Abstract
Biosurfactants constitute amphiphilic molecules, receiving increased attention as environmentally benign, biodegradable alternatives to substitute for the petroleum derived counterparts in food, pharmaceutical and cosmetics applications. However, their high production cost hinders industrial production. In this study, fifty GRAS lactobacilli strains were screened for their ability to produce biosurfactants, implementing different substrates. Cheese whey permeate (CWP) was also assessed as a low-cost and inherent lactobacilli substrate, aiming to mitigate its polluting impact, expand valorization strategies, alleviate costs deriving from commercial supplements and enhance overall sustainability. Surface tension, emulsification activity (E24) and oil displacement were deployed to identify the most promising candidates. Results reveal surface tension as the most robust method and underline the effect of substrate on biosurfactant synthesis. Likewise, this study indicates the fundamental role of including the final fermentation substrate (CWP) during strain selection to avoid misinterpretation of results and enhance subsequent bioprocess integration.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biosurfactants are characterised as amphiphilic (or amphipathic) molecules, containing a hydrophobic (e.g. long-chain fatty acid, hydroxyl fatty acid) and a hydrophilic moiety (e.g. a carbohydrate, an amino acid, peptides, phosphate, alcohol) [1]. The significance of biosurfactants ascribes on the emulsification properties, the ability to reduce surface tension in the mixture of two liquids (e.g. oil in water) along with wetting and foaming abilities [2, 3]. Biosurfactants are obtained by microbial entities, mainly yeast, fungi and bacteria, thereby being biodegradable and conforming with the principles of green chemistry, making them environmentally benign alternatives to substitute for the petroleum derived formulated counterparts [4]. Several reviews have previously described the applications and future prospects of microbially obtained surfactants, highlighting the great potential of these compounds on food and agricultural sector along with biomedical and pharmaceutical applications [1, 4, 5].
Regardless the several advantages and the numerous applications, industrial scale-up of biosurfactants is hindered by the high total cost of production, deriving primarily from the cost of raw materials and the downstream processing [6,7,8,9]. To overcome the latter impediments and render the large scale production of biosurfactants economically feasible, several strategies have been undertaken that exhibit diversified targets. For instance, one approach entails the evaluation of various agro-industrial waste as fermentation supplements to formulate biosurfactants. Cheese whey exhibits a dairy by-product stream often employed in fermentation processes that will target value added products for food applications [10]. Recently, several bioprocessing strategies targeting the holistic valorization of cheese whey through consolidated biorefining with specific focus on food applications were demonstrated [11]. On the other hand, recovery and purification of biosurfactants merit equally significant research attention, to avoid the use of organic solvents often required for biosurfactants extraction and purification [2, 12]. The isolation and screening of new strains along with the implementation of genetic and metabolic engineering to develop overproducing strains constitute alternative concepts that could mediate the high production cost [12,13,14].
Within the concept of identifying new isolates, the initial step comprises the combination of screening techniques, either quantitative or qualitative, to assess the ability to produce biosurfactants [13]. These include the haemolytic assay (blood agar test), the N-Cetyl-N,N,N-trimethylammoniumn bromide (CTAB) agar plate method, drop collapse test, the oil displacement test, the emulsification activity and surface tension measurements [15]. However, previous studies have evidenced that the comparison of the results obtained from independent screening tests might exhibit inconsistencies, thereby leading to false estimations on biosurfactants producing strains [16, 17]. On top of that, the majority of studies in the open literature perform the screening experiments using conventional supplements and most frequently De Man, Rogosa and Sharpe (MRS) in the case lactobacilli are employed.
Lactobacilli strains are Generally Recognised As Safe (GRAS) strains, thereby were selected in this study to facilitate and expand future applications, particularly to include also the food sector, in contrast to the biosurfactants produced by pathogenic strains, e.g. Pseudomonas sp. [18,19,20]. Cheese whey was included as a low-cost fermentation feedstock not only to alleviate cost impairments of conventional supplements but also to mediate the environmental burden deriving from this polluting stream. Furthermore, cheese whey elicits a native substrate to lactic acid bacteria, thereby fostering the development of process integration that could facilitate closing the loop of food waste valorization schemes towards zero waste processes. The identification of robust lactobacilli strains producing biosurfactants constitutes the principal steps of a project encompassing a holistic implementation of cheese whey as a low-cost onset feedstock to generate high added value products that could applied de novo in food formulations.
Hence, the aim of this study was the deployment and the interpretation of several methods of the open literature to evaluate lactobacilli strains as potential biosurfactant producers. To the best of our knowledge, this is one of the few studies to correlate and evaluate different screening methods in such a large number of lactobacilli strains using both conventional media and cheese whey as a low-cost fermentation medium. Surface tension, emulsification activity and oil displacement were employed to identify the most promising candidates. Worth noting, our results elucidate that it is fundamental to conduct the strain selection on the final fermentation substrate as well (e.g. cheese whey permeate), to avoid misinterpretation of results, a critical cornerstone for subsequent process optimization, thereby highlighting the novelty of this study.
Materials and Methods
Microorganisms and Culture Conditions
Numerous lactobacilli strains (Table 1) were assessed in the screening experiments. The strains were selected from four different culture collections, namely, Spanish Type Culture Collection, Universitat de Valencia, Spain (CECT), Culture Collection of the Laboratory of Dairy Research, Agricultural University of Athens, Greece (ACA-DC), Culture Collection of the Laboratory of Food Quality Control and Hygiene, Agricultural University of Athens, Greece (LQC) and Culture Collection of the Laboratory of Food Microbiology and Biotechnology, Agricultural University of Athens, Greece (FMCC). Strains were stored at − 80 °C in previously autoclaved (121 °C, 20 min) De Man, Rogosa and Sharpe (MRS) broth (Difco™, USA) supplemented with autoclaved glycerol (1:1, v/v). Prior to each use, strains were streaked on MRS agar plates and incubated overnight in the designated temperature for each strain (30 °C for L. pentosus CECT 4023 and 37 °C for all the remaining strains). A loopful of bacteria was inoculated in screw capped glass tubes containing 5 mL of sterile MRS broth and used as pre-culture. Bacterial stock solutions were kept at 4 °C and resuscitated each week.
For the screening experiments, three different media, namely, MRS broth, Medium 2 (M2) and cheese whey permeate (CWP) were employed. For the preparation of modified MRS, 20 g L−1 lactose was used instead of glucose. Medium 2 (M2) contained the following: glucose (20 g L−1); yeast extract (8 g L−1); (NH4)2SO4 (5 g L−1); K2HPO4 (1 g L−1); MgSO4 7H2O (0.3 g L−1); C2H3NaO2 (5 g L−1). The strains were inoculated in glass tubes (1500 mm × 15 mm) and incubated for 72 h at the designated temperature. In the first phase, fermentation time was chosen following previous studies of the literature where it was observed that biosurfactant (BS) production occurs at the stationary phase. At the end of the fermentation, samples were collected and centrifuged at 10,394 g for 10 min (NÜVE, Turkey). The supernatants were stored at − 20 °C prior to be utilized in screening tests. During this first study, only the extracellular biosurfactant production was taken into consideration as the main target was to identify the candidate strains for subsequent experiments along with evaluating and developing several screening methods of the literature. Subsequently, in the second phase, fermentations were conducted in 120 mL final volume, in Erlenmeyer flask (250 mL), using M2 medium and CWP and samples were withdrawn both at 24 and 72 h. For this case, both cell-bound and extracellular biosurfactant production were evaluated. Samples were collected as previously in the case of supernatants whereas bacterial cell mass was further treated to extract cell-bound biosurfactants. All chemicals and reagents were purchased from Sigma-Aldrich (USA), except for glucose (PENTA, Czech Republic) and yeast extract (Conda Laboratories, Spain).
Cheese Whey Permeate Preparation
Cheese whey, kindly provided by “Galiatsatos” dairy company (Kefalonia, Greece), was implemented as a low-cost substrate in bioconversion experiments for biosurfactant production. Prior to the experiments, cheese whey was de-proteinised following a previously described method [21] to obtain cheese whey permeate (CWP), as supernatant. CWP was diluted to obtain an initial lactose concentration of approximately 20 g L−1. The pH value was adjusted to 6.8 and autoclaved prior to fermentation (121 °C, 15 min). During this study, lactose and pH values were set to simulate the composition of MRS broth (pH:6.8).
Haemolytic Activity
The haemolytic activity was applied as a preliminary tool for biosurfactant production using the blood agar test [17, 22]. Briefly, 40 μL of sample, deriving from 72 h incubation in MRS broth, were spotted on Columbia sheep blood agar plates (5% w/v) and incubated for 48 h at 30 °C and 37 °C, depending on the lactobacilli strain. Plates were inspected after 48 h of incubation and the test was deemed positive when a halo was formed around the spot. Tests were carried out in duplicate for each strain.
Oil Displacement Test
The oil displacement test was performed by carefully dropping 0.1 mL of motor oil (type 20 W-50) on top of 50 mL of deionised water in a glass Petri dish (15 cm in diameter) to form a thin layer [15, 22]. Subsequently, 10 μL of each sample were placed on top of the oil layer and the diameter of the clearing zone was measured. MRS, M2 and CWP were employed as fermentation substrates to evaluate oil displacement whereas each supplement was also used as negative control. Oil displacement tests were performed in triplicates (n = 3) and the results represent the mean values.
Emulsification Index (E 24 )
The emulsification index was employed following methods previously reported [22, 23] (Fig. 1). During this study, different types of oils (namely 20 W-50, soybean oil, olive oil and paraffin oil) were assessed to develop the most suitable method to estimate the emulsification activity. In all cases, 2 mL of cell-free sample were mixed with 2 mL of oil in screw capped tubes, followed by vigorous vortex in high speed for 1 min. Tubes were placed under static conditions in dark environment and the emulsification index (E24) was calculated using the following formula:
where Hemulsion corresponds to the height of the emulsified layer (in mm) and Htotal height of the liquid column represents the total height of the emulsified layer and the oil (in mm) [24]. The emulsification activity was measured after 24, 48 and 72 h to also evaluate the stability of the emulsified layer (E24, E48 and E72 respectively).
Surface Tension Measurement
Whole cell cultures, cell free supernatants and extracted crude biosurfactants obtained after cultivation of lactobacilli strains on MRS broth, M2 medium and CWP, were employed to measure the surface activity and evaluate the production of biosurfactants. Surface tension was estimated via the Wilhelmy plate method, at 25 °C, using a tensiometer (K20 Krüss tensiometer, KRÜSS GmbH, Germany) equipped with a 1.9 cm platinum plate (wetted length: 40.20 mm) [25]. The tensiometer was calibrated with distilled water and the production of biosurfactants was determined based on the decrease of surface tension compared to the control sample (distilled water and phosphate saline buffer, PBS). A reduction of more than 8 mN/m was considered the positive threshold to characterise the strain as biosurfactant producer [26]. All measurements were conducted in triplicate (n = 3) and the results represent the mean values.
Extraction of Biosurfactants
Extraction of cell-bound biosurfactants, from the experiments carried out on M2 medium and CWP, was conducted using a modified method previously reported [6, 27]. Briefly, at the end of fermentation, samples were centrifuged to recover the cells, washed twice with distilled water and resuspended in phosphate buffer saline (PBS, pH 7.0) (6:1 fermentation broth:PBS). The solutions were placed under gentle stirring in demineralised water at 4 °C overnight to allow release of biosurfactants. The suspension was centrifuged to remove bacteria cells; the supernatant was collected and filtered (0.22 μm pore-size filter, Millipore) and further dialyzed with membranes (Cellu-Sep® molecular weight cutoff 6–8 kDa) prior to surface tension measurements.
Analytical Methods
Lactose was determined using High Performance Liquid Chromatography analysis (HPLC, Agilent), equipped with a ROA-organic acid H + (300 mm × 7.8 mm, Phenomenex) column coupled to a differential refractometer (RID). Operating conditions were as follows: sample volume 10 μL; mobile phase 0.010 mM H2SO4; flow rate 0.6 mL min−1; column temperature 65 °C. Samples were diluted and filtered (Whatman®, 0.2 μm) prior to analysis. Total dry weight (TDW, g L−1) was determined gravimetrically by drying the cell pellet after centrifugation at 65 °C until a constant weight was obtained.
Statistical Analysis
Statistical analysis was performed using Microsoft Excel 2018, and values are presented as average ± standard deviation. Statistical correlations of the screening methods were carried out by calculating Pearson’s correlation coefficient and Spearman rank correlation coefficient, rs ranging from − 1 (strong negative correlation) to 1 (strong positive correlation). Moreover, Student’s t test was performed to evaluate significant differences (significance level α = 0.05) between the surface tension values obtained at the same timepoints for different fermentation substrates but also between different timepoints within the same substrate.
Results and Discussion
Results of Haemolytic Activity Assay
The majority of the lactobacilli strains presented a greenish halo during the blood agar test as can be seen in Table 2. The appearance of green coloured zones is related to the production of biosurfactants since lysis of erythrocytes is induced. However, non-conclusive results could be obtained due to the low specificity of this method [15, 16]. Moreover, during the experimental setup, crude biosurfactants were assessed; thus, other virulence compounds (e.g. hydrogen peroxide) could lead to blood cell lysis [24]. Another reason associates with the low diffusion of the biosurfactant on the agar medium, rendering it not adequate to lyse erythrocytes, thereby impairing on biosurfactant detection [16]. Regardless of the drawbacks, the blood agar test constitutes a rapid experimental test, still employed in the majority of the studies dealing with isolation and screening of biosurfactant producing strains. It is crucial however to be combined with more accurate methods to convey reliable results, particularly in the case that biosurfactants would be introduced in food applications. For this reason, the blood agar test was not further implemented in the following experiments.
Evaluation of Oil Displacement Test and Surface Tension on MRS
Oil displacement test was applied in all lactobacilli strains after incubation in MRS medium for 72 h. A solution of 0.5% (w/v) Triton X-100 was used as a positive control, whereas sterile MRS was included as the negative control. Oil displacement was measured after applying the sample on top of the oil layer and the obtained value was subtracted from the corresponding value of the negative control, so that comparison was conducted using net values (Δd, cm), and the results are presented in Table 2. It can be easily observed that all strains demonstrated a positive response, apart from the strains CECT 903, LQC 780, LQC 824, LQC 2030, LQC 2014.
In an effort to establish a threshold value to be included as a selection criterion, the size of the droplet was considered positive when Δd > 1 cm, following previous studies. The majority of the strains illustrating a positive response belongs to the species L. plantarum and L. rhamnosus. Similar to the haemolytic activity, oil displacement test indicates an indirect, yet quick and easy method to assess biosurfactant production, but should be also associated with other methods [28].
The first phase of screening experiments included subsequently the measurement of surface tension for all lactobacilli strains using MRS broth and the results are presented in Table 2. In this case, only the supernatants were employed and distilled water (71.6 ± 0.04 mN/m) was used as the control value, whereas MRS was found equal to 40.8 ± 0.09 mN/m. It should be noted that the composition of MRS includes sorbitan mono-oleate that acts as an emulsifier, thus potentially impedes with surface tension measurements, when extracellular biosurfactants were considered. In any case, Rodrigues et al. [17] had also measured surface tension at the supernatants of L. casei, L rhamnosus, L. pentosus and L. coryniformis after fermentation on MRS broth and the results obtained are in accordance with the values of the present study (Table 2). Thereby, for the second phase of screening experiments, another nutrient supplement was selected, namely, M2 medium, prior to the utilisation of cheese whey permeate, and the results will be presented in a following section.
Correlation of Emulsification Activity (E24) and Biosurfactant Production
Emulsification activity or emulsification index has been widely applied in the literature to estimate biosurfactant production. In most cases, measurements are taken after 24 h; thus, emulsification activity is often denoted as E24. Several methods include the utilisation of different types of oil in the experimental setup [29, 30]. Thereby to develop a suitable method, first we employed the evaluation of several oils, particularly 20 W-50, olive oil, soybean oil and paraffin oil, along with different times of vigorous mixing via vortex (e.g. 10 s, 1 and 2 min), whereas in this case, both whole cell cultures and the supernatants were tested (data not shown). The application of whole cell cultures entailed the formation of an obscure-cloudy layer that did not enable clear measurements of the emulsified layer. Therefore, in the following experiments, only the supernatant was employed, whereas soybean and paraffin oil proved to perform better thus were included for further experiments, with 1 min of vortexing. Initially, incubations were conducted using MRS broth and the E24 values were measured after 24, 48 and 72 h (data not shown). Similarly, the composition of MRS seemed to interfere with precise measurement of emulsification activity. Thus, M2 media was used in subsequent experiments (72 h of incubation) to overcome the interference deriving from MRS and establish the method setup. Figure 2 illustrates the results of the E24 (%) from all lactobacilli strains both in soybean and paraffin oil in comparison. Apparently, in all cases examined, high emulsification activity was observed and more specifically E24 was higher than 30% for all strains, rendering it difficult to exclude some strains from our further investigation. For this reason, the next set of experiments was conducted with paraffin oil and M2 media (Fig. 2). Evidently, it can be noted that the application of paraffin oil demonstrated more conclusive observations with respect to E24, regardless the lower values generally achieved compared to soybean oil. Therefore the establishment of a threshold value within the context of outlining important biosurfactant producers was allowed.
Maximum values were attained for the strains of ACA-DC culture collection, namely, ACA-DC 179 and ACA-DC 183 (L. fermentum), ACA-DC 270 and ACA-DC 287 (L. plantarum). Also, notable E24 values were demonstrated by the LQC strains 752 (L. coryniformis) 780 (L. sakei) and 842 (L. plantarum). On the other hand, the strains LQC 763, 841 and 855, all belonging to L. casei group and also ACA-DC 201 (L. plantarum) that exhibited the lowest values in E24 test, had previously presented positive response to haemolytic activity and oil displacement tests.
In a similar manner, Youssef et al. [16] studied the ability of 205 strains to produce biosurfactants via drop collapse assay, oil spreading and blood agar lysis. The results indicated that strains not lysing blood agar were found to be positive in the oil spreading method, whereas strains that fulfilled the criteria of blood agar lysis gave negative results in oil displacement. The ability of Ochrobactrum anthropi HM-1 and Citrobacter freundii HM-2 to produce biosurfactants using several screening tests, including blood agar lysis, oil displacement and emulsification activity, was also studied [30]. Among the results, it was stated that the produced biosurfactants could emulsify engine oil, diesel oil, crude oil, sunflower oil, olive oil but no emulsification activity was achieved on hexadecane and kerosene. Similarly, Asfhar et al. [31], showed that 31% of the screened strains (32 strains in total) exhibited emulsification properties on n-hexane, whereby 94% of them resulted in a positive response when crude oil was used. Recently, several hydrophobic substrates were evaluated on the emulsification activity of biosurfactants deriving from two lactobacilli strains [29], and diversified responses were attained depending on the strain examined and the substrate used. Even though there is not a molecular mechanistic study until date, it could be speculated that the differences obtained during emulsification activity could be attributed on the different composition of the substrates and the specificity of the produced biosurfactant structures. For instance, soybean oil is mainly composed of neutral lipids, i.e. triglycerides, free sterols and fatty acids, whereas paraffin oil is a mixture of hydrocarbons, consisting of high molecular weight alkanes.
Evaluation of Surface Tension Measurements in Different Timepoints on M2 and CWP
As previously stated, the majority of studies in the open literature dealing with the screening of potential biosurfactant producers often withdrew samples after 72 h of incubation. This is probably based on the fact that LAB biosurfactant production was first reported to occur in the stationary phase [32]. This commonly applied practice was also employed in the current study in the first phase of experimental setup. Nonetheless, other studies have evidenced that biosurfactant production is growth associated, contingent with the strain employed [17, 33]. For this reason, the second phase of the analysis of the screening methods included the use of M2 medium and CWP whereas an additional sample was withdrawn and analysed at 24 h.
Surface tension was measured for both cell-bound and extracellular biosurfactants and the results obtained after 24 h are presented in Table 3. For extracellular biosurfactants, M2 (49.0 ± 0.01) and CWP (45.5 ± 0.01) were used as negative controls, whereas the obtained values for cell-bound biosurfactants were compared to PBS (72.1 ± 0.01). The lowest value in the case of M2 medium was obtained for FMCC E10 (L. plantarum) followed by ACA-DC 0125 (L. delbrueckii) and ACA-DC 0201 (L. plantarum), corresponding to a surface tension reduction of approximately 30 mN/m. On the other hand, when CWP was used, the higher surface tension reduction was obtained with the strains CECT 4023 (L. pentosus), ACA-DC 0280 (L. plantarum) and ACA-DC 0713 (L. rhamnosus). In fact, these observations indicated the substrate-strain specificity to induce biosurfactant synthesis. When extracellular biosurfactants were considered, lower values were accomplished, yet the control solutions already demonstrated low surface tension probably related to nitrogen content. Moreover, as previous studies have stated the LAB biosurfactants are primarily cell-bound, eventually focus was directed to assess the results deriving from PBS extracts.
Figure 2a and b illustrate the surface tension values observed after 24 and 72 h of incubation on commercial M2 medium, whereas 2c and 2d correspond to CWP. It can be easily observed that several strains perform contrastively depending on the fermentation substrate. For instance, strains FMCC E10, CECT 903, ACA-DC 0125 and ACA-DC 0183 were favoured by glucose in M2 medium (p < 0.05), whereas the majority of LQC strains performed better on lactose, specifically after 72 h of fermentation (p < 0.05).
Apparently, no clear pattern was attained neither for M2 or CWP to determine whether biosurfactant production occurs in the exponential or in the stationary phase. As a matter of fact, when CWP was used, 34% of the examined strains displayed higher surface tension reduction after 24 h, whereas for 19 of the 50 strains, it could be speculated that biosurfactant production occurs in the stationary phase.
The criterion to characterise a strain as biosurfactant producer is to achieve a reduction higher than 8 mN/m compared to that of distilled water or PBS [26]. However, several research studies employing lactobacilli or even other types of microbial entities have reported significantly higher values for surface tension reduction. In order to identify robust strains for our future study, the threshold value to include or exclude biosurfactant producers was set at 20 mN/m. In particular, in the case of M2 medium, eleven strains reduced the surface tension more than 20 mN/m, whereas when CWP was used, twenty strains were identified as potential biosurfactant producers, requiring further optimization studies. In particular, the strains L. rhamnosus ACA-DC 270, CECT 278 and LQC 2014, L. pentosus CECT 4023, L. coryniformis LQC 752 and LQC 753, L. sakei LQC 780 and L. fermentum ACA-DC 183 were among the strains displaying the higher values of surface reduction, either after 24 or 72 h of fermentation. The correlation of high surface tension reduction did not prove to coincide with emulsification activity or positive results in the oil displacement test. For instance, the strains CECT 278 and LQC 761 displayed high oil displacement activity at 24 h, where maximum surface tension was achieved, but E24 (%) was higher after 72 h. On the other hand, LQC 2014 indicated high E24 (48%) after 72 h parallel to high surface tension reduction.
In an attempt to better describe these results, the screening methods were correlated via Pearson and Spearman correlation coefficients, using the results obtained for both fermentation timepoints. More specifically, each method was compared with the other within the same timepoint, for the same substrate, separately for cell-bound or extracellular biosurfactant production. For instance, when glucose was used, a weak positive coefficient was achieved (rs, 0.25–0.28) among surface tension and E24 (%) for cell-bound and extracellular biosurfactants after 24 h.
On the other hand, when CWP was used as fermentation feedstock, in all the examined cases, a negative correlation was observed for surface tension measurements and oil spreading method, contrary to preceding studies that reported a positive correlation of surface tension and oil spreading method [16, 31]. A similar pattern was observed for surface tension and emulsification index when cell-bound biosurfactants were considered, coinciding with previous reports [13], whereas in the case of extracellularly produced biosurfactants, a weak positive correlation was obtained. A positive Pearson coefficient was obtained correlating surface tension in the supernatants with lactose consumption in the case of extracellular biosurfactant production.
Overall, the statistical analysis strongly indicates that each method and technique employed during the screening process should be carefully examined in strain-specific level to achieve the optimum interpretation of the results and carefully select the most promising biosurfactant producing strains. Thus, since surface tension elicits the most robust method, it is suggested that the secondary screening method should precede from a correlation between the different methods in strain-specific level.
Substrate Effect on Screening Process
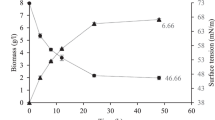
Evidently, the ultimate target of this study was to identify the most promising lactobacilli strains that will be further studied in bioreactor fermentations to enhance biosurfactant production via the implementation of several bioprocessing strategies. Hence, lactose consumption in the case of CWP was included in the selection criteria, to utilize CWP as the onset feedstock. Figure 3a–d illustrates lactose consumption after 24 and 72 h along with the reduction of surface tension for the PBS extracted biosurfactants. After 24 h of incubation, average lactose consumption was 28%, whereas the strains LQC 842, LQC 846, LQC 2020 and ACA-DC 287 had consumed more than 40% of the initial substrate. Similarly, after 72 h, average lactose consumption increased to 50.6%, whereas strains CECT 278, CECT 903, ACA-DC 270, ACA-DC 710 and ACA-DC 759 had consumed more than 75% of the substrate. Nevertheless, the higher lactose consumption did not coincide with the higher values in surface tension reduction for all strains. For instance, after 24 h, strains FMCC E10, ACA-DC 0081 and ACA-DC 0713 that displayed the highest reduction values had lower lactose consumption (~ 30%). In the case of 72 h samples, strains LQC 2014 and ACA-DC 0182 had consumed ~ 70% of starting lactose concentration.
Correlation between lactose consumption and surface tension reduction of cell-bound BS using CWP as fermentation sunbstrate; (
 ) lactose consumption (24 h), (
) lactose consumption (24 h), (
 ) ST reduction (24 h), (
) ST reduction (24 h), (
 ) lactose consumption (72 h), (■) ST reduction (72 h). a FMCC, CECT and LQC strains, b LQC strains, c ACA-DC strains, d ACA-DC strains. *Denotes statistically significant differences between 24 and 72 h of fermentation in surface tension reduction (p < 0.05)
) lactose consumption (72 h), (■) ST reduction (72 h). a FMCC, CECT and LQC strains, b LQC strains, c ACA-DC strains, d ACA-DC strains. *Denotes statistically significant differences between 24 and 72 h of fermentation in surface tension reduction (p < 0.05)
It is well established that lactobacilli are fastidious strains, often requiring specific nitrogen sources and micronutrients to support microbial proliferation. Previous studies have reported on the supplementation of cheese whey with yeast, peptone and meat extract and their combination [34, 35]. Regardless the fact that glucose is the most easily metabolised substrate, the results of the current study evidence the significance to additionally carry out the screening for strain selection on the final fermentation substrate (CWP). The aim is to avoid reaching on misleading results and excluding strains that could perform better in optimised media to be used in subsequent benchtop bioreactor studies.
Technological Consideration of Results
In the context of transitioning from a linear to circular economy, we need to foster policies like the Bioeconomy Strategy and the Circular Economy plan [36]. The development of consolidated and sustainable bioprocesses confers one of the routes to mitigate agricultural and food waste streams treatment, entailing the recovery and production of value-added compounds (e.g. biomolecules and active components) [37]. Sustainability and economic feasibility relate to multiproduct formation, integration in existing facilities but also the end-product applications, particularly in the food industry [36, 38].
Unequivocally, cheese whey exhibits one of the most polluting streams of dairy manufacture, demonstrating high organic load, thereby rendering significant challenges for its disposal. Likewise, numerous research studies have utilized CWP in the development of bioprocesses, including the production of biosurfactants, as high-added value compounds. In this frame, CWP (an inherent substrate for GRAS lactobacilli) was implemented in our study to circumvent the environmental impact of CWP via the production of biosurfactants that could be further reintroduced in food applications, to expand the valorization strategies for CWP, enhance process integration and also the circularity of the process. Evidently, to reach optimum results during the first stages of bioprocess development, it is critical to employ also CWP as the screening substrate, within the concept of avoiding misleading results that could hinder optimization and process scale-up.
Conclusions
The present study implemented the evaluation of several screening methods for biosurfactant production using numerous lactobacilli strains. Results indicate the assessment of surface tension as the most robust technique. Likewise, we conclude and suggest that screening experiments should be also conducted in the final substrate, e.g. cheese whey permeate, instead of only conventional supplements, to approach realistic results and avoid misinterpretations that could exclude potential biosurfactant producer strains. Ultimately, the implementation of CWP will alleviate cost impediments of commercial supplements, mitigate its polluting impact and also broaden the valorization strategies for CWP, expand potential applications and enhance overall process integration.
References
Markande, A. R., Patel, D., & Varjani, S. (2021). A review on biosurfactants: properties, applications and current developments. Bioresource Technology, 330(January). https://doi.org/10.1016/j.biortech.2021.124963
Marchant, R., & Banat, I. M. (2012). Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends in Biotechnology, 30(11), 558–565. https://doi.org/10.1016/j.tibtech.2012.07.003
Sharma, D., Saharan, B. S., & Kapil, S. (2016). Substrates and production of biosurfactants, 61–72. https://doi.org/10.1007/978-3-319-26215-4_5
Nitschke, M., & Silva, S. S. E. (2018). Recent food applications of microbial surfactants. Critical Reviews in Food Science and Nutrition, 58(4), 631–638. https://doi.org/10.1080/10408398.2016.1208635
Naughton, P. J., Marchant, R., Naughton, V., & Banat, I. M. (2019). Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. Journal of Applied Microbiology, 127(1), 12–28. https://doi.org/10.1111/jam.14243
Vecino, X., Rodríguez-López, L., Gudiña, E. J., Cruz, J. M., Moldes, A. B., & Rodrigues, L. R. (2017). Vineyard pruning waste as an alternative carbon source to produce novel biosurfactants by Lactobacillus paracasei. Journal of Industrial and Engineering Chemistry, 55, 40–49. https://doi.org/10.1016/j.jiec.2017.06.014
Sood, U., Singh, D. N., Hira, P., Lee, J.-K., Kalia, V. C., Lal, R., & Shakarad, M. (2020). Rapid and solitary production of mono-rhamnolipid biosurfactant and biofilm inhibiting pyocyanin by a taxonomic outlier Pseudomonas aeruginosa strain CR1. Journal of Biotechnology, 307, 98–106. https://doi.org/10.1016/j.jbiotec.2019.11.004
Müller, M. M., Kügler, J. H., Henkel, M., Gerlitzki, M., Hörmann, B., Pöhnlein, M., … Hausmann, R. (2012). Rhamnolipids-Next generation surfactants? Journal of Biotechnology, 162(4), 366–380. https://doi.org/10.1016/j.jbiotec.2012.05.022
Pathania, A. S., & Jana, A. K. (2020). Improvement in production of rhamnolipids using fried oil with hydrophilic co-substrate by indigenous Pseudomonas aeruginosa NJ2 and characterizations. Applied Biochemistry and Biotechnology, 191(3), 1223–1246. https://doi.org/10.1007/s12010-019-03221-9
Zotta, T., Solieri, L., Iacumin, L., Picozzi, C., & Gullo, M. (2020). Valorization of cheese whey using microbial fermentations. Applied Microbiology and Biotechnology, 104(7), 2749–2764. https://doi.org/10.1007/s00253-020-10408-2
Lappa, I. K., Papadaki, A., Kachrimanidou, V., Terpou, A., Koulougliotis, D., Eriotou, E., & Kopsahelis, N. (2019). Cheese whey processing: Integrated biorefinery concepts and emerging food applications. Foods, 8(8). 10.3390/foods8080347
Banat, I. M., Franzetti, A., Gandolfi, I., Bestetti, G., Martinotti, M. G., Fracchia, L., … Marchant, R. (2010). Microbial biosurfactants production, applications and future potential. Applied Microbiology and Biotechnology, 87(2), 427–444. https://doi.org/10.1007/s00253-010-2589-0
Varjani, S. J., & Upasani, V. N. (2017). Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Bioresource Technology, 232, 389–397. https://doi.org/10.1016/j.biortech.2017.02.047
Dobler, L., Vilela, L. F., Almeida, R. V., & Neves, B. C. (2016). Rhamnolipids in perspective : Gene regulatory pathways, metabolic engineering, production and technological forecasting. New Biotechnology, 33(1), 123–135. https://doi.org/10.1016/j.nbt.2015.09.005
Walter, V., Syldatk, C., & Hausmann, R. (2010). Screening concepts for the isolation of biosurfactant producing microorganisms BT - biosurfactants. In R. Sen (Ed.), (pp. 1–13). Springer New York. https://doi.org/10.1007/978-1-4419-5979-9_1
Youssef, N. H., Duncan, K. E., Nagle, D. P., Savage, K. N., Knapp, R. M., & McInerney, M. J. (2004). Comparison of methods to detect biosurfactant production by diverse microorganisms. Journal of Microbiological Methods, 56(3), 339–347. https://doi.org/10.1016/j.mimet.2003.11.001
Rodrigues, L., Moldes, A., Teixeira, J., & Oliveira, R. (2006). Kinetic study of fermentative biosurfactant production by Lactobacillus strains. Biochemical Engineering Journal, 28(2), 109–116. https://doi.org/10.1016/j.bej.2005.06.001
Patowary, R., Patowary, K., Kalita, M. C., & Deka, S. (2016). Utilization of paneer whey waste for cost-effective production of rhamnolipid biosurfactant. Applied Biochemistry and Biotechnology, 180(3), 383–399. https://doi.org/10.1007/s12010-016-2105-9
Samykannu, M., & Achary, A. (2017). Utilization of agro-industry residue for rhamnolipid production by P. aeruginosa AMB AS7 and its application in chromium removal. Applied Biochemistry and Biotechnology, 183(1), 70–90. https://doi.org/10.1007/s12010-017-2431-6
Jimoh, A. A., & Lin, J. (2020). Biotechnological applications of Paenibacillus sp D9 lipopeptide biosurfactant produced in low-cost substrates. Applied Biochemistry and Biotechnology, 191(3), 921–941. https://doi.org/10.1007/s12010-020-03246-5
Sharma, D., Saharan, B. S., Chauhan, N., Bansal, A., & Procha, S. (2014). Production and structural characterization of Lactobacillus helveticus derived biosurfactant. The Scientific World Journal, 2014, 493548. https://doi.org/10.1155/2014/493548
Freitas, B. G., Brito, J. G. M., Brasileiro, P. P. F., Rufino, R. D., Luna, J. M., Santos, V. A., & Sarubbo, L. A. (2016). Formulation of a commercial biosurfactant for application as a dispersant of petroleum and by-products spilled in oceans. Frontiers in Microbiology, 7(OCT), 1–9. https://doi.org/10.3389/fmicb.2016.01646
Thavasi, R., Jayalakshmi, S., & Banat, I. M. (2011). Application of biosurfactant produced from peanut oil cake by Lactobacillus delbrueckii in biodegradation of crude oil. Bioresource Technology, 102(3), 3366–3372. https://doi.org/10.1016/j.biortech.2010.11.071
Alvarez, V. M., Jurelevicius, D., Marques, J. M., de Souza, P. M., de Araújo, L. V., Barros, T. G., … Seldin, L. (2015). Bacillus amyloliquefaciens TSBSO 3.8, a biosurfactant-producing strain with biotechnological potential for microbial enhanced oil recovery. Colloids and Surfaces B: Biointerfaces, 136, 14–21. https://doi.org/10.1016/j.colsurfb.2015.08.046
Mouafo, T. H., Mbawala, A., & Ndjouenkeu, R. (2018). Effect of different carbon sources on biosurfactants’ production by three strains of Lactobacillus spp. BioMed Research International, 2018, 5034783. https://doi.org/10.1155/2018/5034783
Rodríguez, N., Salgado, J. M., Cortés, S., & Domínguez, J. M. (2010). Alternatives for biosurfactants and bacteriocins extraction from Lactococcus lactis cultures produced under different pH conditions. Letters in Applied Microbiology, 51(2), 226–233. https://doi.org/10.1111/j.1472-765X.2010.02882.x
Moldes, A. B., Paradelo, R., Vecino, X., Cruz, J. M., Gudiña, E., Rodrigues, L., … Barral, M. T. (2013). Partial characterization of biosurfactant from Lactobacillus pentosus and comparison with sodium dodecyl sulphate for the bioremediation of hydrocarbon contaminated soil. BioMed Research International, 2013, 961842. https://doi.org/10.1155/2013/961842
Madhu, A. N., & Prapulla, S. G. (2014). Evaluation and functional characterization of a biosurfactant produced by Lactobacillus plantarum CFR 2194. Applied Biochemistry and Biotechnology, 172(4), 1777–1789. https://doi.org/10.1007/s12010-013-0649-5
Morais, I. M. C., Cordeiro, A. L., Teixeira, G. S., Domingues, V. S., Nardi, R. M. D., Monteiro, A. S., … Santos, V. L. (2017). Biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P6A and Lactobacillus gasseri P65. Microbial Cell Factories, 16(1), 155. https://doi.org/10.1186/s12934-017-0769-7
Ibrahim, H. M. M. (2018). Characterization of biosurfactants produced by novel strains of Ochrobactrum anthropi HM-1 and Citrobacter freundii HM-2 from used engine oil-contaminated soil. Egyptian Journal of Petroleum, 27(1), 21–29. https://doi.org/10.1016/j.ejpe.2016.12.005
Afshar, S., Lotfabad, T. B., Roostaazad, R., Najafabadi, A. R., & Noghabi, K. A. (2008). Comparative approach for detection of biosurfactant-producing bacteria isolated from Ahvaz petroleum excavation areas in south of Iran. Annals of Microbiology, 58(3), 555–559. https://doi.org/10.1007/bf03175557
Velraeds, M. M. C., Van der Mei, H. C., Reid, G., & Busscher, H. J. (1997). Inhibition of initial adhesion of uropathogenic Enterococcus faecalis to solid substrata by an adsorbed biosurfactant layer from Lactobacillus acidophilus. Urology, 49(5), 790–794. https://doi.org/10.1016/S0090-4295(97)00065-4
Gudiña, E. J., Teixeira, J. A., & Rodrigues, L. R. (2011). Biosurfactant-producing lactobacilli: Screening, production profiles, and effect of medium composition. Applied and Environmental Soil Science, 2011, 1–9. https://doi.org/10.1155/2011/201254
Rodrigues, L., Teixeira, J., Oliveira, R., & Van Der Mei, H. C. (2006). Response surface optimization of the medium components for the production of biosurfactants by probiotic bacteria. Process Biochemistry, 41(1), 1–10. https://doi.org/10.1016/j.procbio.2005.01.030
Gudiña, E. J., Teixeira, J. A., & Rodrigues, L. R. (2010). Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloids and Surfaces B: Biointerfaces, 76(1), 298–304. https://doi.org/10.1016/j.colsurfb.2009.11.008
Caldeira, C., Vlysidis, A., Fiore, G., De Laurentiis, V., Vignali, G., & Sala, S. (2020). Sustainability of food waste biorefinery: a review on valorisation pathways, techno-economic constraints, and environmental assessment. Bioresource Technology, 312(May). https://doi.org/10.1016/j.biortech.2020.123575
Lee, J. K., Patel, S. K. S., Sung, B. H., & Kalia, V. C. (2020). Biomolecules from municipal and food industry wastes: an overview. Bioresource Technology, 298(September 2019), 122346. https://doi.org/10.1016/j.biortech.2019.122346
Kachrimanidou, V., Ioannidou, S. M., Ladakis, D., Papapostolou, H., Kopsahelis, N., Koutinas, A. A., & Kookos, I. K. (2021). Techno-economic evaluation and life-cycle assessment of poly(3-hydroxybutyrate) production within a biorefinery concept using sunflower-based biodiesel industry by-products. Bioresource Technology, 326. https://doi.org/10.1016/j.biortech.2021.124711
Acknowledgements
We acknowledge the Laboratory of Dairy Research (Agricultural University of Athens, Greece) for kindly providing the ACA-DC culture collection strains, the Laboratory of Food Quality Control and Hygiene (Agricultural University of Athens, Greece) for kindly providing the LQC culture collection strains and the Laboratory of Food Microbiology and Biotechnology (Agricultural University of Athens, Greece) for kindly providing the FMCC culture collection strains.
Funding
This work is supported by the project “Research Infrastructure on Food Bioprocessing Development and Innovation Exploitation – Food Innovation RI” (MIS 5027222), which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure”, funded by the Operational Programme "Competitiveness, Entrepreneurship and Innovation" (NSRF 2014–2020) and co-financed by Greece and the European Union (European Regional Development Fund).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
All authors have given the consent to participate in the present research concept.
Consent for Publication
All authors have read and gave the consent for publishing the manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kachrimanidou, V., Papadaki, A., Lappa, I. et al. Biosurfactant Production from Lactobacilli: an Insight on the Interpretation of Prevailing Assessment Methods. Appl Biochem Biotechnol 194, 882–900 (2022). https://doi.org/10.1007/s12010-021-03686-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03686-7


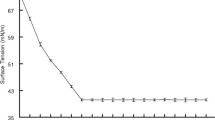


 )and 72 h (■) of incubation on M2 medium: a FMCC, CECT and LQC strains on M2 medium, b ACA-DC strains on M2 medium, c FMCC, CECT and LQC strains on cheese whey permeate, d ACA-DC strains on cheese whey permeate
)and 72 h (■) of incubation on M2 medium: a FMCC, CECT and LQC strains on M2 medium, b ACA-DC strains on M2 medium, c FMCC, CECT and LQC strains on cheese whey permeate, d ACA-DC strains on cheese whey permeate