Abstract
Allium jesdianum Boiss. & Buhse. is the most important species of the Amaryllidaceae family with various pharmacological properties. Three subsequent experiments (germination, callogenesis, and elicitation) were carried out as a completely randomized design with six replication. At the first study, the highest seed germination (78.33%) was achieved at chemical pre-treatment including the combination of α-naphthalene acetic acid (1 mg L−1) and benzylaminopurine (3 mg L−1) under in vitro condition. The highest callus induction (86.7%) was observed at MS/2 media, which was supplemented by NAA (1 mg L−1) and BAP (3 mg L−1) from hypocotyl explants. Then, two chemical elicitors including methyl jasmonate (MeJ) (0, 25, 50, and 100 µM) and putrescine (Pu) (0, 0.5, and 1 mM) were used to investigate their effects on different biochemical traits under callus culture. The results showed the superiority of MeJ over Pu for increasing the secondary metabolites and antioxidant activity in calluses of Allium jesdianum, compared to the control. The highest contents for total phenolics (6.02 mg GAE g−1 FW), total flavonoids (0.52 mg QE g−1 FW), and total flavonols (0.39 mg QE g−1 FW) were observed under 50 µM of MeJ. Meanwhile, the highest value for anthocyanin (8.99 µ mol g−1 FW) was achieved at 25 µM of MeJ. The highest 2,2-diphenyl-1-picrylhydrazyl activities were observed at 50 and 100 µM of MeJ. Putrescine (0.5 mM) elicitation showed only superiority for callus growth rate (0.53 mm day−1). Enhancement of desired secondary metabolites at 50 µM MeJ could be suitable for future studies in biotechnological aspects of this medicinal plant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The discovery of natural products with medicinal characteristics is the subject of intense research [1]. Plants with medicinal and nutritional properties are traditionally considered among the main sources of secondary metabolites (SMs) and natural drugs with antioxidant properties [1]. These SMs are important ingredients for producing different cosmetics, fragrances, flavors, food additives, and pharmaceutical and industrial products [2, 3].
The technique of plant cell culture is based on a set of biotechnological methods for producing valuable plant-specific SMs on large scale, independent from environmental conditions [4, 5]. In vitro culture techniques have been used for more producing of different phytochemical compounds as a promising bio-production way for desired and/or rare natural products with elite pharmacological properties [1, 6]. The production of these compounds is a dynamic defense response exhibited by plant cells when challenged by an elicitor. The scavenging of the reactive oxygen species (ROS) is triggered sufficiently under in vitro elicitation [7]. In vitro production of worthy compounds using callus culture has been very well recognized and is being used widely for different industrial applications [8].
The subsequent modulating and production of plant SMs occurs by regulating the expression of genes that are involved in producing key enzymes implicated in the biosynthesis of different defense-related compounds as phytoalexins, flavones, flavonoids, phenolics, and other bioactive compounds [2] under the elicitation process.
Elicitors can be divided into two types including abiotic and biotic [2, 8]. In about abiotic elicitors with non-biological origin they are grouped in chemical, physical and hormonal factors [8]. Among abiotic elicitors, chemical elicitors have been widely applied in recent years as an effective technique to enhance the production of different SMs with valuable pharmacological properties [8]. Chemical elicitors grouped into different types as heavy metals and hormones [8].
Jasmonates (JA) are considered an important chemical messenger for different biologic pathways such as elicitation for the biosynthesis of SMs and participating in enzyme activation under environmental stresses [9, 10]. Methyl jasmonate (MeJ) is an essential signaling compound in the intracellular signal cascades that ultimately leads to the hyper production of various SMs [8, 11]. According to previous studies in the stimulation of flavonoids and phenolic compounds production, jasmonates and their derivatives such as MeJ have been used in many plant families as elicitors under cell cultures [12,13,14].
Polyamines (PAs), as a group of phytohormone-like aliphatic amine natural compounds, play critical roles in the defense mechanism of plants against environmental stresses [15]. Polyamines are beneficial for protein homeostasis, detoxification of ROS, activation of the antioxidative machinery, and providing broad-spectrum tolerance against a variety of environmental stresses [16]. The PAs are also involved in many important cellular processes such as cell viability, cell division, and its prolongation, protein synthesis, replication of DNA, regulation of different physiological processes such as embryogenesis, floral development, and fruit ripening and senescence [15, 17, 18].
The putrescine (Pu) is used as one of the chemical plant elicitors which is in polyamines group [17]. This organic compound is considered as the primary source of other PAS species such as spermidine and spermine [17, 18]. The polyamines play a role in membrane fluidity, signal transduction, and RNA processing [19]. Many studies have shown progressing effects of PAS as elicitors on increasing the plant tolerance to environmental stresses such as drought and salinity stresses [16, 20]. But few studies have been reported that the effect of exogenous PAs is enhanced for somatic embryogenesis and shoot regeneration in some plants such as Cucumis anguria L. [21], Dactylis glomerata L. [22], and Nigella damascena L. [23] and also callus induction in some plant species as Hancornia speciosa Gomes [24]. On the other hand, some studies have done regarding the effects of Pu as an elicitor on callus level [19, 25].
Allium jesdianum Boiss (A. jesdianum) is an important endemic, threatened, and underutilized herbal species of Iran (known locally as “Bonsorkh” and “Yazdi onion”), which belongs to Allium genus in the Amaryllidaceae family. It grows in high altitudes (1800–2600 m) of Zagros Mountains in west and northwest regions of Iran [26, 27]. This species is eaten raw or as a cooked vegetable or is used as a flavor additive to fresh or cooked foods in Iran. In the field cultivation, its propagation rate is very slow, and it takes years to produce a new variety. In folk medicine of Iran, bulbs and leaves of this vegetable are used for the treatment of cold, kidney problems, and rheumatic pains [27]. Also, other medical properties as antibacterial [28] and anticancer [28] are reported for this valuable species. In this regard, many attempts have been made to isolate and exploit the active medicinal compounds from it.
The study of seed germination of medicinal plant species has received special attention due to the increased demand for these plants in the pharmacological industries [29]. Considering the potential medical values of A. jesdianum, there is an urgent need to develop strategies for the conservation of this genus by the breaking of its seed dormancy and callus induction methods for future exploitation aims through cell culture elicitations.
The present work aimed to optimize an efficient procedure for seed germination and callus induction of A. jesdianum, by the first. Then it aimed to evaluate the elicitation effects and suitable concentration of used elicitors (methy jasmonate and putrescine) on various physio-biochemical traits of A. jesdianum under callus culture.
Materials and Methods
Seed Collection
A. jesdianum seeds were gathered from the highland of Sabzkooh province (31° 27′ N 52° 40′ E mountain about 2,140 m height), in Chaharmahal-o-Bakhtiari region of Iran. A. jesdianum seeds were identified by the experts at the department of Natural Resources in Isfahan University of Technology using the Flora Iranica [30] and registered under the voucher name RIBB/AJ-1/2019 at the Herbarium of Research Institute of Biotechnology and Bioengineering, Isfahan University of Technology (IUT), Isfahan, Iran.
In Vitro Germination and Callus Induction
For in vitro evaluation studies, the seeds of A. jesdianum were disinfected with 70% (v/v) ethanol for 90 s and then surface sterilized thoroughly with 2% (v/v) sodium hypochlorite for 15 min. The seeds were then rinsed three times with sterile water to remove the remaining amount of disinfection liquid. For in vitro germination, the Murashige and Skoog [31] basal medium (Duchefa, Haarlem, the Netherlands) was used. The MS media adjusted to pH 5.8, which was supplemented with 3% sucrose (Merck Com.) (30 g L −1) agar (8 g L −1) (Sigma-Aldrich) and different physio-chemical treatments (Table 1). Eight number of seeds were incubated in each petri dishes (10 mm) under 16/8 day/night photoperiod at a light intensity of 110 µmol m−2 s−1, temperature of 24 ± 2 °C and 60% air humidity under aeration for 2 months. Six replicates were considered for each treatment in germination section.
For callus induction, two different explants (seed and hypocotyl) were used. For preparation of hypocotyl explants, the best treatment for seed germination was used (Table 1). Ten number of hypocotyls (4–6 mm long) and sterile seeds as explants were cultured in each replicates containing MS basal medium, 3% (w/v) sucrose and 0.8% (w/v) agar, which was supplemented with various combination of plant growth regulators (PGRs). The treatments for PGRs included [1 mg L−1 1-naphthaleneacetic acid (NAA) + 3 mg L−1 6-Benzylaminopurine (BAP)], [3 mg L−1 NAA + 1 mg L−1 BAP], [1 mg L−1 BAP + 2 mg L−1 2,4-dichlorophenoxyacetic (2, 4-D)] and [2 mg L−1 BAP + 1 mg L−1 2, 4-D]. The pH of the culture media was adjusted to 5.8 before autoclaving for 20 min at a pressure of 1.06 kg/cm2. Ten numbers of explants were cultured in each replicates for each medium. The six numbers of replicates were considered for each treatment in callus induction experiment. The cultures were incubated under 16 h light/8 h dark photoperiod at 25 ± 2 °C and photon flux of 90 µmol m−2 s−1 for 2 months in the culture chamber. The callus induction percent (CI) and callus growth rate (CGR) were measured. To calculate CI, callus induction was measured using the following equation: [(n/N) × 100], at the end of 2 months from the initial of callus induction. In this formulae, “n” represents the total number of callus explants, and “N” is the total number of cultured explants. Callus growth rate was calculated according to the means of callus growth rates (mm day−1) at every 15 days in 2 months period [32].
Methyl Jasmonate and Putrescine Elicitation
Two independent experiments were done to study the effects of different concentration of MeJ and Pu on phytochemical traits of A. jesdianum. Methyl jasmonate (Sigma-Aldrich, USA) was dissolved in pure ethanol and filtered using a micro filter of 0.22 μ pore size and then added to the MS medium with different concentrations. Putrescine (Sigma-Aldrich, USA) was dissolved double sterile water and then added to the MS medium with different concentrations through a micro filter of 0.22 μ pore size. For elicitation purposes, after 2 months of callus induction, the friable and white callus as equal fragments were transferred to new MS media containing 3 mg L−1 of BAP and 1 mg L−1 of NAA under different concentrations of MeJ (0, 25, 50, and 100 µM) and Pu (0, 0.5, and 1 mM). All treatments were incubated in the 16 h light/8 h dark photoperiod at 24 ± 2℃ and photon flux of 90 µmol m−2 s−1 in the culture chamber. Different phytochemical characteristics were measured under MeJ and Put elicitation after 4 weeks of incubation under six number of replicates per treatment.
Studied Traits Under Elicitation Process
Callus-Related Characteristics
Callus relative fresh weight (RFW) was according to this formulae: RFW = (FW2 − FW1)/days, where, FW1 is the fresh weight of the callus at the initiation of the elicitation and FW2 is the final fresh weight of the callus at the final day post of elicitation process. Callus growth rate was calculated at 15, 30, 45, and 60 day post callus transferring to elicitation media.
Lipid Peroxidation Assay
The amount of cell membrane damage was determined by measuring malondialdehyde (MDA) as the end product of peroxidation of membrane lipids. For this assay, 0.2 g of fresh calli was homogenized with 3 mL trichloroacetic acid (TCA) (0.1% w/v) (Merck, Com.). The homogenate was centrifuged for 30 min at 4000 rpm. Then the supernatant (5 mL) was collected and mixed with 4 mL of thiobarbituric acid (TBA) (0.5% w/v) and 20% TCA (w/v). Then, the sample was heated at 95 °C for 25 min and then placed on ice bath. Its absorbance was measured at 532 and 600 nm using a UV–Vis spectrophotometer (Unico-UV 2100). The content of MDA was calculated by an extinction coefficient of 155 mM−1 cm−1 and expressed as µmol g−1 FW [33].
Methanolic Callus Extract
At first, 0.5 g of completely dried callus were powdered and added to 10 mL of diethyl ether [(C2H5)2O]. The mixture was well mixed and stored in a refrigerator for 24 h. For complete evaporation of diethyl ether, the supernatant was transferred to dryer. Then 10 mL of 80% methanol were added to that purified supernatant and then filtered using a 0.4 μm filter.
Determination of Total Phenolic Content
Based on the Folin-Ciocalteu reagent method described by Singleton et al. [34], total phenolic content (TPC) was determined. In brief, 0.2 g fresh weight of celli was homogenized with 3 mL CH3OH (Merck. Com) and centrifuged at 4000 rpm for 25 min. Then, methanolic extract (0.5 mL) was mixed with 2.5 mL of Folin-Ciocalteu reagent (Sigma-Aldrich, Com), followed by the addition of 2.0 mL of 7% Na2CO3 solution. The mixture was disposed in the dark for 90 min at room temperature. The absorbance of the supernatant was recorded at 765 nm against the reagent blank by a spectrophotometer. The amount of TPC was then quantified by the method of calibration curve using gallic acid (Sigma-Aldrich, Com) as standard.
Determination of Total Flavonoid and Total Flavonol Content
Total flavonoid (TFD) and total flavonol (TFL) contents were determined by spectrophoto metrically using the method of Miliauskas et al. [35]. In brief, 0.2 g fresh calli was ground in 3 mL of methanol and centrifuged at 4000·rpm for 25 min. Then, supernatant was used to assay TFD and TFL contents. For estimation of TFL, 0.5 mL of methanolic extract, 0.5 mL of AlCl3 (2% w/v) solution, and 1.5 mL of sodium acetate (5% w/v) were mixed and after 90 min of incubation at room temperature, and the absorbance of mixture was measured at 445 nm. For total flavonoid 0.5 mL of methanolic extract, 125 µL of AlCl3 solution (%10 w/v) and 125 µL of CH3COOK (1 M) were mixed, and the samples were kept at room temperature for 30 min. A spectrophotometer was used to measure the absorbance of the reaction mixture at 415 nm with. The contents of total flavonoids and flavonols were expressed as mg quercetin (QE) equivalents per gram of fresh mass (mg QE g−1 FW).
Determination of Anthocyanin
At the first step, 2 mL acidified methanol (1% HCI) was used for homogenizing the fresh calli (0.2 g) at room temperature [36]. After one day, the total extract was centrifuged for 25 min at 4000 rpm. Then, the content of anthocyanin was determined by spectrophotometer at wave length of 511 nm based on the extinction coefficient of Raphanusin (33,000 M−1 cm−1).
DPPH Assay
Radical scavenging activities of safflower calli were determined by DPPH assay [37]. The methanolic extract (20 μL) was added to 1 mL of 50 μM DPPH (Sigma-Aldrich) solution in methanol. The prepared extracts ranged from 0 to 250 μg m L−1. The mixtures were mixed and incubated in the dark condition for 20 min. The reduction of DPPH absorption was measured at 515 nm. The positive control was ascorbic acid (Sigma-Aldrich). The DPPH radical scavenging activity was calculated using the equation: Inhibition concentration (inhibition percentage: IP %) = (absorbance control – absorbance sample)/(absorbance control) × 100.
Statistical Analysis
This study elicitation section was carried out as a factorial based on a completely randomized design with six replications. The germination and callus induction experiments were carried out as a completely randomized design. The analysis of variance is done using SAS software version 9.3 (SAS Institute, 2011). Then, mean comparisons (± standard deviations) were carried out using Fisher’s least significant difference (LSD 5%) test.
Results
Seed Germination
Different treatments including mechanical (scarification), chemical (different combination of plant growth regulators), and thermal conditions (cold temperature) were applied to enhance the germination ratio in A. jesdianum (Table 1). Evaluation of seed germination under different treatments showed extensive variation in GP in about 30–45 days after initiating the treatment. There was a statistically significant difference (P < 0.01) in GP and between different pre-germination treatments (Table 1). According to the data in Table 1, the maximum GP (78.33%) was observed in seeds treated with treatment including PGRs as NAA (1 mg L−1) + BAP (3 mg L−1). On the other hand, the minimum of GP (9.12%) was observed in control condition (Table 1).
Callus Induction
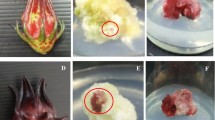
In the present investigation, callus initiation from explants (hypocotyl and seeds) was observed within 14–18 days on all medium compositions. The induced calli grew into yellow-to-greenish color with a semi-friable structure on four different media. The growth of calli was fast after their induction.
Analysis of variance showed a significant effect of treatments on CI and CGR (Table S2). Callus induction in all tested PGRs and explants were effective (Table 2). The effects of different PGRs (2,4-D, NAA, and BAP) and explants (seed and hypocotyl) on callus induction (%) and callus growth rate of A. jesdianum are presented at Table 2. As seems, this is the first report for callus induction in A. jesdianum. According to Table 2, MS/2 media supplemented with 1 mg L−1 NAA and 3 mg L−1 BAP were recognized as the best combination for highest callus induction (83.92%) in seeds explants of A. jesdianum (Table 2), which showed no significant difference with MS/2 media supplemented with NAA (1 mg L−1) + BAP (3 mg L−1) from hypocotyl explants (Table 2). The least value for CI (42.01%) was denoted to MS/2 media which was supplemented by 2 mg L−1 BAP and 1 mg L−1 2,4-D (Table 2). The highest CGR (0.48 mm day−1) was observed at seed explants (Table 2).
Elicitation Effects on Different Studied Traits Under Callus Culture
Callus Growth Traits
Overall, a decrease in relative fresh weight was observed in response to most of the treatments compared to the control (Table 3). The least content for RFW (0.60) was observed at 100 µM concentration of MeJ rather than control (0.42) (Table 3). The CGR showed variation from 0.17 (mm day−1) under 100 µM MeJ to 0.53 (mm day−1) at 0.5 mM Pu to (Table 3). For CGR, all elicitations resulted in a significant reduction compared to the control, except for 0.5 (mM) of Pu (Table 3).
Lipid Peroxidation
Callus evaluation revealed a significant increase in MDA content under treatments of MeJ and Pu (Table 3). This increase varied from 0.18 μmol g−1 FW in the control to 0.53 μmol g−1 FW in the samples treated with 1 mM Pu (Table 3). The MDA content showed a 2.94-fold increase (P < 0.05) in the calluses exposed to 1 mM (Pu) compared with the control cultures. According to the results, MDA content under 1 mM (Pu) was not significantly different from the control (Table 3).
Total Phenolic Content
According to the analysis of variance (ANOVA) results, there was a statistically significant difference for all studied traits under elicitation (Table S3). According to the findings, using MeJ and Pu resulted in a significant increase in TPC compared to control (Table 3). The highest (6.02 mg GAE g−1 FW) and the least (2.86 mg GAE g−1 FW) contents of TPC were observed at treatments of 50 mM MeJ and control treatments, respectively (Table 3). In callus cultures treated with 25, 50, and 100 µM of MeJ, the TPC showed 1.32-, 2.10-, and 1.01-fold increases compared to control, respectively (Table 3). When treated with 50 µM MeJ, callus cultures induced maximum corresponding TPC levels of 6.02 mg GAE g−1 FW, while 100 µM MeJ resulted in inhibited levels with the value 2.89 mg GAE g−1 FW on callus culture (Table 3). The callus treated with 0.5- and 1-mM Pu indicated 1.23-fold and 1.05-fold increases in TPC content, respectively (Table 3).
Total Flavonoid and Flavonol Contents
The TFD content showed a significant increase compared to the control (0.2 mg QE g−1 FW) up to 0.52 (mg QE g−1 FW) under 50 mM of MeJ (Table 3). For Pu elicitation, only the lower concentration level (0.5 mM) showed a significant difference with control (Table 3). The content of TFL showed variation from 0.24 (mg QE g−1 FW) in control to 0.39 (mg QE g−1 FW) under 50 µM MeJ (Table 3).
Anthocyanin Content
In the present study, elicitation by MeJ and Pu increased the in vitro accumulation of anthocyanin in the A. jesdianum calli (Table 3). Its values varied from 2.43 µmol g−1 FW at control condition to 8.99 µmol g−1 FW at 25 mM MeJ. Higher concentrations of MeJ (50 and 100 µM) showed a descending trend for Ant rather than 25 µM (MeJ) (Table 3). The concentration of 0.5 mM Pu led to a 1.21-fold increase in anthocyanins rather than control, but the higher concentration (1 mM Pu) did not have any significant effect compared to the control (Table 3).
Antioxidant Activity
In this study, antioxidant activity was determined as DPPH radical scavenging activity. The highest (86.40%) and the least (66.31%) radical scavenging activities were found in callus cultures treated with 100 mM MeJ and control conditions, respectively (Fig. 1). Callus cultures treated with 50 mM and 100 mM of MeJ showed no significant difference for reactive scavenging ability through the DPPH method. The DPPH activity under two different concentrations of Pu showed no significant effect for DPPH activity compared to control conditions (Fig. 1). It was observed that A. jesdianum calluses had a dose-dependent DPPH radical scavenging activity under MeJ elicitation (Fig. 1).
Correlation Between Different Traits
The simple correlation among studied traits is presented in Table 4. A negative and significant correlation was found between RWC with TFD (− 0.46**) and TFL (− 0.35**). The lipid peroxidation content as MDA showed a negative and significant correlation with RWC (− 0.64**) and CGR (− 0.53**) (Table 4).
Discussion
The phenomenon of seed dormancy in seeds of some medicinal plants is usually linked with internal factors such as physiological dormancy or physical barriers that cover the seed coat and/or the enclosed embryo [38]. The principle factors that influence seed dormancy include certain PGR, which GAs participate mainly in the termination of seed dormancy [39]. External application of PGRs to seeds could break seed dormancy and enhance seedling establishment of many medicinal plants [40]. The effect of different PGRs including 2,4-D, ethephon, GA3, IBA, and NAA on seed germination of some medicinal species as Ocimum basilicum [40], Coriandrum sativum [40], and Mentha arvensis [41] has been reported. Some treatments such as scarification and darkness helped break the dormancy of some Allium genus as Allium suworowii Regel., Allium aflatunense B. Fedtsch, and Allium altissimum Regel. at cold temperatures [42]. Enhancement of seed germination through breaking the seed dormancy is considered as a major gap in A. jesdianum. To the best of our knowledge, no previous study has reported regarding the improvement of seed germination in A. jesdianum through application of different pre-treatments. The differences in the GP of A. jesdianum seeds under different treatments suggest the significant impact of plant growth regulators including NAA and BAP on breaking the seed dormancy in this species. Therefore, from the above findings, it can be inferred that dormancy of the seeds of A. jesdianum was probably associated with the enclosed chemical factors around the embryo, which could be broken significantly through applying PGRs, specially NAA and BAP. It seems that the ecological background of the natural habitat for seed collection region and harvesting time plays predominant roles in seed germination capacity in A. jesdianum.
Despite the role of A. jesdianum as an important medicinal plant, no research has focused on using in vitro cultures in this neglected species. Callus induction was optimized by the first as the necessary step for developing callus cultures in further experiments, here. The findings showed no callus induction on complete MS media for A. jesdianum. It could be resulted that lower concentrations of macro and micro elements were better for cells differentiation from explants in this genus to callus production. The better responses of hypocotyl explants than seed ones for callus induction could be due to homogenous cell nature and easy availability of growth substances to each cell of homogenous tissues in hypocotyl explants. The combined effects of auxins and cytokinins have been considered as essential factors for callus induction in A. jesdianum. Comparison of four different treatment implied that higher concentrations of BAP (as cytokinin group) than 2,4-D and NAA (as auxins) could be promotive for more frequency in callus initiation and its growth in A. jesdianum. This finding was similar to previous reports in Allium schoenoprasum L [43] and Allium ampeloprasum L. [44]. Different with this finding, necessity of higher doses of auxins rather than cytokinins is emphasized for callus induction in other species in Amaryllidaceae family as Allium sativum [45] and Allium cepa [46] and micropropagation in Allium neapolitanum Cirillo [47]. According to reports of Khar et al. [48], lonely application of 2,4-D (0.5 mg L−1) was optimized for maximum callus formation in Allium cepa L. These inconsistencies can be due to the effects of many factors on callus induction in Allium species such as genus, genotypes, type of explants, medium composition, and plant growth used [47]. The highest (0.48 mm day−1) callus growth rate were observed in MS/2 media supplemented with 1 mg L−1 NAA and 3 mg L−1 BAP (Table 2), but the lowest one (0.18 mm day−1) was observed at MS/2 media which was supported by 2 mg L−1 BAP + 1 mg L−1 2,4-D (Table 2). This implied at a positive interactive effects of these concentrations of BAP and 2,4-D on used explant for enhancing the callus growth.
After elicitation process, the effects of different concentrations of MeJ and Pu on callus-related traits, MDA content as a sign of lipid peroxidation damage on cell walls, different phenolic compounds (TPC, TFD, TFL, and anthocyanins), photosynthetic pigments (chlorophyll and carotenoids), and antioxidant activity were evaluated. When studying the callus growth, the responses of relative fresh weight and callus growth rate were evaluated to different doses of MeJ and Pu. In view point of callus-related traits, the stressor effects of both used elicitors (especially at higher concentrations) reduced the RFW of calluses. This is probably due to the inhibitory effects of elicitors on cell growth and capacity of cell osmotic adjustment, which increase the requirement for maintaining turgor of the growing cells, consuming energy, and decrease in callus growth [32]. Similarly, higher concentrations of MeJ [49] and other elicitors as phenylalanine and mevalonic acid in Zingiber officinale [50] and chitosan in safflower [32] reduced the fresh weight of the callus. Similarly, an optimized concentration of MeJ (20 µM) produced the highest growth rate (98.21 mg/day) in callus culture of Dianthus caryophyllus L. (49). Such a response is different with findings of cell growth and callus diameter in Hypericum perforatum under MeJ elicitation [51]. According to this finding different concentrations of MeJ (125, 250, and 500 µM) reduced the callus diameter and fresh weigh of Hypericum perforatum calli [51]. These discrepancies could be compromised by the differences in elicitor concentration and plant species [2, 8]. The higher CGR at lower concentration of Pu (0.5 mM) indicates the beneficiary effects of lower concentrations of it on motivating callus growth. According to the reports of Bais et al. [52], putrescine application at 1.5 mM concentration increased the biomass of hairy roots of Beta vulgaris and Tagetes patula as 1.4-fold and 1.30-fold, respectively. It is well established that Pu stimulates not only callus cell extension but also its cell division and biomass at cellular level. Elicitation by these elicitors changed the color of calli to yellow-to-pale brown and pale brown after the elicitation period. The calli under Pu were more turbid. The elicitation process diminishes the friable structure of the calluses to an approximate extent.
During the elicitation process, some processes such as lipid peroxidation of membranes lead to enhancing antioxidant enzyme activity and the activation of SMs production [53]. Lipid peroxidation produces MDA as a sign of oxidative damage to scavenging ROS under elicitation [53]. In agreement with this study, exposure to MeJ significantly increased the MDA content in Panax ginseng [54] through suspension culture and other elicitors such as chitosan [32] through callus culture. The non-significant difference between MDA content at control and Pu (1 mM) could interestingly indicating at effective participating roles of Pu in sustaining membrane integrity in plant cells [55]. In confirmation of this finding, it could be mentioned at involving role of Pu in ROS signaling in plants and/or direct interaction of it with ROS under elicitation. Similarly, it has been reported that exogenous application of Pu reduces the severity of oxidative damage in welsh onion by increasing the antioxidant capacity of the plant [55]. This may suggest that a higher concentration (> 0.5 mM Pu) could act as a stressor agent on calli cells.
Plants employ a different variety of defense response pathways in response to environmental stresses such as elicitor exposure at cellular levels [56]. These cellular responses lead to the more production and accumulation of several SMs such as total phenolics and their derivatives (total flavonoids, total flavonols and total anthocyanins) [2, 3, 56]. Among chemical elicitors, MeJ has been used widely for in vitro elicitation studies as callus culture systems [10, 49, 54, 57], but fewer studies demonstrated the effects of Pu elicitation on accumulation of different SMs under callus [19, 25] and hairy root cultures [52].
In Allium species, some studies have reported the effects of elicitation on some SMs and their content in the whole plant [27, 58], but to the best of our knowledge, no study has evaluated changes in SMs under elicitation process in A. jesdianum at callus level. Similar to this finding, the enhancing effect of lower concentrations of MeJ (50 µM) on increase of TPC was reported on other species through callus cultures of Zanthoxylum stenophyllum [59] and suspension cultures in Artemisia absinthium L. [60], Ajuga bracteosa [61], Polygonum multiflorum [9], Thevetia peruviana [14], and Panax ginseng [54]. The positive effects of elicitation by MeJ are also reported an increase of and some specific metabolites as ursolic acid and oleanolic acid [57] and eugenol content in Dianthus caryophyllus L. [49].
The effect of in vitro elicitation of Pu on TFD accumulation has been rarely reported in literature review; however, the results showed its positive effect on increasing some phenolic acids (e.g., ferulic, caffeoylquinic, and sinapic acids) in Gardenia jasminoides L. by the concentration of 0.5 mg L−1 of it [62]. Our finding was also like the positive effects of MeJ on increase in TFD of Thevetia peruviana [14] and Hypericum perforatum [63] through callus culture and cell suspension culture of Panax ginseng [54], Taxus baccata [64], and Ajuga bracteosa [61]. This significant increase can be attributed to the possible positive effects of used elicitors at specific concentration on the enhanced expression of the genes, contributing to the synthesis pathway of total flavonoids (e.g., phenylpropanoid) [65]. The incidence of non-significant differences between the two levels of Pu with control for the TFL content may be concluded that Pu elicitation did not provide adequate efficiency for increasing TFL in calluses of A. jesdianum and thus other concentrations should be applied.
Polyamines as putrescine had a significant effect on accumulation of flavonols, hydroxybenzoic, and hydroxycinnamic acid derivatives in Cucumis anguria L. [21]. Similarly, the increasing effects of polyamines are reported on the amount of withanolides in regenerated Withania somnifera plants [66]. In this regards, different concentrations of putrescine (0.1, 0.2, and 0.4 mg L−1) were applied for significant increase of capsaicin in Capsicum annuum L. through cell suspension cultures [25]. In jojoba plants, putrescine (50 ppm) significantly increased TPC, TFD, tannin content, and antioxidant activity [67]. Gharib and Hanafy Ahmed [68] reported that putrescine foliar application (1–2 ppm) improved total phenolic compounds in Pisum sativum shoots [68]. Reviewing the literature shows no study on the elicitation effects of Pu (as a compound in polyamines) on TFD and TPC under callus culture.
Anthocyanins are a group of water-soluble flavonoids that are produced at one end of the flavonoid biosynthesis pathway in the cytoplasm and enter the cells actively or separately by glutathione pump [69]. Anthocyanins production is affected under different environmental elicitors [69]. The additional result obtained indicated that a concentration of 50 µM MeJ was the best that could induce the highest anthocyanin production in this study. The results of this study are consistent with those of Ram et al. [70] and Suan See et al. [71], who reported that the quantity of anthocyanin accumulation depends on MeJ concentration in Rosa hybrida and Melastoma malabathricum, respectively. According to Suan See et al. [71], biosynthesis and accumulation of anthocyanin could be stimulated by MeJ elicitation via induction of particular enzymes such as catalyzes. Besides, synthetic pathways for anthocyanin biosynthesis or increase the gene(s) expression of this specialized metabolite biosynthesis are triggered by MeJ elicitation [70]. The supportive effect of MeJ for anthocyanin accumulation was similar to the significant effects of MeJ on in vitro enhancement of anthocyanin in Daucus carota under callus culture [72]. Therefore, at higher concentrations, both elicitors had an inhibitory or non-significant effect on Ant content in A. jesdianum. Among these two elicitors, MeJ acted as a better inducer of anthocyanin biosynthesis in A. jesdianum callus cultures than Pu. The novel results from the present study show that callus cultures of A. jesdianum under elicitation could be considered as an efficient way for these valuable pigments production.
To neutralize the effect of stresses caused through the elicitation process, the plant cells employ antioxidative activities in different ways such as enhancement of SMs [10, 32]. The findings based on DPPH assay suggest that A. jesdianum is a good free radical scavenger under elicitation. Similarly, the increase in antioxidant activity of callus cells (e.g., DPPH method) was observed under MeJ elicitation in different cell culture techniques [13]. The significant increase in DPPH activity is reported under root suspension cultures in Panax ginseng [54].
Totally, the medium concentration of MeJ (50 µM) and a lower concentration of Pu (0.5 mM) showed an enhancement effect on the contents of phenolics, flavonoids, flavonols, and anthocyanin. This result could be attributed to higher production of trans-cinnamate as a product of L-phenylalanine deamination by phenylalanine ammonia-lyase enzyme and more convention of trans-cinnamate to different SMs under lower concentrations of used elicitors [73]. In this regard, the following factors have significant effects on the trends of the elicitation process: the differences in chemical characteristics of elicitors, its preparation source and concentration, exposure time of elicitation, age and type of explant for culture, growth regulations in media culture and nutrition composition, and its interaction with plant species and genotypes [2, 8]. Considering that the production of plant SMs is strongly controlled by signaling events and environmental factors, a fine and one-by-one analysis might be required to obtain a deeper understanding of the type of their modulation effects in different plant species at the callus level. The significant increase in different phenolic content may be compromised by the inducing effect on trigging effects of MeJ and Pu on signal transduction pathways. These effects, in turn, speed up enzyme catalysis and lead to the formation of specific compounds such as flavonoids, flavonols, and anthocyanins in A. jesdianum.
Correlation studies between different biochemical traits showed the importance of cell wall integrity on retaining cell water content and its growth under cellular conditions. The antioxidant activity (DPPH method) showed positive and significant correlation with TFL (0.43**) and TPC (0.33**). The antioxidant activity through the DPPH method was found to be mainly TPC (0.33**) and TFL (0.43**), depending on callus cultures treated with different elicitors. Furthermore, a positive correlation between TPC and TFL with DPPH suggests that the elicitation process increased the antioxidant activity of A. jesdianum through more accumulation of TPC and TFL in callus culture.
Conclusion
Plant cell cultures have been perceived as an attractive method for producing SMs under controlled conditions. This study optimized seed germination, callus induction, elicitation effects of two different elicitors (MeJ and Pu) on the enhancement of different secondary metabolites, callus-related traits, and antioxidant activity in A. jesdianum, by the first. The findings showed higher efficiency of MeJ than Pu for increasing different secondary metabolites and antioxidant activity in calli of A. jesdianum. The Pu elicitation showed superiority on increase of callus growth. The concentration of 50 µM MeJ showed the best selective dose for enhancing the contents of total phenolics (total flavonoids and total flavonols) in A. jesdianum through callus culture. This study could lead to introducing a new elicitation method to improve the production of the beneficial phytochemical classes from undifferentiated callus cells in the threatened medicinal plant of A. jesdianum. It is suggested to evaluate the commercially production of specific metabolites in these major classes through bioreactor or cell suspension culture techniques in future.
Availability of Data and Materials
No applicable.
References
Yue, W., Ming, Q., Lin, B., Rahman, K., Zheng, C. J., Han, T., & Qin, L. P. (2016). Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Critical Reviews in Biotechnology, 36(2), 215–232.
Namdeo, A. G. (2007). Plant cell elicitation for production of secondary metabolites: A review. Pharmacognosy Reviews, 1(1), 69–79.
Erb, M., & Kliebenstein, D. J. (2020). Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiology, 184(1), 39–52.
Smetanska, I. (2008). Production of secondary metabolites using plant cell cultures. Food Biotechnology, 111, 187–228.
Vanisree, M., Lee, C. Y., Lo, S. F., Nalawade, S. M., Lin, C. Y., & Tsay, H. S. (2004). Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Botanical Bulletin of Academia Sinica, 45(1), 1–22.
Dias, M. I., Sousa, M. J., Alves, R. C., & Ferreira, I. C. (2016). Exploring plant tissue culture to improve the production of phenolic compounds: A review. Industrial Crops and Products, 82, 9–22.
Matkowski, A. (2008). Plant in vitro culture for the production of antioxidants-a review. Biotechnology Advances, 26(6), 548–560.
Narayani, M., & Srivastava, S. (2017). Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochemistry Reviews, 16(6), 1227–1252.
Thiruvengadam, M., Rekha, K., Rajakumar, G., Lee, T. J., Kim, S. H., & Chung, I. M. (2016). Enhanced production of anthraquinones and phenolic compounds and biological activities in the cell suspension cultures of Polygonum multiflorum. International Journal of Molecular Sciences, 17(11), 1912.
Nabi, N., Singh, S., & Saffeullah, P. (2021). Responses of in vitro cell cultures to elicitation: regulatory role of jasmonic acid and methyl jasmonate: a review. In Vitro Cellular and Developmental Biology - Plant, 1–15.
Singh, A., & Dwivedi, P. (2018). Methyl-jasmonate and salicylic acid as potent elicitors for secondary metabolite production in medicinal plants: A review. Journal of Pharmacognosy Phytochemistry, 7(1), 750–757.
Gabr, A. M., Ghareeb, H., El Shabrawi, H. M., Smetanska, I., & Bekheet, S. A. (2016). Enhancement of silymarin and phenolic compound accumulation in tissue culture of milk thistle using elicitor feeding and hairy root cultures. Journal of Genetic Engineering and Biotechnology, 14(2), 327–333.
Ho, T. T., Murthy, H. N., & Park, S. Y. (2020). Methyl jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell and organ cultures. International Journal of Molecular Sciences, 21(3), 716.
Mendoza, D., Arias, J. P., Cuaspud, O., Ruiz, O., & Arias, M. (2018). Methyl jasmonate/salicylic acid enhanced flavonoid production and change metabolites profile in Thevetia peruviana cell culture. In Vitro Cellular and Developmental Biology- Plant, 54, 4–27.
Chen, D., Shao, Q., Yin, L., Younis, A., & Zheng, B. (2019). Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Frontiers in Plant Science, 9, 1945.
Alcazar, R., Bueno, M., & Tiburcio, A. F. (2020). Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells, 9(11), 2373.
Jimenez Bremont, J. F., Marina, M., Guerrero-Gonzalez, M. D. L. L., Rossi, F. R., Sanchez-Rangel, D., Rodriguez-Kessler, M., Rui, O. A., & Garriz, A. (2014). Physiological and molecular implications of plant polyamine metabolism during biotic interactions. Frontiers in Plant Science, 5, 95.
Rangan, P., Subramani, R., Kumar, R., Singh, A. K., & Singh, R. (2014). Recent advances in polyamine metabolism and abiotic stress tolerance. BioMed Research International, 2014, 1–9.
Rakesh, B., Sudheer, W. N., & Nagella, P. (2021). Role of polyamines in plant tissue culture: An overview. Plant Cell Tissue and Organ Culture, 1–20.
Mustafavi, S. H., Badi, H. N., Sękara, A., Mehrafarin, A., Janda, T., Ghorbanpour, M., & Rafiee, H. (2018). Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiologiae Plantarum, 40, 102.
Thiruvengadam, M., & Chung, I. M. (2015). Phenolic compound production and biological activities from in vitro regenerated plants of gherkin (Cucumis anguria L.). Electronic Journal of Biotechnology, 18(4), 295–301.
Li, Z., & Burritt, D. J. (2003). Changes in endogenous polyamines during the formation of somatic embryos from isogenic lines of Dactylis glomerata L. with different regenerative capacities. Plant Growth Regulation, 40, 65–74.
Klimek-Chodacka, M., Kadluczka, D., Lukasiewicz, A., Malec-Pala, A., Baranski, R., & Grzebelus, E. (2020). Effective callus induction and plant regeneration in callus and protoplast cultures of Nigella damascena L. Plant Cell Tissue and Organ Culture, 143(3), 693–707.
Fraguas, C. B., Villa, F., & Lima, G. P. P. (2009). Evaluation of exogenous application of polyamines on callus growth of Mangaba tree (Hancornia speciosa Gomes). Revista Brasileira de Fruticultura, 31(4), 1206–1210.
Esra, K., Işlek, C., & Karayigit, B. (2020). Effects of spermine and putrescine polyamines on capsaicin accumulation in Capsicum annuum L. cell suspension cultures. Acta Agriculturae Slovenica, 115(2), 369–375.
Kalantari, H., Shamsi Ehsan, T., Samimi, A., Kheradmand, P., Shirani, M., & Zeidooni, L. (2018). Histopathological and biomedical parameters determination in the protective effect of hydroalcoholic extract of Allium jesdianum on hepatotoxicity induced by bromobenzene in mice. Iranian Journal of Pharmaceutical Sciences, 14(2), 15–24.
Pirbalouti, A. G. (2019). Phytochemical and bioactivity diversity in the extracts from bulbs and leaves of different populations of Allium jesdianum, a valuable underutilized vegetable. Acta scientiarum Polonorum. Hortorum cultus, 18(2), 115–122.
Dorosti, N., Zarabi, S., Ahmadi, S., Rostami, R., & Rashidi Pour, M. (2017). Anticancer activity evaluation of methanolic extract of Allium Jesdianum and Nectaroscordeum Coelzi against HeLa and K562 cell lines. Yafte, 19(1), 31–41.
Rasool Hassan, B. A. (2012). Medicinal plants (importance and uses). Pharmaceutica Analytica Acta, 3(10), 2153–2435.
Rechinger, K. H. 1963. Flora Iranica. No. 158. Akademische Druke-U, Verlagsanstalt Wien Austria. 49–71.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473–497.
Golkar, P., Taghizadeh, M., & Yousefian, Z. (2019). The effects of chitosan and salicylic acid on elicitation of secondary metabolites and antioxidant activity of safflower under in vitro salinity stress. Plant Cell Tissue and Organ Culture, 137(3), 575–585.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125(1), 189–198.
Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology, 299, 152–178.
Miliauskas, G., Venskutonis, P., & Van Beek, T. (2004). Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chemistry, 85(2), 231–237.
Hara, M., Oki, K., Hoshino, K., & Kuboi, T. (2003). Enhancement of anthocyanin biosynthesis by sugar in radish (Raphanus sativus) hypocotyl. Plant Science, 164(2), 259–265.
Golkar, P., & Taghizadeh, M. (2018). In vitro evaluation of phenolic and osmolite compounds, ionic content, and antioxidant activity in safflower (Carthamus tinctorius L.) under salinity stress. Plant Cell Tissue Organ Culture, 134(3), 357–368.
Abbas, M. S., El-Shabrawi, H. M., Soliman, A. S., & Selim, M. A. (2018). Optimization of germination, callus induction, and cell suspension culture of African locust beans Parkia biglobosa (Jacq) Benth. Journal of Genetic Engineering and Biotechnology, 16(1), 191–201.
Dewir, Y. H., El-Mahrouk, M. E. S., & Naidoo, Y. (2011). Effects of some mechanical and chemical treatments on seed germination of Sabal palmetto and Thrinax morrisii palms. Australian Journal of Crop Science, 5(3), 248–253.
Elhindi, K. M., Dewir, Y. H., Asrar, A. W., Abdel-Salam, E., El-Din, A. S., & Ali, M. (2016). Improvement of seed germination in three medicinal plant species by plant growth regulators. Horticultural Science, 51(7), 887–891.
Wen-hao, N., & Yan, Z. (2012). Effects of different hormone pretreatments on the germination of Mentha arvensis (peppermint) seeds. Medicinal Plant, 3(11), 64.
Kamenetsky, R., & Gutterman, Y. (2000). Germination strategies of some Allium species of the subgenus Melanocrommuyum from arid zone of Central Asia. Journal of Arid Environments, 45(1), 61–71.
Zdravkovic-Korac, S., Milojevic, J., Tubić, L., Calic-Dragosavac, D., Mitic, N., & Vinterhalter, B. (2010). Somatic embryogenesis and plant regeneration from root sections of Allium schoenoprasum L. Plant Cell Tissue and Organ Culture, 101(2), 237–244.
Monemi, M. B., Kazemitabar, S. K., Khaniki, G. B., Yasari, E., Sohrevardi, F., & Pourbagher, R. (2014). Tissue culture study of the medicinal plant leek (Allium Ampeloprasum L). International Journal of Molecular and Cellular Medicine, 3(2), 118.
Dixit, V., Rai, S. P., & Chaudary, B. R. (2013). Allium sativum: Four-step approach to efficient micropropagation. International Journal of Innovative Biology Research, 2(1), 6–14.
Bekheet, S. A., Taha, H. S., & Solliman, M. E. (2006). Salt tolerance in tissue culture of onion (Allium cepa L.). Arab Journal of Biotechnology, 9(3), 467–476.
Stelmaszczuk, M., & Kozak, D. (2013). Micropropagation of Allium neapolitanum Cirillo. Acta Scientiarum Polonorum Hortorum Cultus, 12(5), 193–206.
Khar, A., Bhutani, R. D., Yadav, N., & Chowdhury, V. K. (2005). Effect of explant and genotype on callus culture and on regeneration in onion (Allium Cepa L.). Akdeniz üniversitesi ziraat fakültesi dergisi., 18(3), 397–404.
Matter, M. A., Hanafy, M. S., & Aly, U. I. (2017). Effect of methyl jasmonate and mannitol application on growth and eugenol content in callus cultures of carnation. Plant Tissue Culture and Biotechnoloy, 27(2), 227–240.
El-Nabarawy, M. A., El-Kafafi, S. H., Hamza, M. A., & Omar, M. A. (2015). The effect of some factors on stimulating the growth and production of active substances in Zingiber officinale callus cultures. Annals of Agricultural Science, 60(1), 1–9.
Abdollahipoor, M., Kalantari, S., Azizi, M., & Saadat, Y. A. (2017). Effects of methyl jasmonate and chitosan on shoot and callus growth of Iranian Hypericum perforatum L. in vitro Cultures. Journal of Medicinal Plants by- Products, 6(2), 165–172.
Bais, H. P., Madhusudhan, R., Bhagyalakshmi, N., Rajasekaran, T., Ramesh, B. S., & Ravishankar, G. A. (2000). Influence of polyamines on growth and formation of secondary metabolites in hairy root cultures of Beta vulgaris and Tagetes patula. Acta Physiologiae Plantarum, 22(2), 151–158.
Das, K., & Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science, 2, 53.
Ali, M. B., Hahn, E. J., & Paek, K. Y. (2007). Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension culture. Molecules, 12(3), 607–621.
Yiu, J. C., Juang, L. D., Fang, D. Y. T., Liu, C. W., & Wu, S. J. (2009). Exogenous putrescine reduces flooding-induced oxidative damage by increasing the antioxidant properties of welsh onion. Scientia Horticulturae, 120(3), 306–314.
Ramirez-Estrada, K., Vidal-Limon, H., Hidalgo, D., Moyano, E., Golenioswki, M., Cusidó, R. M., & Palazon, J. (2016). Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules, 21(2), 182.
Martinez, V. M. V., Estrada-Soto, S. E., de Jesus Arellano-Garcia, J., Rivera-Leyva, J. C., Castillo-Espana, P., Flores, A. F., Cardoso-Taketa, A. T., & Perea-Arango, I. (2017). Methyl jasmonate and salicylic acid enhanced the production of ursolic and oleanolic acid in callus cultures of Lepechinia caulescens. Pharmacognosy Magazine, 13(4), S886.
Alwan, A. H., Twaij, B. M., & Alwan, B. H. (2020). Induction of Allium sativum tissue culture by l-methionine and gibberellic acid and study of the effect of extract against fungal plant pathogens. Plant Archives, 20(1), 2839–2844.
Biondi, S., Antognoni, F., Perellino, N. C., Sacchetti, G., Minghetti, A., & Poli, F. (2004). Medium composition and methyl jasmonate influence the amount and spectrum of secondary metabolites in callus cultures of Zanthoxylum stenophyllum Hemsl. Plant Biosystem, 138(2), 117–124.
Ali, M., Abbasi, B. H., & Ali, G. S. (2015). Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant Cell Tissue and Organ Culture, 120(3), 1099–1106.
Saeed, S., Ali, H., Khan, T., Kayani, W., & Khan, M. A. (2017). Impacts of methyl jasmonate and phenyl acetic acid on biomass accumulation and antioxidant potential in adventitious roots of Ajuga bracteosa Wall ex Benth., a high valued endangered medicinal plant. Physiology and Molecular Biology of Plants, 23(1), 229–237.
Al-Maameri, A. A. K., & Almyali, A. A. H. (2020). Effect of putrescine and type of light in callus of Gardenia Jasminoides L. content from some effective medical compounds. In IOP Conference Series: Materials Science and Engineering (Vol. 871, No. 1, p. 012017). IOP Publishing.
Wang, J., Qian, J., Yao, L., & Lu, Y. (2015). Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresources and Bioprocessing, 2(1), 1–9.
Jalalpour, Z., Shabani, L., Afghani, L., Sharifi-Tehrani, M., & Amini, S. A. (2014). Stimulatory effect of methyl jasmonate and squalestatin on phenolic metabolism through induction of LOX activity in cell suspension culture of yew. Turkish Journal of Biology, 38(1), 76–82.
Talukder, P., Talapatra, S., Ghoshal, N., & Sen Raychaudhuri, S. (2016). Antioxidant activity and high-performance liquid chromatographic analysis of phenolic compounds during in vitro callus culture of Plantago ovata Forsk. and effect of exogenous additives on accumulation of phenolic compounds. Journal of the Science of Food and Agriculture, 96(1), 232–244.
Sivanandhan, G., Mariashibu, T. S., Arun, M., Rajesh, M., Kasthurirengan, S., Selvaraj, N., & Ganapathi, A. (2011). The effect of polyamines on the efficiency of multiplication and rooting of Withania somnifera (L.) Dunal and content of some withanolides in obtained plants. Acta Physiologiae Plant, 33(6), 2279.
Taha, L. S., Taie, H. A., & Hussein, M. (2015). Antioxidant properties, secondary metabolites and growth as affected by application of putrescine and moringa leaves extract on jojoba plants. Journal of Applied Pharmaceutical Science, 5(01), 030–036.
Gharib, A. A., & Hanafy Ahmed, A. H. (2005). Response of pea (Pisum sativum, L.) to foliar application of putrescine, glucose, folia feed D and silicon. Journal of Agricultural Science Mansoura University, 30(12), 7563–7579.
Castaneda-Ovando, A., de Lourdes Pacheco-Hernandez, M., Paez-Hernandez, M. E., Rodriguez, J. A., & Galan-Vidal, C. A. (2009). Chemical studies of anthocyanins: A review. Food Chemistry, 113(4), 859–871.
Ram, M., Prasad, K. V., Singh, S. K., Hada, B. S., & Kumar, S. (2013). Influence of salicylic acid and methyl jasmonate elicitation on anthocyanin production in callus cultures of Rosa hybrida L. Plant Cell Tissue and Organ Culture, 113(3), 459–467.
Suan See, K., Bhatt, A., & Lai Keng, C. (2011). Effect of sucrose and methyl jasmonate on biomass and anthocyanin production in cell suspension culture of Melastoma malabathricum (Melastomaceae). Revista de Biologia Tropical, 59(2), 597–606.
Sudha, G., & Ravishankar, G. A. (2003). Elicitation of anthocyanin production in callus cultures of Daucus carota and the involvement of methyl jasmonate and salicylic acid. Acta Physiologiae Plantarum, 25(3), 249–256.
Alrawaiq, N. S., & Abdullah, A. (2014). A review of flavonoid quercetin: Metabolism, bioactivity and antioxidant properties. International Journal PharmTech Research, 6(3), 933–941.
Acknowledgements
The authors would like to thank Research Institute for Biotechnology and Bioengineering, Isfahan University of Technology, Isfahan, Iran, for supporting the practical part of this work. The authors would also thanks from Mostafa Abdollahi Bakhtiari for helping us in experimental sections of elicitation section.
Author information
Authors and Affiliations
Contributions
Esmat Yazdanian done the experimental sections of the study. Dr. Pooran Golkar done the conceptualization, data analysis, material preparation, writing original draft, reviewing, and final editing. Under the supervision of Dr. Mohammad Reza Vahabi, conceptualization and experimental sections were done. Dr. Marzieh Taghizadeh contributed on traits measurement and writing original draft of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
No applicable.
Consent to Participate
No applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yazdanian, E., Golkar, P., Vahabi, M.R. et al. Elicitation Effects on Some Secondary Metabolites and Antioxidant Activity in Callus Cultures of Allium jesdianum Boiss. & Buhse.: Methyl Jasmonate and Putrescine. Appl Biochem Biotechnol 194, 601–619 (2022). https://doi.org/10.1007/s12010-021-03643-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03643-4