Abstract
The present study assesses the Paenibacillus sp. D9 lipopeptide biosurfactant synthesis in cheap substrates including functional properties and applicability for varying biotechnological processes. Different experimental setups were made for oil dispersion, heavy metals removals from contaminated environments, and washing performance. The study revealed surface tension activities of 31.7–32.7 mN/m, and maximum biosurfactant yield of more than 8 g/L. Removals of 85.90%, 98.68%, 99.97%, 63.28%, 99.93%, and 94.22% were obtained for Ca, Cu, Fe, Mg, Ni, and Zn, respectively from acid mine effluents. In comparison with chemical surfactants, there was pronounced removal of heavy metals from wastewater, contaminated sands, and vegetable matter, as well as improved oil dispersing activity. A comparative study revealed that biosurfactant was more efficient (> 60%) for removal of tomato sauce and coffee stains than chemical surfactants (< 50%). Thus, lipopeptide biosurfactants are green biomolecules reducing hazards and contaminations within the environment. The future use of this lipopeptide biosurfactant is greatly promising in biotechnology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At the present time, there is great emphasis directed toward the devastating effects and severity of the usage of synthetic surfactants on the environment [1]. This is based on their great toxicity, persistence, and non-biodegradable properties [2]. Environmental dangers associated with oil spills and heavy metals have limited biotechnological advancements which ultimately creates a market for alternative “greener” technology [1]. A more sustainable development involving the biomolecules surfactants (BioSs) has been given increased recognition over the years [3, 4]. The desirable functional properties of BioSs have driven their use over the past few decades. They are specific, emulsifiers, have acceptable biocompatibility, and are suitable for wetting, foaming, and are overall biodegradable [5]. In comparison with manufactured surfactants, they offer a better and more eco-friendly approach because of their minimal toxicity as recommended by the World Health Organization [6]. The stability of a BioS under extraordinary environmental conditions (excellent thermal stability and great salt tolerance) as a result of their retained surface-active properties suggesting their possibility for usage in oil recovery, heavy metal bioremediation, and the food industry [7, 8].
The possible forms of oil contaminants and hydrocarbons vary in the environment such as desorption in water, soil particles adsorption, and/or absorption in soil particles, or presentation at separate phases, which could be either solid or liquid [9]. The use of surfactants to improve solubilization is one of the viable approaches required to increase the mobilization of hydrophobic contaminants. Additionally, the affiliated excessive expense and toxicity of the synthetic tensioactive agents forestall far-reaching use of surfactants for oil recovery [5]. Besides hydrocarbons, major persistent soil contaminants are heavy metals, posing threats to the ecosystem and human wellbeing indirectly through regular lifestyle or by direct contact to the pollutant [5]. Since heavy metals are not biodegradable, their removal from soil is particularly challenging, and the conventional remediation usually involves the excavation and transport to landfill, which is an extremely costly process that poses many disadvantages [10]. Another issue associated with heavy metal contamination in the soil is the contamination of plants and the subsequent biomagnification throughout the food chain [11]. Continuous ingestion of foods contaminated with heavy metals may lead to detrimental and severe health risks in both humans and other animals, as this can result in successive accumulation [12]. In another context, chemical surfactants such as household and laundry cleaning products necessitate innovation of eco-friendly products, because of the toxic and persistent nature of detergents on the environment. This need is heightened further because chemical surfactants comprise between 10 and 50% of the composition of detergent products, with the rest being additives such as fabric softener, enzymes, and bleaching agents [13].
Despite the abundance of microorganisms producing various BioSs, that demonstrate a variety of applications, there are still a few challenges to overcome in terms of cost and production yield. There is a need, therefore, to be addressed before BioSs can be considered as commercially viable [14]. Exploitation of various low-cost substrates is a means of overcoming the challenges associated with combating the financial implications of the bioprocess [15]. Most microorganisms can grow and sustain themselves using the nutrients present in many cheap substrates and waste products, thus minimizing the cost involved [5]. Cost-effective and renewable carbon sources like molasses, soybean oil, waste frying oil, palm oil, and agricultural residues are now being used for BioS production because of the excessive cost in producing these compounds when using glucose, glycerol, hydrocarbons, and other substrates [5].
This finding together with the current movement toward sustainability ultimately creates a demand for new formulations and improvements in the environmental and biotechnological industry. It has been demonstrated that lipopeptide BioS produced by Paenibacillus sp. D9 exhibited good performance in the degradation of highly toxic and hydrophobic pollutants over 21 days of incubation [16, 17]. Having considered the different prospects of BioS in improving environmental and biotechnological sustainability, the present research was involved with evaluating the effects of low-cost substrates on Paenibacillus sp. D9 BioS synthesis and its potential use in oil dispersal, detergent/washing formulations, heavy metal removal from vegetables and contaminated environments. Furthermore, the toxicity and efficiency of the BioS were evaluated in survival trials with Brassica oleracea, Lactuca sativa, and brine shrimps.
Materials and Methods
Materials, Chemicals, and Reagents
All chemicals were purchased from Sigma-Aldrich, Co, USA. Sodium tripolyphosphate and sodium sulfate utilized as builder and filler were of analytical grade. Waste frying oils (sunflower and coconut) were acquired from different restaurants in the city of Durban, Kwazulu-Natal, Republic of South Africa. Waste frying oil of plant, animal, or synthetic fat was previously used in frying, baking, and other types of cooking. They were stored in the laboratory until further usage. The waste oils are vegetative carbon source, lipidic in nature (16–20 carbon atom chains) comprised majorly of saturated or unsaturated fatty acids. Two available commercially detergent was purchased from the Durban market, South Africa. Chemical surfactants, sodium dodecyl sulfate (SDS), and Triton X-100 were purchased from Sigma-Aldrich, USA, for a comparative study. The contaminated samples (primary effluent) used in the experiments were obtained from acid mine drainage, northern KZN, South Africa.
Growth and Maintenance of Paenibacillus sp. D9
A culture of Paenibacillus sp. D9 was obtained from Microbiology Department, School of Life Sciences, University of KwaZulu Natal, Westville Campus. A single colony of the bacterial culture was placed in a 5-mL tube for growth overnight at 30 °C. The extract was then centrifuged at 10, 000 rpm for 10 min and the pellet washed twice with phosphate-buffered saline of the composition: (g/L) 0.24 KH2PO4, 1.42 Na2HPO4, 8.0 NaCl, 0.2 KCl with pH adjusted to 7.6 ± 0.2. The remaining pellet in the Bushnell Haas (BH) medium was then suspended and the optical density (OD) value adjusted to 1.0 at 600 nm. The Paenibacillus inocula were kept at 4 °C until further use.
Biosurfactant Production, Extraction, and Recovery
BioS production was carried out in BH medium composition (g/L): K2HPO4 1.00, KH2PO4 1.00, MgSO4·7H2O 0.20, FeCl3 0.05, CaCl2 0.05, CaCl2 0.02, NH4NO3 1.00, pH of 7.0 ± 0.2. Waste coconut (5.0%) and sunflower oil (5.0%) were utilized as low-cost substrates in a 500-mL flask, with the variation of inoculum sizes ranging from 1 to 3 mL. The flasks were incubated at 30 °C for 7 days, the solutions were centrifuged, and the culture supernatants were used in the experiments. The increase or decrease in OD was determined using a spectrophotometer (Shimadzu Model, Japan) at 600-nm wavelength. The production medium allowed for clear separation of hydrophobic layer containing the substrates and hydrophilic layer containing the bacterial cells. The spectrophotometer was blanked with the medium containing the waste substrate mixtures during the measurement of OD600 value.
At the end of the production period, the crude BioS was extracted as described in our previous study [17]. The crude BioS was purified according to the procedures defined below [18]. The sample was then liquefied in methanol, mixed with silica gel (230–400 mesh) and subsequently oven-dried at 50 °C. The silica gel was further mixed with chloroform and then loaded onto a chromatography column (50 cm × 2.8 cm). A mixture of ethyl acetate/chloroform in different proportions (100 to 0% with 10% interval) was used in the sequential washing of the loaded column at a flow rate of 0.5 mL/min. A UV spectrophotometer (Cary 60, Agilent Technologies Australia) with a range of 200–800 nm was used to monitor the absorption wavelength of the mixtures to determine the greatest fractions containing the BioS. The eluents (20 mL) were collected and the fractions showing oil displacement activity were thoroughly mixed. This was followed by evaporation at 80 °C to acquire purified sample [18]. The purified BioS was confirmed for surface properties before its further usage. The CFS and the purified BioS described above were utilized for different application setups.
Surface Tension
Surface tension (ST) was determined with a K6 Tensiometer (KRÜSS GmbH, Germany) using 1.9-cm De Noüy platinum ring at room temperature. The culture media were centrifuged at 13,500×g for 20 min to obtain a 40 mL CFS [17]. For calibration, the ST of distilled water was first measured. The ST of BH medium supplemented with the waste frying oils (sunflower and coconut) was analyzed and determined as controls. All recordings were made as three independent experiments with mean ST value used.
Critical Micelle Concentration
Critical micelle concentration (CMC) was analyzed by measuring the ST sequences of a series of dilutions of BioS concentrations using Tris-HCl buffer solution, pH 8 [19]. A stock solution of the crude BioS (1 g/L) was prepared and various dilutions were made to obtain a range of the concentrations from 10 to 1000 mg/L. The common experimental procedure was used to determine the intersection point of two straight lines that best fit through the CMC (pre- and post-) data and BioS concentration.
Heavy Metal Removal from Contaminated Acid Mine Drainage Effluents
Removal of heavy metals from acid mine drainage samples was evaluated using previously developed methods [20]. Ten milliliters of each contaminated sample was transferred to different falcon tubes and approximately 10 mL of the BioS (500 mg/mL; ST; 30.9 mN/m), sodium dodecyl sulfate (SDS) (500 mg/mL; ST; 30.9 mN/m), and CFS (ST; 30.9 mN/m) was added separately to the experimental setup. The samples were incubated at room temperature for 24 h, and subsequently, each sample was filtered through 0.42-μm membrane filter. The detection of the concentrated metals was performed using a multi-element, inductively coupled plasma-optical emission spectrometer (Perkin Elmer) with sample atomization in acetylene flame and compressed air. The initial heavy metal effluent composition included Ca 177.88 ppm, Cu 157.67 ppm, Fe 273.6 ppm, Mg 119.83 ppm, Ni 114.03 ppm, and Zn 315.1 ppm, respectively. The control experiment was achieved without the introduction of the biomolecule treatments. The percentage of heavy metals removed was determined based on the metal content (control) in the aqueous solution using the following equation:
where HMC is the concentration of heavy metals (control, i.e., without treatment) and HMF is the final concentration of heavy metals (after treatment by lipopeptide BioS).
Determination of Physicochemical Properties of Contaminated Effluent
Physicochemical analysis of the contaminated acid mine drainage effluents was performed to analyze the effect of some factors, which play a vital role in the heavy metal removal process. The measurement of parameters such as pH, electrical conductivity, salinity, and total dissolved solids concentration was carried out according to standard procedures. Electrical conductivity, salinity, and total dissolved solids were measured as per the instruction manual supplied with the instrument Hatch HQd Portable Meter. The sample pH was determined with the aid of 3510 pH meter (Lasec, Jenway). Phosphate and sulfate concentration was determined according to the American Public Health Association standard. Following treatment with Paenibacillus sp. D9 BioS, cell-free broth, and SDS, the parameters were measured as stated earlier. All readings were performed in triplicate and deionized water was used as control.
Heavy Metal Removal from the Different Vegetables
The different biomass samples (potato, cucumber, tomato, onion) were washed extensively with running tap water for 30–40 min to remove the particulate matter and dirt. The external parts were pulverized into small pieces, and subsequently immersed in 1:1 HCl solution for 10 min. The different biomass was further washed with twofold deionized water [11]. A stock solution of cadmium chloride (1000 mg/L) was prepared in Milli Q for the detection of cadmium (Cd). Upon the introduction of diphenyl carbazide, a violet color was developed and was measured at an absorbance of 540 nm. Vegetables were exposed to cadmium chloride (0.40, 0.60, and 0.80 mg/mL) for 30 min. Diphenyl carbazide was added to develop the violet color, and the change in cadmium concentration resulting from absorption was determined by the absorbance at 540 nm. The vegetables from the same stock were treated with BioS, to allow the absorption of cadmium chloride with the biomolecule. The cadmium ion removal percentage by adsorption was determined as follows:
where Ci is the initial concentration of cadmium (mg/mL), and Cf is the final concentration of cadmium (mg/mL) [11].
Preparation of Standard Sand with Heavy Metals
A metal solution [Pb (NO3)2 + ZnSO4. 7H2O + CuSO4. 7H2O)] was used to contaminate artificial standard sand. The final concentration of 1000 mg/L was achieved through the addition of separate salts dissolved initially in deionized water without pH adjustment. The sand and salt solutions containing heavy metal were left in contact for proper mixing in an orbital shaker (200 rpm, 25 °C) for 2 days. The non-adsorbed metals present in the solutions were removed by centrifugation for 10 min at 5000 rpm. The contaminated sands were further dried in an oven at 55 °C for 24 h while the supernatant obtained was discarded. The initial and the final weight of the sand was determined to confirm the adsorption of the heavy metals on the contaminated sand.
Treatment of Contaminated Sand with Biosurfactant
The sequential treatment of contaminated sand was determined utilizing the purified BioS at full CMC, as well as, crude BioS and CFS. Chemical surfactant (SDS) and distilled water were both used as controls. The experiment was also conducted with a 1% HCl solution which was performed both individually and in combination with BioS and CFS. Fifty milliliters of the solution was introduced at different CMC concentrations and 10 g of sand was subsequently transferred to make a final experimental setup in a 500-mL Erlenmeyer flask. The samples were incubated in an orbital shaker for 48 h at 25 °C, followed by centrifugation at 5000×g for 10 min. The supernatants collected were analyzed for the residual heavy metal concentration using multi-element, inductively coupled plasma-optical emission spectrometer (Perkin Elmer). The percentage of heavy metals removed was determined based on the metals content (control) in the aqueous solution of the contaminated sand as described above.
Biosurfactant Treatment of Synthetic Wastewater Contaminated with Heavy Metals
The BioS produced was tested for its capability of removing heavy metals from Phoenix wastewater effluent. The synthetic wastewater after analysis contained substantial amount of Pb and Zn. The concentrated wastewater was treated separately through the addition of BioS at ½ CMC, full CMC, 2× CMC, and crude BioS to test the ability of the biomolecule to bind to heavy metals present in the aqueous solution [21]. The conductivity of the resulting solution was measured using the instrument Hatch HQd Portable Meter after removing the metal-BioS precipitate. The Hatch HQd Portable Meter was calibrated with deionized water, prior to the measurement of each sample. The percentage of heavy metals removed was also determined based on the metals content (control) in the aqueous solution (synthetic wastewater) as described in the equation above.
Oil Dispersion Assay
The BioS extracted from Paenibacillus sp. D9 was used for its oil dispersing ability according to described methodology [22]. A thin layer of oil on the water surface was formed by the addition of 250 μL of engine oil to the center of 40 mL of distilled water in a petri dish (10 cm). The formation of a clear zone was a positive result for the presence of the BioSs oil dispersing properties. SDS and Triton X-100 were also tested since they are well-known chemical surfactants capable of dispersing oil. The supernatant from the culture was also tested for this property. The oil displacement rate (expressed in %) was attained by measuring the displacement diameter after 30 s, relative to the diameter of the petri dish. The rate of oil displacement was calculated as
Results were conducted in triplicate and compared relative to negative control of distilled water.
Evaluation of Wash Performance and Detergent Formulation
White clean dry white cotton cloth cut into 5 cm2 pieces was stained with 1.25 mL of sunflower oil-tomato sauce and coffee subsequently dried at 40 °C overnight. To test the wash experiments, stained white cloth was either was dipped in any one of the flasks containing as stated containing (i) 50 mL of tap water (control), (ii) 40 mL tap water, and 10 mL of 1.0% (v/v) of each detergent solution, (iii) 40 mL tap water and 10 mL of 1.0% (v/v) biocommercial detergent solution, (iv) 40 mL tap water and 10 mL of 1.0% (v/v) BioS solution, or (v) 40 mL tap water and 5 mL each of 1.0% (v/v) detergent and BioS solutions (Table 1).
Flasks were rotated at 200 rpm for 40 min at room temperature (25 °C), followed by the removal of cloth pieces from the flasks, and the remaining wash solution was decanted carefully to avoid soap bubbles. This post- wash water was used to determine the removal of stain from the white fabric cloths. The percentage of stain removal from the white cotton was calculated with the following equations.
Where, A = initial weight of the flask before washing, B = weight of the flask + addition of stained white cotton, and C = Final weight of the flask after washing.
Toxicity of Formulated Biosurfactant Against Brassica oleracea and Lactuca sativa
The phytotoxicity of the produced BioS was assessed by a static test including the seed germination and root elongation of cabbage (Brassica oleracea) and lettuce (Lactuca sativa) [23]. Distilled water was used to prepare isolated BioS in different concentrations of 1 to 200 mg/L (CMC). The toxicity experiment was determined in sterilized Petri dishes (1 cm × 10 cm) containing filter paper. Twenty-five seeds were inoculated after pre-treatment in each Petri dish containing 5 mL of the test solutions. The seed germination, root elongation (≥ 5 mm), and germination index (GI) were determined below after 7 days of incubation (20 °C).
where ns is the number of seeds germinated in the sample and nc that in the control,
where Ls is the sample root length (mean) and Lc that in the control,
Biosurfactant Toxicity to Brine Shrimp
Brine shrimp (Artemia salina) was used as a toxicity indicator with different concentrations of isolated BioS. Different concentrations of BioS solutions such as, 0, 1, 10, 100, and 200 mg/L (CMC) were tested in this experiment. The assays were carried out using 10 brine shrimp larvae contained in 5 mL aqueous solution (33.3 g/L marine salt solution) in a total of 10-mL glass tubes. Subsequently, 10 mL of each BioS solution at concentrations listed above was introduced in each tube containing the brine shrimp larvae. The tubes were observed for 24 h to determine the rate of mortality. The 50% lethal concentration (LC50) to kill brine shrimp within 24 h was defined as the toxicity threshold concentration.
Statistical Analysis
All the experimental data were expressed in terms of arithmetic averages of at least three independent replicates, with standard deviation (±). Significance was ascribed using ANOVA at the 95% confidence level using Graph Pad Prism statistical tool.
Results and Discussion
Biosurfactant Production in Combination of Low-cost Substrates
The capability of Paenibacillus sp. D9 to utilize a combination of low-cost substrates for maximum production yield was determined (Table 2). However, because of the concentration of substrate used (10%), inoculum conditions were varied to ascertain the ability of Paenibacillus sp. D9 to withstand selective pressure and concentration. At the end of the experiment, there was an increase in OD of the medium indicating growth-associated BioS production. Results reveal ST activities of between 31.7 and 32.7 mN/m, and maximum Paenibacillus sp. D9 BioS yield of more than 8 g/L, regardless of the inoculum size used (Table 2). The outcomes showed significance in relative to control samples with no production of BioS yield. This however, ruled out any possibilities of the substrates co-precipitating with the isolated BioS. Pronounced reduction in ST from the low-cost production media indicates high production of BioS; thus, the great yield obtained. There was no significant difference (p > 0.05) between the BioS activity output (ST and BioS yield); thus, the differences in inoculum size are inversely proportional to the increased concentrations of the substrates used. The ST achieved in this research showed high influence of the BioS synthesized as the control sample containing the low-cost substrates only reduced from 71.4 to 67.8 mN/m. Conversely, a greater rhamnolipid BioS yield of 13.93 g/L was achieved by a non-pathogenic microorganism Pseudomonas sp. SWP-4 utilizing waste cooking oil [24]. Also, the utilization of another low-cost substrate, soybean oil refinery waste, by Pseudomonas aeruginosa MR01 led to maximum production yield of 9.64 g/L [25]. Improvement in production procedures and use of inexpensive substrates reduces the initial costs with double the benefit of reducing the pollutants while producing useful products. The probable usage of low-cost substrates for improved BioS yield is of great significance to counter the high cost of production. The present work assesses a few residuals from food restaurants to deliver BioS by Paenibacillus sp. D9. The waste frying oils were obtained at essentially no expense as an alternative medium. The source will significantly diminish the costs associated with large-scale production of BioS. The present investigation sheds light on the elective usage of waste cooking oil as a high-vitality source for synthesis of great value products as lipopeptide BioS.
Physicochemical Analysis of Contaminated Acid Mine Drainage Samples
Determination of the physicochemical properties in contaminated samples is significant because these properties may impact the function of biomolecules, and thus impact their ability for use in applications like heavy metal removal [26]. Therefore, by recording the physicochemical parameters before and after treatment with the BioS, one may determine whether treatment affected the use of these molecules as well as whether any alterations in the parameters occurred (Table 3). The initial readings of the physiochemical parameters for the heavy metal effluents revealed that it was very acidic, with a pH of 1.17 and the salinity of the effluent was relatively minimal at 3%. After treatment with BioS, the pH increased significantly to 6.03 from the BioS structural composition. There was a further surge in salinity to 14.34%, and the electrical conductivity increased from 6.52 to 23.83 μs/cm. In comparison with BioS, SDS caused a remarkable reduction in the TDS which is owed in part to its detergent properties. The TDS represents a measure of inorganic salts, organic matter, and other dissolved materials, and in lowering the TDS, the BioS was also able to clear the effluent of these particles [27]. From this result, addition of deionized water which served as a control did not change the physiochemical properties of the contaminated wastewater (Table 3). However, results following treatment of the effluent with CFS revealed a reduction in electrical conductivity and salinity, and reduced TDS more effectively as compared with BioS and SDS. The increase in pH ultimately demonstrated the ability of the BioS to treat heavy metal effluent. By raising the pH, the impact of the effluent had become milder and thus more suitable for the environment as opposed to the acidic pH impact it previously had.
Still, of great concern to the environment is the incidence of elevated phosphate and sulfate resulting from the contaminated effluents. The BioS and the CFS were both efficient in removal of phosphate and sulfate as compared with the chemical surfactant (Table 3). There was a reduction from initial high concentrations of sulfate and phosphate to 617.12 and 2.27 ppm (purified BioS), and 622.04 and 3.72 ppm (CFS) respectively. However, there was little influence on phosphate and sulfate removal by CFS and purified BioS. Increasing concentrations of pollutants (sulfate and phosphate) subsequently leading to their release into ground water can create long-term effects such as algal blooms termed “eutrophication.” Additionally, inorganic sources, for example, nitrates, phosphates, and sulfates, are some essential contaminants, so it is important to diminish the output levels before releasing. The produced BioS provided great potential since there is a huge market for removing these pollutants, thus highlighting the usefulness of this biomolecule in environmental sustainability. As such, the toxic and harmful contaminants present in the effluents were converted into a less toxic or non-toxic state owing to the complete removal of various heavy metals. This approach also provides an ecologically safer and cost-effective alternative to the conventional method since CFS produced similar efficiency as the purified BioS.
Removal of Heavy Metals from the Acid Mine Drainage Effluent
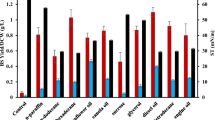
The extraction of heavy metals by BioS is facilitated through different mechanisms, which include dissolution, ion exchange, precipitation, and association. The capability for reducing heavy metals, for example, calcium, copper, iron, magnesium, nickel, and zinc, and zinc, was explored additionally, and the outcomes displayed (Fig. 1). As compared with the chemically synthesized surfactant (SDS), a critical decrease of metal resulted after adding the lipopeptide BioS. Removals of 85.90%, 98.68%, 99.97%, 63.28%, 99.93%, and 94.22% were obtained for Ca, Cu, Fe, Mg, Ni, and Zn, respectively, when the purified BioS was used. The results were similar to those for the cell-free BioS-containing solution removing 81.18% Ca, 97.9% Cu, 99.65% Fe, 99.79% Ni, 52.15% Mg, and 94.22% Zn from acid mine drainage effluents respectively. These heavy metals become toxic as their ionic species and as such, they become difficult to dissociate from the environment [28]. The percentages observed indicate that the removal resulted from the electrostatic interaction between the molecules of the BioS and the metals. The BioS-metal complex was absorbed from the solution because of the reduction in surface and interfacial tension. Similar to the CFS, the produced BioS allowed a greater percentage reduction over time, reducing the toxicity of heavy metals. Similar to the present research, there was a removal order of Cd = Cr > Pb = Cu > Ni from a multi-element contaminated soil by a di-rhamnolipid produced by Pseudomonas aeruginosa BS2 [29].
Removal of heavy metals (Ca, Cu, Fe, Mg, Ni, Zn) on contaminated effluent by cell-free supernatant, purified biosurfactant synthesized by Paenibacillus sp. D9, and chemical surfactant. CFS, cell-free supernatant; BioS, Paenibacillus sp. D9 biosurfactant; SDS, sodium dodecyl sulfate. Values represent mean ± standard deviation (n = 3). The a–c superscript letters above histogram bars reflect significant difference (p < 0.05) if the letters are different
Addition of both BioS and CFS promotes heavy metal desorption from acid mine drainage effluents through complexation. Still, heavy metals are cations, and this enables their attraction to the negatively charged functional groups present in the biomolecule (CFS and BioS), as this explains their similarities in activity. Thus, the usage of CFS allows for a reduction in production costs, since it does not require extraction, recovery, and purification processes which account for 30 ̶ 50% of the total production cost. Previous reports have mentioned the efficient role of BioS in removing heavy metals from polluted effluents [28, 30, 31]. However, the present report is the first to show the effective advantage of BioS in removing heavy metal from acid mine drainage contaminated effluents, and the different physiochemical parameters, including pH, phosphate, and nitrate. The present report is therefore of significance to maximize health and environmental benefits associated with treatment using the produced BioS. The BioS can be tested further in future environmental applications that involve wastewater from different sources, as the foremost synthetic contaminants being heavy metals, nitrogen, phosphorus, pesticides, detergents, and hydrocarbons [32].
Additionally, in soil polluted with Cd and Zn, rhamnolipid BioS increased metal phytoextraction without the conceivable increment of metal mobility in the long term [33]. However, heavy metals are cations, and this regulates their sorption to negatively charged functional groups present in biomolecule, residual hydroxides (OH−), humic acid, and anionic salts, such as PO4− and SO4− [28]. The result is novel and significant; hence, the controlled stimulation of the surface-active agent supports the removal of toxic heavy metals and acid mine drainage pollutants, enabling us to have a safer and cleaner environment.
Heavy Metal Removal from Vegetables
Human health risks have resulted from consumption of metal polluted vegetables [17]. Different concentrations of cadmium were selectively removed from various vegetables like potato, tomato, cucumber, and onion by Paenibacillus sp. D9 BioS (Table 4). The BioS eliminated a substantial amount of heavy metal from polluted food samples; thus, the BioS synthesized could be used economically, enabling its usefulness for the health of humans and other living beings in the environment. The concentrations of heavy metal (cadmium) introduced to vegetables in proportion do not have an influence on the percentage removal capacities of the BioS (Table 4). From the present study, the BioS selectively removed cadmium from contaminated vegetables in the order of onion = tomato > cucumber > potato. The greater Cd removal ability observed on tomato (71.38%, 73.46%, 74.28%) and onion (65.12%, 66.01%, 67.08%) could result from their greater absorption to this heavy metal. A comparable result was obtained with the BioS synthesized from Bacillus sp. MTCC 5877 removed 61.03% Cd from contaminated onion [11].
The final reaction between different cadmium concentrations (0.4, 0.6, 0.8 mg/mL) and 1,5-diphenylcarbazide (C13H14N4O) gives a violet color in the different vegetables setup (Fig. 2a). The use of a chemical surfactant (SDS) showed no effect on the color absorption of the heavy metals of the different vegetables tested (Fig. 2b). It is necessary to control adherence of the biomolecule to food contact surfaces, and it is critical to provide healthy and safe food products to the consumers at large. Thus, the utilization of this BioS could be an imperative apparatus for the food industry as the excessive intake of heavy metals through food is dangerous to human health. Successively, the introduction of BioS to the experimental setup was effective as there was a substantial color change from violet to colorless, confirming the BioS ability to remove the metal absorption from the different vegetables (Fig. 2c). Compared with the control, a significant reduction of the heavy metals was observed with the introduction of the BioS. In this regard, the measured usage of the surface-active compound will support the improved washing of the compounds from surfaces of vegetable and food crops present in the soil environment. Similar to this research, the removal of 47%, 61.0%, 62.5%, and 73% Cd, respectively from different vegetables was achieved by a BioS produced from Bacillus sp. MTCC 5877 [11]. Also, the rhamnolipid BioS produced from Pseudomonas putida might play a great part in the removal of these toxic heavy metals, as 50% zinc and iron were both removed from the contaminated medium [34]. The Bacillus licheniformis VS16 BioS likewise reduced cadmium (Cd) from contaminated vegetables namely ginger, carrot, radish, and potato with the greatest removal being 60.98% [35]. Thus, Paenibacillus sp. D9 BioS also demonstrates its capacity as a washing agent in heavy metal removal from both contaminated acid mine effluents and vegetables when compared with a synthetic surfactant. This enabled its usefulness in the world market as a bioremediation agent and important tool in biotechnological and environmental sustainability.
Removal of Heavy Metals from Contaminated Sand
There is increased interest in the discovery of novel washing procedures and bioproducts, such as amphiphilic BioSs equipped for attaching metals and without presenting danger to nature [21]. Solutions of purified BioS at various concentrations [1/2 × CMC (0.1%), CMC (0.2%), and 2 × CMC (0.4%)] were examined to assess for the removal of metals with and without micelle formation. From the results obtained, Paenibacillus sp. D9 BioS was highly effective in removing copper and lead, with lesser percentages observed for zinc (Table 5). As observed from this result, increasing in BioS concentration tested did not cause proportional removal of heavy metals namely copper, lead, and zinc. The BioS produced possess very little affinity to zinc, giving a poor removal efficiency of ≤ 60% at all the concentrations as compared with greater removal percentages (≥ 60%) of copper and lead respectively. The greater removal observed for both copper and zinc is frequently identified with BioSs’ binding to the constituents of the soil particles. The 1% HCl solution removed 50–55% of the metals adsorbed to the sand and the removal rate was enhanced when acid solutions were combined with purified BioS and CFS. However, the introduction of the HCl solution did not influence the removal capabilities of the BioS with ≤ 60% zinc removal from contaminated sands achieved.
Different BioSs have fluctuating attractions to various metals and are constantly influenced by type and concentration of acids or alkalines, biomolecules, charge of heavy metal, and soil properties [36]. The crude BioS removes a greater percentage of copper and lead from sand demonstrating its utilization, as well as, BioS in the decontamination of heavy metal polluted soils. The downstream procedure to purify BioS could account for 60% of the total cost, and thus crude BioS could be efficient and significant in achieving a cost-effective bioprocess. It was also reported that ∼ 30% heavy metal was removed from contaminated sand, with a further ≥ 80% removal achieved when different additives were introduced [21]. This is similar to the present study in which increasing BioS concentrations was not proportional to the heavy metal removal capacities. Recently, BioS produced by Candida sphaerica demonstrated 95%, 90%, and 79% removal rates for Fe, Zn, and Pb, respectively, from samples, gathered from a car battery industry. The introduction of HCl solutions increased removal rate when utilized with BioS at concentrations of 0.1% and 0.25% [37].
Biosurfactant Ability to Bind Heavy Metals in Aqueous Solution
The ability of the BioS to bind heavy metals (Pb and Zn) present in synthetic wastewater was determined by measuring the conductivities and heavy metal removal capabilities. The initial conductivity of the metal solutions containing concentrations viz., 1/2 CMC, CMC, and 2× CMC was 80 μS/cm, 92 μS/cm, and 76 μS/cm, respectively. Regardless, the conductivity of the solutions comprising zinc (Zn) and lead (Pb) experienced a highlighted decrease when BioS was introduced. The BioS precipitated the positively charged metals from the solution, leading to metal ion reduction and subsequently diminishing its conductivity (Table 6). The removal capabilities were observed with CMC (58.1% Pb, 53.3% Zn) and 2× CMC (77.5% Pb, 57.7% Zn), respectively. There was very little reduction observed with half CMC in terms of conductivity and removal capabilities in both heavy metals. It is notable that high concentration of this BioS eliminated metals in a greater proportion. This outcome displayed more micelles incited less free particles and conductivity was accordingly less than in the solutions with no or little BioS. Generally, the different concentrations achieved a greater removal capability of Pb and Zn in comparison with chemically synthesized surfactant (SDS) and distilled water (negative control). In contrast to this research, no variation in the effect of different BioS concentrations on the conductivity of the metal in synthetic wastewater [21].
Oil Dispersion Assay
Oil dispersion is another technique that demonstrates the capacity of the BioS to remove oil from surfaces using its surface and interfacial tension reducing properties, providing a reason for its application in oil clean-up and control of oil spillages. The Paenibacillus sp. D9 BioS achieved a significant dispersal rate of 60% whereas the SDS and Triton X-100 had 25 and 20%, respectively. The dispersion rate of the CFS was 30% and the negative control had the smallest initial diameter of 1.5 cm, obtaining a dispersal rate of only 15% (Table 7). The oil dispersal technique represents both a means of confirming the presence and screening for BioS production by the microorganism as well as a measure of the surface-active properties. This is because the detection of a zone of clearing indicates that oil has been displaced by the pressure of BioS [38]. The diameter of this zone of clearing positively correlates with the concentration of BioS and depicts oil spreading activity [39]. This illustrates the efficiency of BioS, and the larger the diameter of the dispersal, the greater the activity of the surfactant [39]. The oil dispersion limit of a BioS is of extraordinary significance when the goal is to treat situations polluted with hydrocarbons [40].
When comparing the dispersal rate of the BioS with that of the positive controls SDS, Triton-X, it was evident that Paenibacillus sp. D9 BioS displays the greatest dispersing ability (Fig. S1). Although SDS and Triton-X are good chemical surfactants having been used in many applications around the world, the negative impact of their use on the environment introduces a major drawback. Since the results obtained involved the use of minute volumes of BioS, it demonstrates the potential of this biomolecule to withstand much greater concentrations such as oil spills control and detoxification applications. Likewise in the present report, the BioS acquired from Bacillus licheniformis culture had the most minimal oil spreading activity (23 mm) in the crude oil-liquid medium while Bacillus firmus, Bacillus lentus, Pseudomonas paucimobilis, Serratia marcescens, and Micrococcus kristinae had 45 mm, 30 mm, 27 mm, 38 mm, and 51 mm, respectively [38].
Fabric Wash Performance and Formulations
The washing efficiency of Paenibacillus sp. D9 BioS formulation was compared with two chemical surfactants, namely anionic surfactant (SDS) and non-ionic surfactant (Triton X-100). This latter being anionic both act as a detergent and an emulsifier. In the present study, the synthetic surfactant displayed less washing efficacy in comparison with Paenibacillus sp. D9 BioS formulation. The Paenibacillus sp. D9 BioS influenced washing could eliminate more than 64.3% of tomato sauce and 60.4% of coffee stains while the chemical surfactants removed only 52.2% of tomato sauce, 47.1% of coffee (SDS), and 46.7% tomato sauce, 42.2% of coffee (Triton X-100) from the white cotton fabric respectively. These outcomes are as compared with rhamnolipid BioS formulation by Pseudomonas aeruginosa which was effective in removing whiteboard marker stains which was similar to chemical surfactants [41]. Also, the formulation Bacillus subtilis SPB1 BioS exhibited better cleaning efficiency on oil and tea stains removal as compared with the conventional chemical surfactant [13].
Given the differences in stains (such as a yellow solid containing phenolic, an acrylic group in coffee stain, caffeic acid, and curcuminoids), this makes them notorious and difficult to wash [42]. In this regard, there is an indication of BioS ability to remove most of these stains as efficiently and as well as detergent. For this, it will be important to correspond to Paenibacillus sp. D9 BioS formulation efficiency with two commercial detergents obtainable in Durban, South Africa. In the present study, the two commercial detergents produced a better washing and removal capability of different stains than when the BioS from Paenibacillus sp. D9 BioS was used alone. The results obtained are not far-fetched as the two commercial detergents have been processed industrially with additional chemicals and additives while the BioS was in its isolated pure form, which could have lost some of its cleaning properties during extraction, isolation, and purification processes. The stain removal by Paenibacillus sp. D9 BioS alone was less capable, but also comparable and effective. As observed, 64.3% tomato sauce and 60.4% coffee were removed when using Paenibacillus sp. D9 BioS and commercial detergent A removed 72.9% of tomato sauce and 67.7% of coffee stains, while 72.2% of tomato sauce and 67% of coffee stain were removed by commercial detergent B, respectively (Fig. 3). The biocommercial detergent a percentage similar to that for listed commercial detergent A and commercial detergent B (Fig. 3). Quite similar to the present study, others have described the commercial formulation was more effective than the natural products in stain removal from white cotton materials [43]. Moreover, the ability of sophorolipids, a glycolipid BioS synthesized by Candida bombicola (ATCC22214), was nearly equivalent to detergent in removing four types of stains (espresso, turmeric, oil, and poster) from cotton and polyester fabrics [42]. It has been demonstrated that stain removal by Pseudozma sp. NII 08165 glycolipid BioS alone was effective and practically identical to that of the commercial cleanser [44].
Comparison of the effect of different formulations for stain (coffee and tomato sauce) removal from fabric cotton. BioS, Paenibacillus sp. D9 biosurfactant; DA, detergent A; DB, detergent B; BCD, biocommercial detergent; SDS, sodium dodecyl sulfate. Values represent mean ± standard deviation (n = 3). The a–f and r–x superscript letters above histogram bars reflect significant difference (p < 0.05) if the letters are different
Finally, Paenibacillus sp. D9 BioS was supplemented with each of the two commercial detergents with the ratio of 1:1 (v:v) respectively. The 1:1 (v/v) BioS commercial detergent formulation gave an increase in wash performance (Fig. 3). This demonstrated that Paenibacillus sp. D9 BioS enhanced the positive outcome on the performance of the commercial formulated detergents rather than using commercial detergents alone. Similarly, Jatropha oil-derived sophorolipids BioS and detergent combination lead to an enhanced coffee stain elimination from cotton fabric rather than the detergent alone [42]. There was also substantial synergy on wash performance between Paenibacillus sp. D9 BioS and commercial surfactants in a proportional ratio of 1:1 (w/w) in the role of compost humic acid-like matter in the detergent formulation [45]. The detergent-like properties of the BioS open many applications with respect to laundry and detergent industries. Although lacking the additives present in commercial detergents, BioSs have shown promising results in their ability to reduce stains when compared with commercial detergents [13]. The formulation produced in the present investigation offers a favorable position in the expulsion of hydrophilic stains in contrast with other formulations presented. This lipopeptide BioS can be a productive surfactant and cleanser formulations. The stain eliminating potential of BioS-containing detergent is equivalent to manufactured ones particularly for the removal of hydrophilic and hydrophobic dangerous stains. The present research is noteworthy since microbial BioS can be considered as a substitute for chemical surfactants because of their low or non-toxicity and higher biodegradability.
Paenibacillus sp. D9 Biosurfactant Ecotoxicity
The germination index which combines overall seed germination and relative development of roots was utilized to assess the toxic effect of Paenibacillus sp. D9 BioS to cabbage and lettuce seeds. The proportion of ≤ 80% GI is considered as a positive indicator, thus supporting the absence of phytotoxicity [46]. The present results demonstrate that the different Paenibacillus sp. D9 BioS solutions tested produced inhibition of seed germination nor root development (Table 8). The germination index of Brassica oleracea (cabbage) was relatively higher than Lactuca sativa (lettuce) across all the concentrations of BioS tested. The germination index values of 103.4, 102.9, 104.9, and 117.1% were observed for the former while values of 92.6, 87.8, 89.8, and 94.7% for the latter at a BioS concentration of 1, 10, 100, and 200 mg/L, respectively. The development of auxiliary roots and the rise of leaves were additionally noticed for the different experimental conditions tested on Brassica oleracea and Lactuca sativa. Like the present research, higher GI values of 201, 128, 113, and 113% were observed for cabbage, and values of 189, 110, 105, and 96%, respectively for lettuce against different concentrations of Streptomyces sp. DPUA1566 BioS [23].
The Paenibacillus sp. D9 BioS solutions (in various concentrations 0, 1, 10, 100, 200 mg/L) were assessed for brine shrimp toxicity (Table 9). From this study, experimental tests with brine shrimp revealed that Paenibacillus sp. D9 BioS produced no toxicity at the different concentrations to brine shrimp. As observed, little mortality was observed for concentration close to CMC (100 mg/L) and CMC (200 mg/L). In the short-term bioassay, there was no sign of lethality toward the Artemia salina larvae after 24 h. Similar to the present research, others showed the absence of toxicity of BioS synthesized from Candida tropicalis at different concentrations of 0.25% and 0.5%, respectively [46]. Lipoprotein BioS by Streptomyces sp. DPUA1566 did not exhibit any form of mortality at different concentrations of BioS utilized [23]. In contrast, Bacillus subitillis BioS was shown to exhibit a minimal death rate (under 20%) when utilized at varying concentrations of 12.5, 25, and 50 mg/L, respectively [47]. The non-toxic effect of Paenibacillus sp. D9 BioS supports usefulness in different applications relative to soil and aquatic environments, as the biomolecule was confirmed to be ecological safe and environmentally friendly.
Conclusion
The present study supports Paenibacillus sp. D9 BioS economics by the utilization of possible low-cost materials. These outcomes demonstrated the use of waste frying oils (coconut and sunflower) to be utilized as viable substrates for the economic production of Paenibacillus sp. D9 BioS. The BioS was successful in dispersing engine oil, with further capability in removing different heavy metals from the environments including contaminated effluents, synthetic wastewater, contaminated sands, and food crops. In addition, Paenibacillus sp. D9 BioS can successfully singly or as components of commercial detergent formulation. The efficiency of Paenibacillus sp. D9 BioS-containing detergent is equivalent to commercial products in removing extreme hydrophilic and hydrophobic stains. The study confirmed the fundamental prospect of BioS synthesized by Paenibacillus sp. D9 in environmental and biotechnological applications.
References
De Almeida, D. G., Da Soares Silva, R. C. F., Luna, J. M., Rufino, R. D., Santos, V. A., Banat, I. M., & Sarubbo, L. A. (2016). Biosurfactants: promising molecules for petroleum biotechnology advances. Frontiers in Microbiology, 7, 1718.
Sarubbo, L. A., Lunaa, J. M., & Rufinoa, R. D. (2015). Application of a biosurfactant produced in low-cost substrates in the removal of hydrophobic contaminants. Chemical Engineering, 43, 295–300.
Jimoh, A. A., & Lin, J. (2019). Biosurfactant: a new frontier for greener technology and environmental sustainability. Ecotoxicology and Environmental Safety, 184, 109607.
Jimoh, A. A., & Lin, J. (2019). Heterologous expression of Sfp-type phosphopantetheinyl transferase is indispensable in the biosynthesis of lipopeptide biosurfactant. Molecular Biotechnology, 161(11), 836–851.
Santos, D. K. F., Rufino, R. D., Luna, J. M., Santos, V. A., & Sarubbo, L. A. (2016). Biosurfactants: multifunctional biomolecules of the 21st century. International Journal of Molecular Sciences, 17(3), 401.
Anyanwu, C., Obi, S., & Okolo, B. (2011). Lipopeptide biosurfactant production by Serratia marcescens NSK-1 strain isolated from petroleum-contaminated soil. Journal of Applied Sciences Research, 7, 79–87.
Syahriansyah, U. K. M., & Hamzah, A. (2016). Determination of optimum conditions and stability study of biosurfactant produced by Bacillus subtilis UKMP-4M5. Malaysian Journal of Analytical Sciences, 20, 986–1000.
Zhao, F., Shi, R., Cui, Q., Han, S., Dong, H., & Zhang, Y. (2017). Biosurfactant production under diverse conditions by two kinds of biosurfactant-producing bacteria for microbial enhanced oil recovery. Journal of Petroleum Science and Engineering, 157, 124–130.
Bezza, F. A., & Chirwa, E. M. (2015). Biosurfactant from Paenibacillus dendritiformis and its application in assisting polycyclic aromatic hydrocarbon (PAH) and motor oil sludge removal from contaminated soil and sand media. Process Safety and Environmental Protection, 98, 354–364.
Usman, M. M., Dadrasnia, A., Lim, K. T., Mahmud, A. F., & Ismail, S. (2016). Application of biosurfactants in environmental biotechnology; remediation of oil and heavy metal. AIMS Bioengineering, 3, 289–304.
Anjum, F., Gautam, G., Edgard, G., & Negi, S. (2016). Biosurfactant production through Bacillus sp. MTCC 5877 and its multifarious applications in food industry. Bioresource Technology, 213, 262–269.
Singh, J., & Kalamdhad, A. S. (2011). Effects of heavy metals on soil, plants, human health and aquatic life. International Journal of Research in Chemistry and Environment, 1, 15–21.
Bouassida, M., Fourati, N., Ghazala, I., Ellouze-Chaabouni, S., & Ghribi, D. (2018). Potential application of Bacillus subtilis SPB1 biosurfactants in laundry detergent formulations: compatibility study with detergent ingredients and washing performance. Engineering in Life Sciences, 18, 70–77.
Patowary, K., Patowary, R., Kalita, M. C., & Deka, S. (2017). Characterization of biosurfactant produced during degradation of hydrocarbons using crude oil as sole source of carbon. Frontiers in Microbiology, 8, 279.
Yañez-Ocampo, G., Somoza-Coutiño, G., Blanco-González, C., & Wong-Villarreal, A. (2017). Utilization of agroindustrial waste for biosurfactant production by native bacteria from Chiapas. Open Agriculture, 2, 341–349.
Jimoh, A. A., & Lin, J. (2019). Enhancement of Paenibacillus sp. D9 lipopeptide biosurfactant production through the optimization of medium composition and its application for biodegradation of hydrophobic pollutants. Applied Biochemistry and Biotechnology, 187(3), 724–743.
Jimoh, A. A., & Lin, J. (2019). Production and characterization of lipopeptide biosurfactant producing Paenibacillus sp. D9 and its biodegradation of diesel fuel. International journal of Environmental Science and Technology, 16, 4143–4158.
Deng, M. C., Li, J., Hong, Y. H., Xu, X. M., Chen, W. X., Yuan, J. P., Peng, J., Yi, M., & Wang, J. H. (2016). Characterization of a novel biosurfactant produced by marine hydrocarbon-degrading bacterium Achromobacter sp. HZ01. Journal of Applied Microbiology, 120, 889–899.
Sharma, D., Saharan, B. S., Chauhan, N., Procha, S., & Lal, S. (2015). Isolation and functional characterization of novel biosurfactant produced by Enterococcus faecium. SpringerPlus, 4, 4.
Dahrazma, B., & Mulligan, C. N. (2007). Investigation of the removal of heavy metals from sediments using rhamnolipid in a continuous flow configuration. Chemosphere, 69(5), 705–711.
Santos, D. K. F., Resende, A. H. M., de Almeida, D. G., Soares da Silva, R. C. F., Rufino, R. D., Luna, J. M., Banat, I. M., & Sarubbo, L. A. (2017). Candida lipolytica UCP0988 biosurfactant: Potential as a bioremediation agent and in formulating a commercial related product. Frontiers in Microbiology, 8, 767.
Andrade Silva, N. R., Luna, M. A., Santiago, A. L., Franco, L. O., Silva, G. K., de Souza, P. M., Okada, K., Albuquerque, C. D., Silva, C. A., & Campos-Takaki, G. M. (2014). Biosurfactant-and-bioemulsifier produced by a promising Cunninghamella echinulata isolated from caatinga soil in the northeast of Brazil. International Journal of Molecular Sciences, 15(9), 15377–15395.
Santos, E., Teixeira, M., Converti, A., Porto, A., & Sarubbo, L. (2018). Production of a new lipoprotein biosurfactant by Streptomyces sp. DPUA1566 isolated from lichens collected in the Brazilian Amazon using agroindustry wastes. Biocatalysis and Agricultural Biotechnology, 17, 142–150.
Lan, G., Fan, Q., Liu, Y., Chen, C., Li, G., Liu, Y., & Yin, X. (2015). Rhamnolipid production from waste cooking oil using Pseudomonas SWP-4. Biochemical Engineering Journal, 101, 44–54.
Partovi, M., Lotfabad, T. B., Roostaazad, R., Bahmaei, M., & Tayyebi, S. (2013). Management of soybean oil refinery wastes through recycling them for producing biosurfactant using Pseudomonas aeruginosa MR01. World Journal of Microbiology and Biotechnology, 29(6), 1039–1047.
Velioglu, Z., & Urek, R. O. (2016). Physicochemical and structural characterization of biosurfactant produced by Pleurotus djamor in solid-state fermentation. Biotechnology and Bioprocess Engineering, 21, 430–438.
Kim, J., & Vipulanandan, C. (2006). Removal of lead from contaminated water and clay soil using a biosurfactant. Journal of Environmental Engineering, 132, 777–786.
Sarubbo, L., Rocha Jr., R., Luna, J., Rufino, R., Santos, V., & Banat, I. (2015). Some aspects of heavy metals contamination remediation and role of biosurfactants. Chemistry and Ecology, 31, 707–723.
Juwarkar, A. A., Dubey, K. V., Nair, A., & Singh, S. K. (2008). Bioremediation of multi-metal contaminated soil using biosurfactant—a novel approach. Indian Journal of Microbiology, 48(1), 142–146.
Elouzi, A. A., Akasha, A. A., Elgerbi, A. M., El-Baseir, M., & El Gammudi, B. A. (2012). Removal of heavy metals contamination by bio-surfactants (rhamnolipids). Journal of Chemical and Pharmaceutical Research, 4, 4337–4341.
Hidayati, N., Surtiningsih, T., & Ni’matuzahroh. (2014). Removal of heavy metals Pb, Zn and Cu from sludge waste of paper industries using biosurfactant. Journal of Bioremediation & Biodegradation, 5, 255.
Akpor, O. (2011). Wastewater effluent discharge: effects and treatment processes. International Proceedings of Chemical, Biology, and Environmental Engineering, 20, 85–91.
Wen, J., Stacey, S. P., McLaughlin, M. J., & Kirby, J. K. (2009). Biodegradation of rhamnolipid, EDTA and citric acid in cadmium and zinc contaminated soils. Soil Biology and Biochemistry, 41, 2214–2221.
Meenakshisundaram, M., & Pramila, M. (2017). Detoxification of heavy metals using microbial biosurfactant. International Journal of Current Microbiology and Applied Sciences, 6, 402–411.
Giri, S. S., Sen, S. S., Jun, J. W., Sukumaran, V., & Park, S. C. (2017). Role of Bacillus licheniformis VS16-derived biosurfactant in mediating immune responses in Carp Rohu and its application to the food industry. Frontiers in Microbiology, 8, 514.
Ochoa-Loza, F. J., Noordman, W. H., Jannsen, D. B., Brusseau, M. L., & Maier, R. M. (2007). Effect of clays, metal oxides, and organic matter on rhamnolipid biosurfactant sorption by soil. Chemosphere, 66(9), 1634–1642.
Luna, J. M., Santos Filho, A. S., Rufino, R. D., & Sarubbo, L. A. (2016). Production of biosurfactant from Candida bombicola URM 3718 for environmental applications. Chemical Engineering, 49, 583–588.
Ibrahim, M. L., Ijah, U. J. J., Manga, S. B., Bilbis, L. S., & Umar, S. (2013). Production and partial characterization of biosurfactant produced by crude oil degrading bacteria. International Biodeterioration and Biodegradation, 81, 28–34.
Chandran, P., & Das, N. (2010). Biosurfactant production and diesel oil degradation by yeast species Trichosporon asahii isolated from petroleum hydrocarbon contaminated soil. International Journal of Engineering, Science and Technology, 2, 6942–6953.
Freitas, B. G., Brito, J. M., Brasileiro, P. P., Rufino, R. D., Luna, J. M., Santos, V. A., & Sarubbo, L. A. (2016). Formulation of a commercial biosurfactant for application as a dispersant of petroleum and by-products spilled in oceans. Frontiers in Microbiology, 7, 1646.
Turbekar, R., Malik, N., Dey, D., & Thakare, D. (2014). Development of rhamnolipid based white board cleaner. International Journal of Applied Sciences and Biotechnology, 2, 570–573.
Joshi-Navare, K., Khanvilkar, P., & Prabhune, A. (2013). Jatropha oil derived sophorolipids: production and characterization as laundry detergent additive. Biochemistry Research International, 2013, 11.
Khaje Bafghi, M., & Fazaelipoor, M. H. (2012). Application of rhamnolipid in the formulation of a detergent. Journal of Surfactants and Detergents, 15, 679–684.
Sajna, K. V., Sukumaran, R. K., Jayamurthy, H., Reddy, K. K., Kanjilal, S., Prasad, R. B., & Pandey, A. (2013). Studies on biosurfactants from Pseudozyma sp. NII 08165 and their potential application as laundry detergent additives. Biochemical Engineering Journal, 78, 85–92.
Savarino, P., Montoneri, E., Musso, G., & Boffa, V. (2010). Biosurfactants from urban wastes for detergent formulation: surface activity and washing performance. Journal of Surfactants and Detergents, 13, 59–68.
da Rocha Junior, R. B., Meira, H. M., Almeida, D. G., Rufino, R. D., Luna, J. M., Santos, V. A., & Sarubbo, L. A. (2018). Application of a low-cost biosurfactant in heavy metal remediation processes. Biodegradation, 30(4), 215–233.
de França, Í. W. L., Lima, A. P., Lemos, J. A. M., Lemos, C. G. F., Melo, V. M. M., de Sant’ana, H. B., & Gonçalves, L. R. B. (2015). Production of a biosurfactant by Bacillus subtilis ICA56 aiming bioremediation of impacted soils. Catalysis Today, 255, 10–15.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 3734 kb)
Rights and permissions
About this article
Cite this article
Jimoh, A.A., Lin, J. Biotechnological Applications of Paenibacillus sp. D9 Lipopeptide Biosurfactant Produced in Low-cost Substrates. Appl Biochem Biotechnol 191, 921–941 (2020). https://doi.org/10.1007/s12010-020-03246-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03246-5