A. obliquus is accompanied by an induction of a variety of metabolic changes, some of which directly contribute to metal tolerance capacity of the microalga. Results showed that A. obliquus accumulated Pb in a concentration- and time-dependent manner. This is in agreement with the earlier reports on algae, such as C. vulgaris (Bajguz 2011, Piotrowska-Niczyporuk et al. 2012), C. kesslerii (Andrade et al. 2005), Scenedesmus incrassatulus ( Peña-Castro et al. 2004), and Spyrogyra as well as Cladophora filamentous macroalgae (Lee and Chang 2011). Green algae have attracted the attention of many researchers and environmentalists as organisms to be tested and used as a new analytical reagent to concentrate and adsorb metal ions such as Cu, Cd, Pb, chromium (Cr), and gold (Au) (Fargasova 1993). Fathi et al. (2008) reported that the uptake of cobalt (Co), Cu, and zinc (Zn) from the surrounding medium by water phytoplankton is seldom exactly proportional to the amount present in the environment. Such organisms have been shown to perform very well for this type of application because they have a uniform cell size and a number of different metal binding sites on their cell walls (Wang and Chen 2009). Green algae are mainly cellulose, and a high percentage of the cell wall are proteins bonded to polysaccharides to form glycoproteins. Some functional groups in cell wall have been found to bind metal ions, especially carboxyl group. There are some evidence to confirm that the O-, N-, S-, or P-containing groups participate directly in binding a certain metals. Heavy metal ions such as Pb form strong bond with ligands present in cell wall structure such as CN−, R-S−, −SH−, NH2−, and imidazol, which are groups containing nitrogen and sulfur atoms (Hamdy 2000; Wang and Chen 2009). Toxic metals may also bind in algal cells through low-molecular proteins (e.g., phytochelatins), which are one of the cell’s mechanisms for the detoxification of heavy (Vacchina et al. 1999).
It was determined that toxic effect of Pb on growth and some key metabolic processes in A. obliquus cells is caused by its intracellular accumulation. The most dramatic symptom of phytotoxicity of Pb in green algae was the cessation of culture growth expressed as cell number. As the growth reflects the metabolism of the cell, it has been used as a key indicator of the toxicity of heavy metal ions in microalgae, and it depends on the proper functioning of various metabolic processes, such as photosynthesis, respiration, and nutrient uptake, etc. (Poskuta et al. 1996; Tripathi and Gaur 2006; Tripathi et al. 2006). Growth inhibition in microalgae is related to the amount of heavy metal ions bound to the algal cell surface, in some cases, to the amount of intracellular heavy metal ions and to the chemical nature of the heavy metal ions (Tripathi and Gaur 2006; Yang et al. 2014; Polonini et al. 2015). The suppression of growth by heavy metals was also observed in experiments performed on C. vulgaris cultures treated with Cd, Pb, and Cu (Bajguz 2011; Piotrowska-Niczyporuk et al. 2012). Changes in cell size are common effect of heavy metal toxicity in unicellular eukaryotic algae. However, the kind and intensity of the effects depends on the algal species, the metal, and its concentrations. Our results indicate that Pb at higher concentrations 1–500 μM reduces the size of algal cells, especially at long-term exposure (fifth to seventh day of culture). Similar observations have been documented for algae exposed to pollutants. After pollutant treatment (e.g., Cu; nickel, Ni), the cell size of algal cells Chlorococcum infusionum was reduced during 6 days of culture (Nam and An 2015) and the cell size was far more sensitive than other parameters of algal viability such as cell granularity and autofluorescence of chlorophyll. Similarly, Jamers et al. (2009) reported that Chlamydomonas reinhardtii exhibited a decline in cell size under 5 and 100 mM Cd exposure during 72 h of culture. The decrease in cell volume in response to Pb may be connected with toxic effect of heavy metals on the structure of cell wall and the leakage of ions or cell components through plasma membrane leading to irreversible cell damage and necrosis (Jamers et al. 2009; Nam and An 2015).
The decrease in the content of chlorophylls can be used to monitor the heavy metal-induced cellular damage in A. obliquus cells. Reduction in the contents of chlorophylls might be owing to several reasons such as inhibition of chlorophyll biosynthesis, breakdown of pigments or their precursors, and destruction of chloroplast membrane by lipid peroxidation due to lack of antioxidants. Heavy metals have been found to decrease the total chlorophyll content, and the chlorophyll a/b ratio, and decrease the chlorophyll/carotenoid ratio in plants. Pb may inhibit the biosynthesis of chlorophyll pigments and enzymes involved in this process in higher plants (Pahlsson 1989) as well as green alga Chlorella pyrenoidosa (Poskuta et al. 1996) and C. vulgaris (Bajguz 2011; Piotrowska-Niczyporuk et al. 2012). Studies with in the filamentous green alga Cladophora fracta reported that Pb at high concentrations can destroy the chloroplasts (Lamaia et al. 2005). Moreover, an enhancement of chlorophyll damage occurs in the plants growing in the presence of Pb ions due to increased chlorophyllase activity (Drążkiewicz 1994). The inhibition in photosynthetic pigment accumulation in response to heavy metal stress may be also a consequence of peroxidation of chloroplast membranes via increased rate of ROS production. This observation is in good agreement with the increased rate of H2O2 and lipid peroxidation in A. obliquus cultures treated with Pb. However, the increased carotenoid level in A. obliquus cells exposed to Pb is probably the part of strategy adopted by the green alga to counteract the toxic effect of free radicals generated under abiotic stress, which is in agreement with other reports on higher plants (Singh et al. 2006; Hou et al. 2007) and algae (Bajguz 2011; Piotrowska-Niczyporuk et al. 2012). Carotenoids, which play the part of guard of chlorophyll, serve also as antioxidants to quench or scavenge the free radicals and reduce the damage of cell induced by heavy metal stress (Artetxe et al. 2002).
The reduction in monosaccharide content in A. obliquus cells growing in the presence of Pb may be due to the enhancement in the degradation of photosynthetic pigments contributing to decline in photosynthesis and sugar accumulation. The significant decrease in activities of enzymes involved in CO2 fixation in field grown Avena sativa exposed to heavy metals was reported by Moustakas et al. (1994). Soluble-protein content in plants, an important indicator of reversible and irreversible changes in metabolism, is known to respond to a wide variety of abiotic stressors (Singh et al. 2006). The inability of A. obliquus cells to accumulate proteins in response to Pb application might be caused by acute oxidative stress induced by heavy metal excess in algal cells. The toxicity of this heavy metal may be also explained by their effect on nucleic acid degradation. For example, Cd pollution induces DNA damage such as single- and double-strand breaks, modified bases, abasic sites, DNA-protein cross-links, oxidized bases, 8-hydroxyguanine, and even bulky adducts in Hordeum vulgare seedlings (Liu et al. 2005).
Exposure to heavy metal in general, and to Pb in particular, increases the production of ROS, including H2O2, thereby unbalancing the cellular redox status (Hegedüs et al. 2001; Verma and Dubey 2003; Phang et al. 2011). At high levels, or following acute increases of metal pollutants, algal cells are injured because the resulting ROS levels exceed the capacities of cellular antioxidant protection systems. ROS can react with cellular components, such as lipids, proteins, pigments, and nucleic acids to cause lipid peroxidation, membrane damage, and enzyme inactivation, thus affecting many physiological processes as well as cell viability. Therefore, metal-induced oxidative stress in algae is now receiving increasing attention (Pinto et al. 2003; Tripathi and Gaur 2006; Tripathi et al. 2006; Bajguz 2011; Piotrowska-Niczyporuk et al. 2012). The level of oxidative stress was, in our study, shown directly by increases in MDA level and the content of H2O2 in A. obliquus cells exposed to Pb. MDA production in plants exposed to adverse environmental conditions is an indicator of free radical formation in the tissues and is commonly used as an index of lipid peroxidation in biological systems (Heath and Packer 1968). Probably, the binding of Pb to thiols disrupts redox status of the algal cells thereby enhancing the production of ROS and lipid peroxidation. Additionally, high levels of H2O2 can accelerate processes like Haber-Weiss reaction, resulting in the formation of hydroxyl radicals that can cause lipid peroxidation (Hegedüs et al. 2001; Verma and Dubey 2003). This is clear from the greater degree of lipid peroxidation in A. obliquus up on exposure to Pb stress. A concentration-dependent increase in the level of lipid peroxides occurred in spruce (Picea abies) needles (Radotic et al. 2003), barley (Hordeum vulgare) seedlings (Hegedüs et al. 2001), rice (Oriza sativa) shoots (Verma and Dubey 2003), water hyssop (Bacopa monnieri) (Singh et al. 2006), Wolffia arrhiza fronds (Piotrowska et al. 2010), and green alga C. vulgaris (Bajguz 2011; Piotrowska-Niczyporuk et al. 2012) growing in the presence of increased concentrations of heavy metals. Moreover, in cells, H2O2 may be produced by SOD because this enzyme catalyzes the dismutation of O2 •− into H2O2 in higher plants exposed as well as in Scenedesmus sp. during short- and long-term exposure to Cu and Zn (Tripathi et al. 2006). Therefore, the present results suggest positive correlation between the high activity of SOD and the generation of H2O2 in A. obliquus cells in response to Pb, especially at low concentration of 0.1 μM. But, the enhancement of activity of SOD alone cannot alleviate the burden of ROS. H2O2 is a highly toxic ROS and must be sequestered by the action of APX and CAT. Our results indicate that the activities of these two key antioxidant enzymes increased significantly in response to 0.1 μM Pb, when the oxidative stress was rather mild. Therefore, increased activity of antioxidant enzymes can be expected to reduce oxidative stress in algal cells induced by the presence of low concentrations of Pb in the medium. In fact, other green algae C. vulgaris with enhanced activities of antioxidant enzymes have been shown to be tolerant to oxidative stress induced by heavy metal, such as Cd, Cu, and Pb pollution (Bajguz 2011; Piotrowska-Niczyporuk et al. 2012). Our data show that also GR activity was stimulated appreciably in A. obliquus cultures exposed to Pb stress. GR is connected to APX activity in the Halliwell-Asada cycle, in which glutathione and NAPDH are used to restore the reduced ascorbate pool. Thus, it should be expected that GR activity changes together with that of APX. In addition, we found the increase in the total cellular glutathione pool in A. obliquus cells exposed to 0.1 μM Pb. Taken together, these results suggest that glutathione and related enzymatic pathways seems to be involved in the defense mechanisms of this alga upon heavy metal exposure, under our experimental conditions. These results agree with those of Kumar et al. (2010), who demonstrated that the green alga Ulva lactuca also increased in GR activity after exposure to Cd. On the other hand, Pb at higher concentration range of 10–500 μM inhibited the activity of all studied antioxidant enzymes in A. obliquus culture, which is probably due to the harmful effect of H2O2 overproduction or its poisonous ROS derivatives on proteins.
Whenever ROS are produced, algae accumulate also non-enzymatic antioxidants, such as ascorbate and glutathione responsible for directing removing these highly reactive molecules. The highest increase in ascorbate and glutathione level was found in A. obliquus cells treated with Pb at low concentration (0.1 μM). This is in an accordance with previous results obtained on C. vulgaris treated with heavy metals ( Piotrowska-Niczyporuk et al. 2012). Glutathione can act as a messenger molecule in cellular signal transduction and as a factor in plant defense against oxidative stress (Gill and Tuteja 2010). Our results support the hypotheses that cells of algae elevated glutathione level in response to Pb which induce oxidative stress expressed as H2O2 and lipid peroxidation. Hence, oxidative stress enhanced the ability to synthesis glutathione which may induce the development of resistant cells. High cellular glutathione level in algal cells is associated to mild Pb stress (0.1 μM). In other words, heavy metal exposure has been shown to lead to accelerated glutathione accumulation in A. obliquus cells. Similarly, glutathione accumulation is found to compensate for decreases in the capacity of other antioxidants, and depletion of glutathione increased the sensitivity to oxidative. Hence, the increasing in both of cellular ascorbate and glutathione contents are correlated with algal tolerance to heavy metal-induced stress (Gill and Tuteja 2010). This may be due to the role of ascorbate in reductive detoxification of ROS generated in response of Pb stress, which must therefore, be continuously regenerated from its oxidized form. A major function of glutathione in protection against oxidative stress is reduction of ascorbate in ascorbate-glutathione cycle. In this pathway, glutathione act as a recycled intermediate in the reduction of H2O2 using electrons derived, ultimately from H2O2 (Nakano and Asada 1981; Kampfenkel et al. 1995). In spite of the fact that neither reduced/oxidized ratio of ascorbate and glutathione were assayed in this study, studies of APX and GR activity as well as ascorbate and glutathione level lends support to hypothesis that the H2O2 scavenging ascorbate-glutathione cycle is activated in green microalga under mild Pb stress. Similar results obtained by Artetxe et al. (2002), Rucińska-Sobkowiak and Pukacki (2006) as well as Singh et al. (2006) confirmed that ascorbate accumulation led to enhancement of plant tolerance toward heavy metal stress.
The present study shows that antioxidant enzymes and non-enzymatic antioxidants provide protection to A. obliquus only when Pb-induced oxidative stress is mild, which occurs at low concentrations of this heavy metal. The redox status of cells is maintained by an integrated net constitutive and inducible antioxidant enzyme (e.g., SOD, CAT, APX, and GR) in coordination with fluctuating intercellular levels of low-molecular weight of antioxidant compounds such as glutathione and ascorbate. Algae response to mild heavy metal stress by the increased accumulation of both antioxidants or elevated activity of protective enzymes. If the protective and repair capacities are exceeded, proteins, lipids, and photosynthetic pigment suffer oxidation damage resulting in inhibition of algal growth. On the other hand, green alga A. obliquus exhibited different tolerance and strategies against Pb exposure, which are not only dose dependent, but also, strongly correlated with antioxidant enzyme activities.
Conclusion
In the present study, the accumulation of Pb in green alga A. obliquus resulted in considerable biochemical changes. The data confirm the inhibitory effect of Pb on the growth expressed as the number and the size of cells and the contents of selected primary metabolites in algal cells. The rate of proteins, photosynthetic pigments, and monosaccharide degradation was proportional to increased heavy metal concentration and the most phytotoxic influence of Pb was observed at the highest tested concentration of 500 μM. Treatment with Pb caused also oxidative damage as evidenced by increased lipid peroxidation and H2O2 level. However, to cope with heavy metal toxicity, A. obliquus cells are able to carry out a cellular strategy involving activation of various enzymatic (SOD, CAT, APX, and GR) and non-enzymatic (ascorbate, glutathione) antioxidants serve as important components of mechanism detoxifying heavy metal. The highest enhancement in antioxidant activity was observed in cultures treated with low concentration of Pb (0.1 μM) which may account for higher alga tolerance and acclimation to metal contamination of aquatic environment.
References
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:212–125
Aliexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Andersen RA (2005) Algal Culturing Techniques. Elsevier, USA
Andrade AD, Rollemberg MCE, Nóbrega JA (2005) Proton and metal binding capacity of the green freshwater alga Chaetophora elegans. Process Biochem 40:1931–1936
Artetxe U, García-Plazaola JI, Hernández A, Becerril JM (2002) Low light grown duckweed plants are more protected against the toxicity induced by Zn and Cd. Plant Physiol Biochem 40:859–863
Bajguz A (2011) Suppression of Chlorella vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous brassinolide. Arch Environ Contam Toxicol 60:406–416
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
De Kok LJ, Maas FM, Godeke J, Haaksma AB, Kuiper PJC (1986) Glutathione, a tripeptide which may function as a temporary storage compound of excessive reduced sulphur in H2S fumigated spinach plants. Plant Soil 91:349–352
Drążkiewicz M (1994) Chlorophyll-occurrence, functions, mechanism of action, effects of internal and external factors. Photosynthetica 30:321–331
Fargasova A (1993) Effect of five toxic metals on the alga Scenedesmus quadricauda. Biologia 48:301–304
Fathi AA, El-Shahed AM, Shoulkamy MA, Ibraheim HA, Abdel Rahman OM (2008) Response of Nile water phytoplankton to the toxicity cobalt, copper and zinc. Res J Environ Toxicol 2:67–76
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gin KY, Tangm YZ, Aziz MA (2002) Derivation and application of a new model for heavy metal biosorption by algae. Water Res 36:1313–1323
Hamdy AA (2000) Biosorption of heavy metals by marine algae. Curr Microbiol 41:232–238
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hegedüs A, Erdei S, Horvath G (2001) Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci 160:1085–1093
Hou W, Chen X, Song G, Wang Q, Chang CC (2007) Effect of copper and cadmium on heavy metal polluter waterbody restoration by duckweed (Lemna minor). Plant Physiol Biochem 45:62–69
Hu S, Lau KWK, Wu M (2001) Cadmium sequestration in Chlamydomonas reinhardtii. Plant Sci 161:987–996
Jamers A, Lenjou M, Deraedt P, Bockstaele DV, Blust R, de Coen W (2009) Flow cytometric analysis of the cadmium-exposed green alga Chlamydomonas reinhardtii (Chlorophyceae). Eur J Phycol 44:541–550
Javanbakht V, Alavi SA, Zilouei H (2014) Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci Technol 69:1775–1787
Kampfenkel K, Van Montagu M, Inzé D (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225:165–167
Kumar M, Kumari P, Gupta V, Anisha PA, Reddy CRK, Jha B (2010) Differential responses to cadmium induced oxidative stress in marine macroalga Ulva lactuca (Ulvales, Chlorophyta). Biometals 23:315–325
Lamaia C, Kruatrachuea M, Pokethitiyooka P, Upathamb ES, Soonthornsarathoola V (2005) Toxicity and accumulation of lead and cadmium in the filamentous green alga Cladophora fracta: a laboratory study. Sci Asia 31:121–127
Lee Y-C, Chang S-P (2011) The biosorption of heavy metals from aqueous solution by Spirogyra and Cladophora filamentous macroalgae. Bioresour Technol 102:5297–5304
Liu W, Li PJ, Qi XM, Zhou QX, Zheng L, Sun TH, Yang YS (2005) DNA changes in barley (Hordeum vulgare) seedlings induced by cadmium pollution using RAPD analysis. Chemosphere 61:158–167
Lourie E, Patil V, Gjengedal E (2010) Efficient purification of heavy-metal-contaminated water by micro-algae-activated pine bark. Water Air Soil Poll 210:493–500
Moustakas M, Lanaras T, Symeonidis L, Kartaglis S (1994) Growth and some photosynthetic characteristics of field grown Avena sativa under copper and lead stress. Photosynthetica 30:389–396
Nagalakshmi N, Prasad MNV (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299
Nakano Y, Asada K (1981) Hydrogen peroxidase is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nam S-H, An Y-J (2015) Cell size and the blockage of electron transfer in photosynthesis: Proposed endpoints for algal assays and its application to soil alga Chlorococcum infusionum. Chemosphere 128:85–95
Pahlsson AMB (1989) Toxicity of heavy metals (Zn, Cd, Cu, Pb) to vascular plants. Water Air Soil Pollut 47:287–319
Peña-Castro JM, Martínez-Jerónimo F, Esparza-García F, Cañizares-Villanueva RO (2004) Heavy metals removal by the microalga Scenedesmus incrassatulus in continuous cultures. Bioresour Technol 94:219–222
Phang IC, Leung DW, Taylor HH, Burritt DJ (2011) The protective effect of sodium nitroprusside (SNP) treatment on Arabidopsis thaliana seedlings exposed to toxic level of Pb is not linked to avoidance of Pb uptake. Ecotoxicol Environ Saf 74:1310–1315
Pinto E, Van Nieuwerburgh L, Barros MP, Pedersén M, Colepicolo P, Snoeijs P (2003) Density-dependent patterns of thiamine and pigment production in Nitzschia microcephala. Phytochemistry 63:155–163
Piotrowska A, Bajguz A, Godlewska-Żyłkiewicz B, Zambrzycka E (2010) Changes in growth, biochemical components, and antioxidant activity in aquatic plant Wolffia arrhiza (Lemnaceae) exposed to cadmium and lead. Arch Environ Contam Toxicol 58:594–604
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, Godlewska-Żyłkiewicz B (2012) Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol Biochem 52:52–65
Pirson A, Lorenzen H (1966) Synchronised dividing algae. Ann Rev Plant Physiol 17:439–458
Polonini HC, Brandão HM, Raposo NR, Brandão MA, Mouton L, Couté A, Yéprémian C, Sivry Y, Brayner R (2015) Size-dependent ecotoxicity of barium titanate particles: the case of Chlorella vulgaris green algae. Ecotoxicol 24:938–948
Poskuta JW, Parys E, Romanowska E (1996) Toxicity of lead to photosynthesis, accumulation of chlorophyll, respiration and growth of Chlorella pyrenoidosa. Protective role of dark respiration. Acta Physiol Plant 18:165–171
Radotic K, Ducic T, Mutavdzic D (2000) Changes in peroxidase activity and isoenzymes in spruce needles after exposure to different concentrations of cadmium. Environ Exp Bot 44:105–113
Rajamani S, Torres M, Falcao V, Gray JE, Coury DA, Colepicolo P, Sayre R (2014) Noninvasive evaluation of heavy metal uptake and storage in micoralgae using a fluorescence resonance energy transfer-based heavy metal biosensor. Plant Physiol 164:1059–1067
Rucińska-Sobkowiak R, Pukacki PM (2006) Antioxidative defense system in lupin roots exposed to increasing concentrations of lead. Acta Physiol Plant 28:357–364
Sáez CA, Roncarati F, Moenne A, Moody AJ, Brown MT (2015) Copper-induced intra-specific oxidative damage and antioxidant responses in strains of the brown alga Ectocarpus siliculosus with different pollution histories. Aquatic Toxicol 159:81–89
Schaedle M, Bassham JA (1977) Chloroplast glutathione reductase. Plant Physiol 59:1011–1012
Singh S, Eapen S, D’Souza SF (2006) Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant Bacopa monnieri L. Chemosphere 62:233–246
Somogyi M (1954) Notes on sugar determination. J Biol Chem 195:19–23
Tripathi BN, Gaur JP (2006) Physiological behavior of Scenedesmus sp. during exposure to elevated levels of Cu and Zn and after withdrawal of metal stress. Protoplasma 229:1–9
Tripathi BN, Mehta SK, Amar A, Gaur JP (2006) Oxidative stress in Scenedesmus sp. during short- and long-term exposure to Cu2+ and Zn2+. Chemosphere 62:538–544
Tukaj Z, Pokora W (2006) Individual and combined effect of anthracene, cadmium, and chloridazone on growth and activity of SOD izoformes in three Scenedesmus species. Ecotoxicol Environ Saf 65:323–331
Vacchina V, Chassaigne H, Oven M, Zenk MH, Lobinski R (1999) Characterization and determination of phytochelatins in plant extracts by electrospray tandem mass spectrometry. Analist 124:1425–1430
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotech Adv 27:195–226
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Wilde KL, Stauber JL, Markich S, Franklin JNM, Brown PL (2006) The effect of pH on the uptake and toxicity of copper and zinc in a tropical freshwater alga (Chlorella sp.). Arch Environ Contam Toxicol 51:174–185
Yang J, Cao J, Xing G, Yuan H (2014) Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresour Technol 175C:537–544
Yoshida N, Ikeda R, Okuno T (2006) Identification and characterization of heavy metal-resistant unicellular alga isolated from soil and its potential for phytoremediation. Bioresource Technol 97:1843–1849
Zhou W, Li Y, Min M, Hu B, Chen P, Ruan R (2011) Local bioprospecting for high-lipid producing microalgal strains to be grown on concentrated municipal wastewater for biofuel production. Bioresour Technol 102:6909–6919
Acknowledgments
This project has been financed from the funds of the National Science Centre allocated on the basis of the decision number DEC-2012/05/B/NZ8/00958
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Cinta Porte
Highlights
1. Green alga Acutodesmus obliquus is a good scavenger of Pb ion from aqueous solution.
2. Pb induced inhibition of algal growth and primary metabolite accumulation.
3. Oxidative stress was generated in algal cells in response to Pb.
4. Pb at low concentrations activated antioxidant defense system.
Rights and permissions
About this article
Cite this article
Piotrowska-Niczyporuk, A., Bajguz, A., Talarek, M. et al. The effect of lead on the growth, content of primary metabolites, and antioxidant response of green alga Acutodesmus obliquus (Chlorophyceae). Environ Sci Pollut Res 22, 19112–19123 (2015). https://doi.org/10.1007/s11356-015-5118-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5118-y