Abstract
Antibiotic resistance in urinary tract infections (UTIs) is a growing concern due to extensive antibiotic use. The study explores a drug repurposing approach to find non-antibiotic drugs with antibacterial activity. In the present study, 8 strains of Pseudomonas spp. were used that were clinically isolated from UTI-infected patients. Amlodipine, a cardiovascular drug used in this study, has shown potential antimicrobial effect in reducing the various virulence factors, including swimming and twitching motility, biofilm, rhamnolipid, pyocyanin, and oxidative stress resistance against all the strains. Amlodipine exhibited the most potent antimicrobial activity with MIC in the range of 6.25 to 25 µg/ml. Significant inhibition in biofilm production was seen in the range of 45.75 to 76.70%. A maximum decrease of 54.66% and 59.45% in swimming and twitching motility was observed, respectively. Maximum inhibition of 65.87% of pyocyanin pigment was observed with the effect of amlodipine. Moreover, a significant decrease in rhamnolipids production observed after amlodipine treatment was between 16.5 and 0.001 mg/ml as compared to the control. All bacterial strains exhibited leakage of proteins and nucleic acids from their cell membranes when exposed to amlodipine which suggests the damage of the structural integrity. In conclusion, amlodipine exhibited good antimicrobial activity and can be used as a potential candidate to be repurposed for the treatment of urinary tract infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary tract infections (UTIs) are widespread bacterial infections affecting individuals globally, presenting a significant public health concern. The advancements of multidrug-resistant (MDR) strains of Pseudomonas spp., particularly Pseudomonas aeruginosa, have added a complex layer to the management of UTIs. These MDR pathogens have evolved intricate resistance mechanisms that defy traditional antibiotics, creating a pressing need for innovative treatment strategies. MDR Pseudomonas spp. pose a formidable challenge in healthcare due to their exceptional resistance to multiple antibiotic classes. Their resistance mechanisms, including efflux pumps, antibiotic-modifying enzymes, and membrane alterations, render them impervious to many antimicrobial agents. The proliferation of MDR strains has led to prolonged hospital stays, increased healthcare costs, and elevated morbidity and mortality rates associated with UTIs. Conventional antibiotics, historically the cornerstone of UTI treatment, are becoming increasingly ineffective against MDR Pseudomonas strains, necessitating a reevaluation of treatment approaches. This situation has catalyzed the exploration of innovative strategies, one of which is drug repurposing – the reevaluation of existing non-antibiotic drugs for potential utility in treating bacterial infections. Drug repurposing offers a promising avenue for addressing MDR Pseudomonas UTIs. It leverages the existing library of drugs with established safety profiles, potentially expediting the availability of new treatments while minimizing the risks and costs of developing new antibiotics [1]. This approach has particular relevance given the slow pace of antibiotic development, which can take many years and substantial resources [2].
Non-antibiotic drugs, initially designed for various therapeutic purposes, often possess mechanisms of action distinct from traditional antibiotics. This diversity is a critical advantage. Bacteria primarily develop resistance through genetic mutations or resistance gene exchange. Non-antibiotic drugs, with novel mechanisms of action, can evade these resistance mechanisms, making it challenging for bacteria to evolve resistance. Moreover, when used in combination with antibiotics, non-antibiotic drugs can enhance treatment outcomes and reduce the likelihood of resistance emergence.
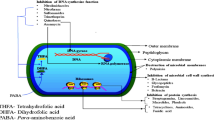
Amlodipine is among the repositioned medications that have been consolidated in the literature to be used with new therapeutic indications. These medications include cardiovascular, psychotropic, antihistamines, anti-inflammatory, local anesthetics, tranquilizers, and anti-hypersensitive [3]. Due to its hydropyridine and calcium channel blocker properties, amlodipine is frequently the first-choice medication for the scientific indication or treatment of arterial hypertension, angina, and cardiac arrhythmias. Investigation on amlodipine has suggested its antifungal properties [4] and parasite-fighting properties [5]. Moreover, it exhibits antineoplastic and antibacterial properties. These properties are not entirely understood, although it is hypothesized that the antibacterial properties arise from the compound's action on macrophages or inhibition of the efflux pump [6]. Antineoplastic effects can be caused via inducing apoptosis, changing the permeability of cells, and suppressing the G1 phase of the life cycle [1]. We propose amlodipine redirection as a successful treatment for a variety of diseases, particularly infections brought on by bacteria that are resistant to drugs, as reported in literature [7].
In conclusion, MDR Pseudomonas UTIs represent a significant healthcare challenge, necessitating novel treatment approaches. Drug repurposing of non-antibiotic drugs emerges as a promising alternative, addressing the urgent need to combat antibiotic resistance, expediting treatment development, and harnessing diverse mechanisms of action.
This paper explores the significance of drug repurposing, delving into its mechanisms with respect to the inhibition of swimming and twitching motilities and biofilm production. The paper contributes to the amlodipine effects on the production of various virulence factors, including rhamnolipid and phycocyanin, and challenges, with a specific focus on its role in managing UTIs caused by Pseudomonas spp. It aims to contribute to our understanding of this innovative approach and its potential in addressing this critical global health concern.
Materials & Methods
Materials
Dimethyl sulfoxide (DMSO), Luria–Bertani (LB) agar and broth, Mueller–Hinton-broth, Mueller Hinton agar, and Tryptone soya broth agar were bought from Sigma (St. Louis, USA). Amlodipine was bought from Dhamtech Pharmaceuticals in Maharashtra, India. All compounds were of pharmacological quality. A total of 8 UTI Pseudomonas strains were taken for study, including Pseudomonas aeruginosa, Pseudomonas Stutzeri, Pseudomonas fluorescens, and Pseudomonas putida and strains were clinically isolated and provided by the Dr. B.Lal Clinical Laboratory, Jaipur, India.
Estimation of MIC (Minimum Inhibitory Concentration)
MIC determination was done by antibacterial susceptibility testing [8]. The assay was performed with the collaboration of broth microdilution test with resazurin dye, which is based on the principle that the reduction of blue resazurin to the pink product resorufin is only done by metabolically active cells. The number of cells visible in pink color is metabolically active and is proportionate to this reduction [9]. Minimum inhibitory concentration (MIC) was determined as the lowest concentration in the resazurin test that showed no live cells, as indicated by a blue color. Bacterial colonies were isolated from the agar plates, which were then placed in Luria broth medium and left to grow overnight. To meet McFarland's criterion of 0.5, the bacterial suspension was adjusted. The cells were diluted with medium to a concentration of 1 × 106 cfu/ml. Amlodipine's antimicrobial properties were examined using the broth microdilution method. To dissolve amlodipine, DMSO was utilized. The chemical was synthesized at two-fold concentrations in the range of 0 µg/ml to 200 µg/ml from the stock solution. As a standard antibiotic, streptomycin was taken. A total of 200 μl each well of a 96-well plate was made by adding 5 μl of test substance and 5 μl of cells to 190 μl of LB medium. The plate was then incubated for 24 h. To ascertain the vitality of the cells, resazurin dye solution was added after a 24-h incubation period [10]. Each well received a volume of 20 μl of Resazurin, which was then incubated for two hours. Where pink showed the presence of living cells, dark purple indicated the presence of dead cells. The absorbance was measured with a microplate reader at 570 and 590 nm. The vitality of cells was calculated and expressed in percentage.
Evaluation of Biofilm Inhibition
Microtiter biofilm inhibition assay was performed to assess the capacity of amlodipine drug to suppress biofilm development by crystal violet method with a slight modification of Abbas et.al. [11]. Aliquots containing 10 µl of each bacterial solution were roughly adjusted to an OD600 of 0.4 and combined with 1 ml of both drug-free and fresh TSB. 200 µl TSB aliquots, either drug-free or with medication, were added to the microtiter plate wells and incubated for the whole night at 37ºC. After the non-adhered planktonic cells were removed, the wells were air-dried and cleaned with phosphate buffer saline (PBS). Following a one-hour air drying period and a 25-min methanol fixation, the adhering cells were stained for fifteen minutes using 0.1% crystal violet. The wells underwent three PBS washes before being redissolved in either glacial acetic acid (33%), or 95% ethanol. Using a micro-titer reader, the OD absorbance at 590 nm analysis was determined three times. The biofilm inhibition percentage was determined according to the formula.
Swimming and Twitching Motilities Inhibition Assay
Modified from the technique of Abbas et al., the impact of amlodipine on twitching and swimming was observed[12].To conduct the swimming experiment, swimming agar plates containing 1% tryptone,0.3% agar, and 0.5% NaCl were produced, both with and without amlodipine. A 24-h incubation period at 37 °C was followed by the insertion of 5 μl into the middle of the agar plates after the preparation and dilution of an overnight culture of Pseudomonas spp. in tryptone broth. The zone of swimming was measured.
In order to perform the twitching experiment, 2 μl of the generated culture was stab-inoculated into 1% LB agar plates containing amlodipine and control plates. The plates were then incubated for 48 h at 37 °C. After air-drying, the plates were subjected to crystal violet staining following agar removal. Subsequently, the dried plates underwent a water wash to eliminate any residual dye. The measurement of twitching zones was then conducted.
Pyocyanin Inhibition Assay
Pyocyanin is one prominently discovered P. aeruginosa virulence factor. The MIC level of pyocyanin synthesis was measured both with and without amlodipine drug. The test was employed to evaluate the effects of amlodipine on P. aeruginosa that was treated with MIC and that was not. The synthesis of pyocyanin by Pseudomonas strains was measured as previously mentioned by Das and Manefield [13]. Specific controls pertaining to drug-treated samples were employed for individual strains. After correcting to an OD600 of 0.5, the strains were cultured for a night before being utilized in the experiment. In MIC, 1 ml of broth containing and excluding amlodipine was mixed with 10 µl aliquots of the bacterial suspensions. After being centrifuged for 10 min at 11,000 rpm, the tubes were incubated for 48 h at 37º C. Pyocyanin synthesis in the supernatant was estimated using a wavelength of 691 nm. The experiment was conducted thrice, and the extent of inhibition was assessed by comparing the drug's inhibitory impact on pyocyanin production with the respective strain-specific controls.
Oxidative Stress Sensitivity Test
The potential of amlodipine to reduce resistance to oxidative stress was assessed using the revised hydrogen peroxide disc test method developed by Hassett et.al. [14]. The diameters of the inhibition zones that developed around hydrogen peroxide-loaded discs on LB agar plates streaked with Pseudomonas species bacteria and with amlodipine drug were measured at its minimum inhibitory concentration (MIC). Pseudomonas cultures in LB were produced overnight, and aliquots of 0.1 ml were evenly distributed on the LB-agar plates surface that contained amlodipine. Sterile paper discs (6 mm) were placed on LB agar plates, and 10 μl of 1.5% H2O2 was administered onto the discs. The same procedures were used to create control plates devoid of amlodipine. Following a 24-h incubation period at 37 °C, the inhibitory zones were identified on the plates.
Rhamnolipid Assay
The rhamnolipid production was assessed using a colorimetric orcinol-sulfuric acid test procedure [15]. To put it briefly, 1 volume of supernatant and 9 volumes of recently made 0.19% orcinol in 53% sulfuric acid were combined. After 30 min of 80ºC incubation, 15 min of RT cooling, and an OD measurement at 421 nm wavelength, the sample was examined. A standard curve between rhamnose and OD421 with a range of 0–50 μg/ml was created using rhamnose. According to the OD value and the standard curve, the rhamnolipid concentration was determined to be three times the rhamnose concentration [16]. In a medium containing 2% glucose or higher as a supplement, the orcinol-sulfuric acid test will state the observed rhamnolipid concentration in the supernatant [17].
Assessment of Amlodipine's Impact on Bacterial Protein Permeability
The approach employed by Kamurai et al. [1] was utilized to determine the possible mode of action that amlodipine employs to inhibit bacterial growth, its effect on protein leakage was investigated. The cells were treated with a concentration of amlodipine at its MIC. To determine how much protein seeped out of bacterial cells after they were exposed to amlodipine, the Bradford technique was utilized. Cells suspended in a 0.9% saline solution at an optical density of 600 (OD600) equal to 1.5, were subjected to incubation at 37ºC with 120 rpm shaking following the addition of amlodipine at its Minimum Inhibitory Concentration (MIC) for 120 min. Afterward, the cell solution (500 μl) was centrifuged at 7000 rpm for two minutes. Protein quantification was executed using Bradford's method. In a brief procedure, 50 µl of the supernatant were mixed with 950 µl of Coomassie Brilliant Blue G-250. The absorbance at 590 nm was then measured with a spectrophotometer after a ten-minute color development period. Controls for comparison included untreated cells and 0.1% SDS. Bovine serum albumin (BSA) served as the standard for determining protein content.
Loss of 260 nm Absorbing Material
Cell lysis can be monitor by the release of UV-absorbing substances. The examination of cytoplasmic membrane leakage was made possible by monitoring the discharge of cellular constituents, including ions, metabolites, and nucleic acids, into the bacterial suspensions at 260 nm by UV spectrophotometer [18]. Pseudomonas spp. broth cultures in the tryptone-broth medium were left overnight and adjusted to OD600. The cells were collected by centrifuging them for 15 min at 400 rpm, discarding the supernatant, and then washing and resuspending the pellet in 1X 7.4 pH PBS (phosphate buffer saline). A MIC of amlodipine was added to the suspension of cells. The positive control was streptomycin (8 mg/ml). Three sets of experiments were conducted. As a control, drug-free cells were used. Every sample underwent a 60-min incubation period at 37 °C. Following treatment, the cell solution was centrifuged for 15 min at 13,400 rpm to extract the supernatant, and the OD 260 value was recorded to determine the proportion of extracellular UV-absorbing components that were released. Three duplicates of each measurement were taken.
Results
Analysis of MIC Values for the Bacterial Strains
The eight UTI clinical isolates of Pseudomonas that were used in this study were subjected to MIC testing using both the antibiotic (streptomycin) and nonantibiotic drug amlodipine (Table 1). Therapeutically significant concentrations of the non-antibiotic medication amlodipine were selected in the range of 3.12 to 200 µg/ml to assess the minimum inhibitory concentrations in order to assess the antimicrobial property of amlodipine against these microorganisms. The compound's compositions are displayed in Fig. 1. A part of the antibiotics was prepared in 10% DMSO and diluted to 0.05% DMSO for testing purposes. The positive control was streptomycin. Amlodipine's MIC ranged from 6.25 to 25 µg/ml. According to Table 1, of the eight test microorganisms, four strains (P. aeruginosa (PA41), P. stutzeri (BE4), P. fluorescence (BE6), and P. putida (Ppt) have demonstrated the MIC at 12.5 µg/ml; three strains (P. aeruginosa (PA9), P. aeruginosa (PA46), and P. stutzeri (BE5) have shown the MIC at 6.25 µg/ml, while P. aeruginosa (PA5) has demonstrated the highest MIC of 25 µg/ml among all.
Assay of Biofilm Inhibition
The measurement of biofilm inhibition demonstrated that the studied medication, amlodipine, could prevent biofilm development at MIC (Fig. 2). The significance of the mean difference between MIC values of amlodipine-treated and untreated Pseudomonas isolates in this triplicate study was determined using the one-way ANOVA. Statistical-significance was inferred for p-values below 0.05. The presented data in Table 2 represent the mean ± standard error, including the percent change from the untreated Pseudomonas control for each parameter. Maximum inhibition was seen in P. putida, P. fluorescens (BE6), and P. stutzeri, at 76.70%, 69.82%, and 57.33%, respectively, although P. aeruginosa showed less inhibition when compared to other strains.
Inhibition of Swimming and Twitching Motilities Assay
The dimensions of Pseudomonas spp. swimming and twitching zones on LB agar plates were assessed both in the presence and absence of amlodipine at its Minimum Inhibitory Concentration (MIC). The experiments were replicated three times for accuracy (Fig. 3). Amlodipine substantially decreased the swimming motilities of P. aeruginosa (PA41) to a maximum of 54.66% and a minimum of 41.90% (PA5). In contrast, to control strains, P. fluorescence (BE6) showed the highest level of twitching motility inhibition at 59.45%, while P. aeruginosa (PA5) showed the lowest level of inhibition at 48.57%.
Pyocyanin Inhibition Assay
One significant P. aeruginosa virulence component that has surfaced is pyocyanin. The MIC level of pyocyanin synthesis was measured both with and without amlodipine drug. The study was conducted thrice, and the one-way ANOVA test was employed to evaluate the effects of amlodipine on P. aeruginosa that was treated with MIC and that was not. The significance level of the data was set at p-values less than 0.05. The findings were displayed as mean standard error of percentage change from untreated P. aeruginosa controls (Fig. 4a). Amlodipine shown a notable capacity to diminish pyocyanin synthesis and effectively reduce pyocyanin pigment production. There was no phycocyanin production seen in P. fluorescens and P. stutzeri, whereas amlodipine showed significant inhibition of phycocyanin production in different strains of P. aeruginosa and P. putida. The percentages of inhibition of pyocyanin pigment in the presence of amlodipine were seen maximum in P. aeruginosa and P. putida, respectively, were 61.25% and 65.87%.
Sensitivity to Oxidative Stress
P. aeruginosa's capacity to survive and proliferate inside cells depends critically on its resistance to oxidative stress. Measurements were made of the widths of the inhibition zones that formed around hydrogen peroxide-loaded discs on LB agar plates that were streaked with bacterial culture of pseudomonas spp. and contained amlodipine drug at its MIC. Amlodipine significantly increased the widths of inhibitory zones in plates, indicating that these medications can reduce Pseudomonas spp. resistance to oxidative stress. However, there was no discernible effect seen in P. stutzeri and P. fluorescens with amlodipine, whereas a considerable effect was seen in Pseudomonas resistance to oxidative stress. The experiment was conducted in triplicate. The results showed the mean ± standard error of inhibitory zones in millimeters for both Pseudomonas controls treated with amlodipine drug and those who were not. (Fig. 5). Amlodipine considerably enhanced the widths of inhibitory zones in plates, indicating that these medications can lessen Pseudomonas spp. resistance to oxidative stress. However, there was no discernible effect was seen in P. stutzeri and P. fluorescens with amlodipine, whereas a considerable effect was seen in P. aeruginosa and P. putida with 64.70% and 54.94% inhibitory activity, respectively.
Assessment of the Impact of Amlodipine on Bacterial Protein Permeability
In order to ascertain the potential mechanism of action utilized by amlodipine to impede bacterial growth, its impact on protein leakage was examined. Amlodipine concentration at its MIC was applied to the cells. The Bradford method was used to calculate the protein amount that leaked out of bacterial cells following their exposure to amlodipine. It was demonstrated that when bacteria were treated with amlodipine, the released protein concentration was raised as compared to control, and there was an increase in protein leakage (Fig. 6a). As per the data obtained, the extracellular protein released in control of P. aeruginosa strains was in the range of 82.76 to 90.55 µg/ml; for P. stutzeri, it was in the range of 87.63 to 103.21 µg/ml, and 94.44 µg/ml, 105.16 µg/ml for P. fluorescens and P. Putida respectively. Whereas the amlodipine-treated cells of P. aeruginosa strains were in the range of 589.07 to 687.43 µg/ml, for P. stutzeri, it was in the range of 535.54 to 556.96 µg/ml, and 600.77 µg/ml, 421.61 µg/ml for P. fluorescens and P. putida respectively Data obtained from amlodipine treated cells demonstrated the increase in extracellular protein released by cells as compared to control that was a clear indication of membrane damage with the effect of the drug.
Rhamnolipid Assay
It is evident from data derived from control strains of Pseudomonas spp. that each strain has the production of rhamnolipids (Fig. 4b). Of all the strains utilized in this study, P. aeruginosa produced the highest amount of rhamnolipids, ranging from 22.9 to 25.4 mg/ml. This is quite similar to the previously published work of Chong et al. [19] and Soberón-Chávez et al. [20]. Rhamnolipid produced by control strains is in the range of 25.6 to 0.005 mg/ml, significant decrease in rhamnolipid production observed after amlodipine treatment was noted between 16.5 and 0.001 mg/ml, according to the data gathered which indicates that amlodipine has the great potential in inhibition of biosynthesis of rhamnolipid that leads to decrease in the virulence of bacterial strains.
Loss of 260 nm absorbing material-
According to Zhou et al. [21], the measurement of UV-absorbing material release serves as an indicator of cell lysi. Analyzing the release of cellular components, such as ions, metabolites, and nucleic acids, into the bacterial suspensions at 260 nm allowed for the analysis of cytoplasmic membrane leakage (Bajpai et al. [22]). As indicated by Table 3 and Fig. 6b, the OD dramatically increased to 0.861 from 0.00 (P value < 0.05) following treatment with amlodipine at MIC. It appears from these findings that amlodipine causes damage to the cytoplasmic membrane, which allows intracellular components to leak out.
Discussion
The main objective of this investigation was to determine which non-antimicrobial drugs would have had the most antibacterial effects against pathogenic Pseudomonas strains that cause urinary tract infections. With its intricate drug resistance mechanism, Pseudomonas is a frequent opportunistic disease found in hospitals that poses a major risk to public health. Therefore, a popular area of study right now is investigating novel anti-infective therapeutic approaches. It was observed in a previously published study that a class of cardiovascular medicines offers intriguing research about drug redirection. Amlodipine was found to have strong antibacterial action on bacteria [23, 24]. Since amlodipine is an antihypertensive medication, it works by blocking long-lasting L-type channels, which are responsible for allowing calcium ions to enter cardiac and vascular muscle cells. Its structure has an incomplete phenothiazine ring and a halogen (chlorine) attached, both of which are essential to the compound's antibacterial action [25].A study by Yan et al. concluded that CCB might be utilized to treat a bacterial infection and can limit the development of Pseudomonas spp. and the expression of its virulence factors. In this existing study, Amlodipine (AML) had MIC in the range of 6.12 μg/ml to 25 μg/ml and exhibited potent antimicrobial activity against all the pathogenic strains of Pseudomonas. The results of this study support the previously published findings that indicate amlodipine has good antibacterial activity [26]. It also appears to be the most potential dihydropyridine Ca2+ channel blocker, which is used to treat hypertension. Amlodipine also demonstrated a remarkable range of MIC90 values of 10–50 μg/ml in vitro against a variety of bacterial strains [24].There can be following reasons behind the mechanism of inhibiting the growth of bacteria by amlodipine. It can be done by Inducing iron starvation response by regulating the calprotectin and by using the CCB for iron channel protein PfeA that directly affects the quorum sensing mechanism and flagellated locomotion in Pseudomonas to exert an antibacterial effect [27]. It may function as antagonizing the Ca2+ by changing the structure of drug efflux membrane protein that includes (OprN, OprM, OprP, and OpdQ) which results in an increase in the time of the drug in bacteria hence providing antibacterial activity[28].This molecule is proposed to possess potential efficacy against bacteria, including Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Salmonella typhimurium, Bacillus cereus, Pseudomonas aeruginosa, and Acinetobacter baumannii [24, 28,29,30,31]. The factors that contribute to the virulence of Pseudomonas include quorum-sensing, biofilm, and flagella, which provide bacterial communications and drug resistance, as well as pilli, LPS, and other processes that help in bacterial adherence and colonization to the host. Moreover, effectors and poisons are introduced into the host by secretion systems [26, 32,33,34,35,36]. P. aeruginosa frequently produces a biofilm in order to initiate a persistent infection; this is related to extracellular polysaccharide and bacterial motility [37]. The creation of biofilms has the potential to multiply bacterial drug resistance hundreds of times [38]. P. aeruginosa's pathogenicity is largely dependent on the synthesis of virulence factors and the synthesis of biofilms [39]. Growth at high Ca2+ levels increases biofilm formation in Pseudomonas and triggers the manufacture of many secreted virulence components, including alginate, extracellular proteases, and pyocyanin, according to research done by Guragain et al. [40,41,42]. According to findings of present study, Amlodipine considerably reduced the biofilm production by Pseudomonas spp. This decrease in biofilm activities can be related to the effect of amlodipine, a calcium channel blocker, in decreasing the Ca2 + uptake, which would lead to pathogenicity and defective cell physiology as calcium is characterized as a regulator of biofilm formation [43], with similar effect of amlodipine was also reported in findings of Gupta et al. 2016 [4]. Another reason that can be considered is the inactivation of the EstA gene that affects cellular motility, which includes swimming, twitching motility, and swarming, as well as rhamnolipid deficiency that directly affects biofilm production. P. aeruginosa secretes a number of lipolytic enzymes, one of these, EstA, is an autoinducer protein found in the outer membrane [44]; it helps give bacteria their virulence function [45]. EstA is a potential enzyme for these kinds of downstream processes. Wilhelm et al.'s study shows that swimming, swarming, and twitching of P. aeruginosa EstA are necessary for biofilm formation in addition to rhamnolipid synthesis since the biofilm generated by the estA mutant differed from the wild-type biofilm. P. aeruginosa forms biofilms by a very complicated process in which the generation of rhamnolipids, feeding, quorum sensing, and cell motility all contribute to the development of mature biofilms [46,47,48,49]. Motility is associated with bacterial adhesion and the establishment of biofilm [50]. MIC amlodipine decreased both twitching and swimming motility in our investigation, which is in line with findings that salicylic acid [51] and azithromycin [52]. Amlodipine showed a significant motility inhibitory activity against all the strains in the range of 48.57 to 59.45% and 41.90 to 54.66% in twitching and swimming motility, respectively, and this may also relate to interference with impairment of bacterial adhesion and biofilm development, which ultimately leads to decrease in virulence.
Pyocyanin is characterized by pigment secreted by P. aeruginosa responsible for virulence and has the capacity to oxidize and decrease other molecules, therefore eliminating competing microorganisms and mammalian cells [53]. Through a type II secretion system, P. aeruginosa secretes PCN into the surrounding environment [54]. It is thought that PCN's zwitter ionic characteristics and low molecular weight make it easy for the toxin to pass across cell membranes [55,56,57]. Because of this, reporting on PCN levels is restricted to the compartments that are directly linked to infection. It has been demonstrated that PCN intercalates with DNA via an oxidative stress-dependent process. This facilitates cell-to-cell contacts between P. aeruginosa cells by modulating their physicochemical interactions and cell surface characteristics. Therefore, by encouraging eDNA, it has been proposed that PCN might help in the production of biofilms and related to virulence [13]. In order to augment the virulence of P. aeruginosa in the present study, amlodipine inhibited the pyocyanin production in the range of 49.7 to 61.25%, although P. aeruginosa strain (PA5) showed lesser inhibition of phycocyanin as compared to other P. aeruginosa strains (PA9, PA41, PA46, BE6) this might be because of more resistance of the strain which can be related to higher MIC and lesser biofilm inhibition. Results of current findings are near about similar to the study done by Kim et al. and Rasamiravaka et al. [58, 59], whereas a higher percentage (78%) of phycocyanin inhibition was seen with the effect of aspirin [42]. We have reported the lesser inhibition (33.33%) of phycocyanin at sub-MIC of paracetamol as compared to amlodipine used in the present study [58]. As per the previous research conducted, it was reported that a multitude of pathogens produces enzymes such as catalase and superoxide dismutase (SOD) to combat the lethal effects of reactive oxygen species. For the treatment of persistent P. aeruginosa infections, disruption of superoxide dismutase activity may be able to overcome multidrug resistance and strengthen the effects of existing bactericidal drugs [59]. In this investigation, the presence of amlodipine's minimum inhibitory concentration (MIC) (42.22–64.70%) increased the effect of H2O2 and decreased tolerance to oxidative stress.
Rhamnolipid is considered an important virulence determinant of Pseudomonas [60]. Moreover, it plays a variety of functions, including cell lysis [61], swarming motility [62], and biofilm formation [47]. It is also a heat-stable hemolysin [63]. As per our results, all strains showed production of rhamnolipid in the range of 0.005 to 25.4 mg/ml (control values), but it was seen after treatment with amlodipine, there was a drastic reduction (0.001 to 16.5 mg/ml) in rhamnolipid supported by the Fig. 4b, justifying the role of amlodipine in inhibition of rhamnolipid production which is ultimately related to decrease in other virulence factors of Pseudomonas like biofilm production. It indicates that amlodipine has some function in inhibition of biosynthesis of rhamnolipids and has shown a greater suppression of rhamnolipid synthesis. As per the data, rhamnolipid inhibition was observed in the range of 35 to 80% for all the strains, where maximum inhibition 80% was observed in P. putida and 35 to 46.72% was observed by P. aeruginosa which is higher as compared to sub-MIC of paracetamol reported by Saleemet al. and in range with the study done by Kim et al. on 6-gingerol [64, 65].
The current work used a protein leakage experiment to investigate the mechanism of action of amlodipine in inhibiting the growth of Pseudomonas spp. This was carried out in order to ascertain how amlodipine affected the bacterial membrane's integrity. One structural element that might be harmed by the antibacterial activity of amlodipine is the bacterial membrane. Bacteria classified as Gram-negative have an exterior membrane that protects their inner membrane or cell wall from antibiotics and drugs, which makes them more resistant. The cytoplasmic membrane serves as a diffusion barrier, and a compromise in its permeability leads to the leakage of proteins and nucleic acids. Targeting the membrane represents a critical strategy in the formulation of antimicrobial medications [66]. Therefore, pharmaceuticals having the capacity to disrupt membranes might be employed as antimicrobial drugs, to combat bacteria that cause chronic infections [67]. Amlodipine treatment releases protein out of bacterial cells, which is a sign of membrane damage. Based on the quantity of liberated cellular components, the extent of damage may be estimated [68]. As a sign of membrane integrity, the discharge of intracellular components. When tiny ions like potassium and phosphate are treated with the right antimicrobial agent, large molecules like DNA, RNA, and other materials seep out. The term "260-nm absorbing materials" refers to the substantial UV absorption of certain materials at 260 nm. This method is widely used to determine membrane integrity measures (Denyer et.al. 1990; Hugo and Snow, 1981). The results of this investigation suggest that amlodipine may influence Pseudomonas species by perforating the bacterial plasma membrane. The leakage of intracellular components supports this. Regarding NSAIDs, Ahmed et al. obtained similar findings [69].
Conclusion
Repurposing FDA-approved drugs is a viable approach to address the issue of rising anti-microbial resistance. In addition to demonstrating the possible suppression of virulence by the cardiovascular drug Amlodipine, our investigation clarified the substantial influence of this drug on biofilm formation, swarming motility, and several other virulence features shown in clinical isolates of Pseudomonas spp. Therefore, treating bacterial infections brought on by MDR strains of bacteria may benefit from the use of amlodipine in addition to antibiotics. It can be concluded that amlodipine has potential and can be further explored for its characteristics for UTI treatments.
Future Prospect
This study clearly demonstrates that the cardiovascular medication amlodipine has a promising antibacterial action, and it may be repurposed for antimicrobial therapy. Further research might support the hypothesis that amlodipine acts as an assistant compound by concentrating within the macrophage, which promotes intracellular killing and prevents mutation responses that lead to resistance because it may boost the antibacterial action. Research on the synergistic combination of amlodipine with other antibiotic and non-antibiotic drugs can greatly expand the scope of prolonged antibiotic therapy in bacterial infections, particularly for drug-resistant bacteria.
Availability of Data and Materials
Not applicable.
References
Kamurai B, Mombeshora M, Mukanganyama S, Khamesipour F (2020) Repurposing of Drugs for Antibacterial Activities on Selected ESKAPE Bacteria Staphylococcus aureus and Pseudomonas aeruginosa. Int J Microbiol 2020:1–9. https://doi.org/10.1155/2020/8885338
Foletto VS, da Rosa TF, Serafin MB et al (2021) Repositioning of non-antibiotic drugs as an alternative to microbial resistance: A systematic review. Int J Antimicrob Agents 58:106380
Bansal KK, Goyal R, Sharma A et al (2023) Repurposing of Drugs for the Treatment of Microbial Diseases. Drug Repurposing for Emerging Infectious Diseases and Cancer. Springer, Cham, pp 347–394
Gupta P, Chanda R, Rai N et al (2016) Antihypertensive, amlodipine besilate inhibits growth and biofilm of human fungal pathogen Candida. Assay Drug Dev Technol 14:291–297
Reimão JQ, Mesquita JT, Ferreira DD, Tempone AG (2016) Investigation of calcium channel blockers as antiprotozoal agents and their interference in the metabolism of Leishmania (L.) infantum. Evid-Based Complement Alternat Med. https://doi.org/10.1155/2016/1523691
Laudy AE, Kulińska E, Tyski S (2017) The impact of efflux pump inhibitors on the activity of selected non-antibiotic medicinal products against Gram-negative bacteria. Molecules 22:114
Coelho SS, da Rosa TF, Rampelotto RF et al (2021) Amlodipine repositioning: scientific studies and synergistic effects. Am J Ther 28:e772–e776
Hegazy WAH, Khayat MT, Ibrahim TS et al (2020) Repurposing anti-diabetic drugs to cripple quorum sensing in pseudomonas aeruginosa. Microorganisms 8:1285. https://doi.org/10.3390/microorganisms8091285
Lescat M, Poirel L, Tinguely C, Nordmann P (2019) A resazurin reduction-based assay for rapid detection of polymyxin resistance in acinetobacter baumannii and Pseudomonas aeruginosa. J Clin Microbiol. https://doi.org/10.1128/JCM.01563-18
Costa P, Gomes ATPC, Braz M et al (2021) Application of the resazurin cell viability assay to monitor Escherichia coli and Salmonella typhimurium inactivation mediated by phages. Antibiotics 10:974
Abbas HA, Hegazy WAH (2020) Repurposing anti-diabetic drug “Sitagliptin” as a novel virulence attenuating agent in Serratia marcescens. PLoS ONE 15:e0231625
Abbas HA, Elsherbini AM, Shaldam MA (2017) Repurposing metformin as a quorum sensing inhibitor in Pseudomonas aeruginosa. African Health Sci 17:808–819
Das T, Manefield M (2012) Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa
Hassett DJ, Schweizer HP, Ohman DE (1995) Pseudomonas aeruginosa sodA and sodB mutants defective in manganese-and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J Bacteriol 177:6330–6337
Irorere VU, Tripathi L, Marchant R et al (2017) Microbial rhamnolipid production: a critical re-evaluation of published data and suggested future publication criteria. Appl Microbiol Biotechnol 101:3941–3951
Abalos A, Pinazo A, Infante MR et al (2001) Physicochemical and Antimicrobial Properties of New Rhamnolipids Produced by Pseudomonas a eruginosa AT10 from soybean oil refinery wastes. Langmuir 17:1367–1371. https://doi.org/10.1021/la0011735
Cheng T, Liang J, He J et al (2017) A novel rhamnolipid-producing Pseudomonas aeruginosa ZS1 isolate derived from petroleum sludge suitable for bioremediation. AMB Express 7:120. https://doi.org/10.1186/s13568-017-0418-x
Devi KP, Nisha SA, Sakthivel R, Pandian SK (2010) Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J Ethnopharmacol 130:107–115
Chong H, Li Q (2017) Microbial production of rhamnolipids: opportunities, challenges and strategies. Microb Cell Fact 16:1–12
Soberón-Chávez G, González-Valdez A, Soto-Aceves MP, Cocotl-Yañez M (2021) Rhamnolipids produced by Pseudomonas: from molecular genetics to the market. Microb Biotechnol 14:136–146
Zhou K, Zhou W, Li P et al (2008) Mode of action of pentocin 31–1: An antilisteria bacteriocin produced by Lactobacillus pentosus from Chinese traditional ham. Food Control 19:817–822
Bajpai VK, Ajay Sharma AS, Baek KwangHyun BK (2014) Antibacterial mode of action of the essential oil obtained from Chamaecyparis obtusa sawdust on the membrane integrity of selected foodborne pathogens. Food Technol Biotechnol 52:109
Almeida HMD e S, Brandão LBS, de Melo TR, Ferreira SB (2022) Anti-Bacterial Perspective of Non-Antibiotic Drugs. In: Medical Sciences Forum. MDPI, p 22
Mazumdar K, Kumar KA, Dutta NK (2010) Potential role of the cardiovascular non-antibiotic (helper compound) amlodipine in the treatment of microbial infections: scope and hope for the future. Int J Antimicrob Agents 36:295–302
Kumar KA, Ganguly K, Mazumdar K et al (2003) Amlodipine: a cardiovascular drug with powerful antimicrobial property. Acta Microbiol Pol 52:285–292
Hauser AR (2009) The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol 7:654–665
Zygiel EM, Nelson CE, Brewer LK et al (2019) The human innate immune protein calprotectin induces iron starvation responses in Pseudomonas aeruginosa. J Biol Chem 294:3549–3562. https://doi.org/10.1074/jbc.RA118.006819
Yan Z, Xia L, Xu X et al (2023) Exploring calcium channel blocker as a candidate drug for Pseudomonas aeruginosa through network pharmacology and experimental validation. Chem Biol Drug Des 102:1353–1366
Kumar R, Kaur M, Bahia MS, Silakari O (2014) Synthesis, cytotoxic study and docking based multidrug resistance modulator potential analysis of 2-(9-oxoacridin-10 (9H)-yl)-N-phenyl acetamides. Eur J Med Chem 80:83–91
Hu C, Li Y, Zhao Z et al (2018) In vitro synergistic effect of amlodipine and imipenem on the expression of the AdeABC efflux pump in multidrug-resistant Acinetobacter baumannii. PLoS ONE 13:e0198061
Elkhatib WF, Haynes VL, Noreddin AM (2009) Microbiological appraisal of levofloxacin activity against Pseudomonas aeruginosa biofilm in combination with different calcium channel blockers in vitro. J Chemother 21:135–143
Veesenmeyer JL, Hauser AR, Lisboa T, Rello J (2009) Pseudomonas aeruginosa virulence and therapy: evolving translational strategies. Crit Care Med 37:1777
Poole K (2011) Pseudomonas aeruginosa: resistance to the max. Front Microbiol 2:65
Gellatly SL, Hancock REW (2013) Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 67:159–173
Newman JW, Floyd RV, Fothergill JL (2017) The contribution of Pseudomonas aeruginosa virulence factors and host factors in the establishment of urinary tract infections. FEMS Microbiol Lett 364:fnx124
Liao C, Huang X, Wang Q et al (2022) Virulence factors of Pseudomonas aeruginosa and antivirulence strategies to combat its drug resistance. Front Cell Infect Microbiol 12:926758
Moradali MF, Ghods S, Rehm BHA (2017) Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol 7:39
Hall CW, Mah T-F (2017) Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev 41:276–301
Wang S, Feng Y, Han X et al (2021) Inhibition of virulence factors and biofilm formation by wogonin attenuates pathogenicity of Pseudomonas aeruginosa PAO1 via targeting pqs quorum-sensing system. Int J Mol Sci 22:12699
Sarkisova S, Patrauchan MA, Berglund D et al (2005) Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa biofilms. J Bacteriol 187:4327–4337
Patrauchan MA, Sarkisova SA, Franklin MJ (2007) Strain-specific proteome responses of Pseudomonas aeruginosa to biofilm-associated growth and to calcium. Microbiology (N Y) 153:3838–3851
Guragain M, Lenaburg DL, Moore FS et al (2013) Calcium homeostasis in Pseudomonas aeruginosa requires multiple transporters and modulates swarming motility. Cell Calcium 54:350–361
Kolodkin-Gal I, Parsek MR, Patrauchan MA (2023) The roles of calcium signaling and calcium deposition in microbial multicellularity. Trends Microbiol. https://doi.org/10.1016/j.tim.2023.06.005
Wilhelm S, Tommassen J, Jaeger K-E (1999) A novel lipolytic enzyme located in the outer membrane of Pseudomonas aeruginosa. J Bacteriol 181:6977–6986
Henderson IR, Nataro JP (2001) Virulence functions of autotransporter proteins. Infect Immun 69:1231–1243
Wilhelm S, Gdynia A, Tielen P et al (2007) The autotransporter esterase EstA of Pseudomonas aeruginosa is required for rhamnolipid production, cell motility, and biofilm formation. J Bacteriol 189:6695–6703
Davey ME, Caiazza NC, O’Toole GA (2003) Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J Bacteriol 185:1027–1036
Klausen M, Aaes-Jørgensen A, Molin S, Tolker-Nielsen T (2003) Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol Microbiol 50:61–68
Klausen M, Heydorn A, Ragas P et al (2003) Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol 48:1511–1524
Arora SK, Neely AN, Blair B et al (2005) Role of motility and flagellin glycosylation in the pathogenesis of Pseudomonas aeruginosa burn wound infections. Infect Immun 73:4395–4398
Guo M, Gamby S, Zheng Y, Sintim H (2013) Small molecule inhibitors of AI-2 signaling in bacteria: state-of-the-art and future perspectives for anti-quorum sensing agents. Int J Mol Sci 14:17694–17728. https://doi.org/10.3390/ijms140917694
Nalca Y, Jänsch L, Bredenbruch F et al (2006) Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrob Agents Chemother 50:1680–1688
Mavrodi DV, Bonsall RF, Delaney SM et al (2001) Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol 183:6454–6465
Hall S, McDermott C, Anoopkumar-Dukie S et al (2016) Cellular effects of pyocyanin, a secreted virulence factor of Pseudomonas aeruginosa. Toxins (Basel) 8:236
Turner JM, Messenger AJ (1986) Occurrence, biochemistry and physiology of phenazine pigment production. Adv Microb Physiol 27:211–275
Lau GW, Hassett DJ, Ran H, Kong F (2004) The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med 10:599–606
Baron SS, Rowe JJ (1981) Antibiotic action of pyocyanin. Antimicrob Agents Chemother 20:814–820
El-Mowafy SA, Abd El Galil KH, El-Messery SM, Shaaban MI (2014) Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb Pathog 74:25–32. https://doi.org/10.1016/j.micpath.2014.07.008
Martins D, McKay G, Sampathkumar G et al (2018) Superoxide dismutase activity confers (p) ppGpp-mediated antibiotic tolerance to stationary-phase Pseudomonas aeruginosa. Proc Natl Acad Sci 115:9797–9802
Reis RS, Pereira AG, Neves BC, Freire DMG (2011) Gene regulation of rhamnolipid production in Pseudomonas aeruginosa–a review. Bioresour Technol 102:6377–6384
Jensen PØ, Bjarnsholt T, Phipps R et al (2007) Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology (N Y) 153:1329–1338
Deziel E, Lepine F, Milot S, Villemur R (2003) rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3-(3-hydroxyalkanoyloxy) alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiology (N Y) 149:2005–2013
Abdel-Mawgoud AM, Lépine F, Déziel E (2010) Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol 86:1323–1336
Seleem NM, Atallah H, Abd El Latif HK et al (2021) Could the analgesic drugs, paracetamol and indomethacin, function as quorum sensing inhibitors? Microb Pathog 158:105097
Kim H-S, Lee S-H, Byun Y, Park H-D (2015) 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci Rep 5:8656
Epand RM, Walker C, Epand RF, Magarvey NA (2016) Molecular mechanisms of membrane targeting antibiotics. Biochimica et Biophysica Acta (BBA) Biomembranes 1858:980–987
Mun S-H, Kim S-B, Kong R et al (2014) Curcumin reverse methicillin resistance in Staphylococcus aureus. Molecules 19:18283–18295
Wu Y, Bai J, Zhong K et al (2016) Antibacterial activity and membrane-disruptive mechanism of 3-p-trans-coumaroyl-2-hydroxyquinic acid, a novel phenolic compound from pine needles of Cedrus deodara, against Staphylococcus aureus. Molecules 21:1084
Ahmed EF, El-Baky RMA, Ahmed ABF et al (2017) Antibacterial activity of some non-steroidal anti-inflammatory drugs against bacteria causing urinary tract infection. Am J Infect Dis Microbiol 5:66–73
Acknowledgements
The authors would like to acknowledge the University of Rajasthan and Dr. B. Lal Institute of Biotechnology for providing technical and lab support. ADT gratefully acknowledge DBT, India for the fellowship support (DBT award no. DBT/JRF/BET-19/I/2019/AL/140). VKC gratefully acknowledges to DHR-MoHFW, Govt of India for support through Young Scientist fellowship Grant R.12014/56/2022-HR.
Funding
Not available.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study. PS: writing original draft, data collection, and literature search; AK: data collection, literature search, editing. ADT and VKC: editing and revision. BC: concept idea, data analysis, and revision. The final paper was reviewed and approved by all contributors.
Corresponding author
Ethics declarations
Conflict of interests
No financial or non-financial interests are reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, P., Kalra, A., Tripathi, A.D. et al. Antimicrobial Proficiency of Amlodipine: Investigating its Impact on Pseudomonas spp. in Urinary Tract Infections. Indian J Microbiol (2024). https://doi.org/10.1007/s12088-024-01280-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12088-024-01280-z