Abstract
This study was undertaken to investigate the effects of salicylic acid (SA) and methyl jasmonate (MeJA) on anthocyanin induction, biomass accumulation, and color value (CV) indices for both pigment content (PC) and pigment production (PP) in callus cultures of Rosa hybrida cv. Pusa Ajay. A concentration-dependent response was exhibited by cultures on SA and MeJA at different concentrations individually or in combinations to Euphorbia millii medium supplemented with 204.5 mM sucrose, 2.45 μM indole butyric acid and 2.33 μM kinetin. There was positive influence on both callus biomass and anthocyanin accumulation. Treatment with 0.5 μM MeJA was most effective in inducing anthocyanin biosynthesis in callus cultures. Anthocyanin accumulation in callus cultures was enhanced with the addition of SA and MeJA, but these did not differ significantly from control for the number of days required for pigment initiation and for color intensification. Moreover, the addition of 0.5 μM MeJA alone resulted in a higher frequency of color response (97.25 %), PC (3.48 ± 0.07 CV g−1 FW), and PP (1.56 ± 0.03 CV test tube−1) over control. In contrast, the presence of higher levels of SA (400 μM) and MeJA (5.0 μM) reduced frequency of color response, as well as levels of PC and PP. MeJA did not increase biomass accumulation but promoted frequency of color response, PC and PP. Hence, it was suggested that 0.5 μM MeJA promoted anthocyanin production in rose callus cultures. Significant correlation was found between frequency of response and each of the PC (r = 0.988) and PP (r = 0.990). Furthermore, PC and PP were also highly correlated (r = 0.998).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthocyanins are water-soluble plant pigments which appear in plants in colors ranging from scarlet to blue. Accumulation of anthocyanins is observed in flowers, fruits, leaves and storage tissues of many plants (Schwinn and Davies 2004; Tanaka et al. 2008). The interesting biological activities of anthocyanin with several well recognized dietary benefits in human health have prompted intensive research on these compounds and their effects on human health. Besides being present in the human diet at relatively high concentrations, anthocyanins are not only used as the alternative to artificial red dyes but also for their medicinal properties (Lila 2004; Stintzing and Carle 2004). Recently, interest in anthocyanins has been intensified because these pigments are not only effective antioxidants but also have multifaceted nutraceutical and pharmaceutical activities. Due to antioxidant capacities these pigments provide health benefits such as antitumor, anticancer activities, suppression of inflammatory responses, protection against age-related decline in cognitive behaviour and neuronal dysfunction (Downham and Collins 2000; Simões et al. 2009; de Pascual-Teresa et al. 2010; Ram et al. 2011).
The extraction of pigments from the fresh plant tissues still has some problems such as low metabolite yield, seasonal availability, fast deterioration, inconsistent product quality and pigment degradation due to extraction and storage process (Lila 2004; Ram et al. 2011). Plant tissue culture technique offers a strategy to minimize all these problems and allows the continuous production of secondary metabolites. Moreover, these also provide enhanced control over the chemical and physical environments to produce a compound of potentially more value for human use (Karuppusamy 2009; Simões et al. 2009). Moreover, the growing demand in today’s marketplace for natural, safe and renewable product from the plant has refocused attention on in vitro plant materials as potential factories for secondary phytochemical products. This paved the way for new research exploring secondary product expression in vitro (Mulabagal and Tsay 2004; Karuppusamy 2009). Therefore, in vitro techniques have been used as the alternative for the production of secondary metabolites including anthocyanins.
Anthocyanin can be produced by using different biotechnological approaches, such as cell/callus culture techniques (Jedinák et al. 2004) and there have been several reports regarding the production of anthocyanins using plant tissue cultures. It has been reported that anthocyanin production in cell/callus cultures is stimulated by various culture conditions (Ram et al. 2011). Exogenous application of jasmonates greatly stimulated the biosynthesis of a wide range of secondary metabolites in cell suspension cultures (Blechert et al. 1995; Curtin et al. 2003) and in intact plants (Rudell and Mattheis 2008). Elicitation has been shown to be the most efficient strategy that direct to the enhancement in anthocyanin production in plant cell cultures (Zhang and Furusaki 1999). Methyl jasmonate (MeJA) has been successfully used as an elicitor in some plant species for enhancing the production of secondary metabolites in the cell cultures (Thanh et al. 2005; Shimizu et al. 2010). Recently, See et al. (2011) showed that MeJA stimulated anthocyanin production in cell suspension cultures of Melastoma malabathricum.
Similarly, salicylic acid (SA) has also been the focus of intensive research due to its function in plant defense responses against pathogens (Vlot et al. 2009; Vicente and Plasencia 2011). It has also been found that SA, in particular, influences seed germination, seedling establishment, cell growth, responses to abiotic and osmotic stresses, etc. (Vicente and Plasencia 2011). Moreover, SA treatments were found to enhance the in vitro anthocyanin biosynthesis in callus cultures of Daucus carota (Sudha and Ravishankar 2003).
Literature available revealed that the work related to in vitro anthocyanin production in the ornamental crops especially in the rose, which contains a good amount of such pigments and generally do not have food safety concerns. Furthermore, until now there has been no report available regarding the elicitors (SA and MeJA) effect on callus biomass accumulation and anthocyanin production in callus culture of Rosa hybrida L. Therefore, to establish efficient anthocyanin production using callus cultures and to find the optimum level of elicitors, the present study was undertaken aiming to investigate the effect of SA and MeJA on callus culture of R. hybrida cv. Pusa Ajay. The interactive effects between SA and MeJA on anthocyanin production were also evaluated.
Materials and methods
Explant and callus induction
Callus tissue was originally derived from leaf explants of R. hybrida cv. Pusa Ajay cultured in the MS basal medium (Murashige and Skoog 1962) supplemented with 19.60 μM indole-3-butyric acid (IBA), 4.65 μM kinetin (Kin) and 108.6 mM adenine sulphate (AdS) and it has been continuously maintained in the same medium with double quantity of vitamins at 24 ± 1 °C in the complete darkness. The stock callus cultures were maintained under the same physical conditions as described above and were sub-cultured at 21-day interval.
Elicitor treatment for anthocyanin induction
Modified Euphorbia millii (EM, only sucrose level modified, i.e. 204.5 mM) medium (Yamamoto et al. 1989) supplemented with 2.45 μM IBA and 2.33 μM Kin was used for the anthocyanin induction. The concentrations of nitrogen, Fe2+, Na2EDTA, Ca2+, Mn2+, BO3 3−, and Zn2+ in EM medium are less than in MS medium. But the concentration of PO4 3−, K+, Mg2+, and sucrose in EM medium are greater than MS medium. MeJA was dissolved in absolute ethanol, sterilized by micro-filtration (0.22 μm Millipore, USA) and added into the autoclaved medium at 0.05, 0.50 and 5.00 μM concentrations. Likewise, SA was dissolved in a small amount of distilled water: ethanol (50:50, v/v), sterilized by micro-filtration and added into the autoclaved medium at 100, 200 and 400 μM concentrations. Three combinations of SA and MeJA, viz., 100 + 0.05, 200 + 0.50 and 400 + 5.00 were also imposed in the EM medium and callus were subjected to these treatments. Medium pH was adjusted to 5.8 ± 0.1 prior to adding agar–agar (5.5 g l−1, Qualigens, Mumbai), autoclaved (121 °C) for 15 min and dispensed into test tubes (25 ml of culture medium per test tube). Cultures were incubated in a culture room at 24 ± 1 °C under 16/8 h (105.7 μmol. photons m−2 s−1 light/dark) photoperiod regime using cool-white fluorescent tubes.
Callus growth and frequency of color response determination
The anthocyanins biosynthesis in callus cultures were measured for different parameters such as frequency of color response = (total number of cultures showing pigmentation/total number of cultured cultures) × 100, number of days taken for pigment initiation and intensification which was visually observed. Callus biomass accumulation was measured by determining the fresh weight (FW) of callus, after 15-day of cultures.
Quantification of anthocyanin
The color value index was estimated as described by Zhang et al. (2002) and Simões et al. (2009) with minor modifications. Samples of pigmented and non-pigmented callus (100 mg) were macerated using acidified methanol (methanol and 1 % HCl, v/v), the best extraction solvent. The extracts were centrifuged at 12,000×g for 10 min in a refrigerated centrifuge (Sigma 3K30, Sigma, Osterode, Germany). Optical density (OD) of the supernatant of each sample was measured in a quartz cuvette having the 1-cm-path-length at 525 nm using a UV–VIS double-beam spectrophotometer (Thermo Electron Corp, MA, USA) against the blank which consisted of the solvent. The CV index was calculated for pigment content (PC) {CV g−1 fresh weight (FW)} and pigment production (PP) (CV test-tube−1) with the following equations:
In the above-described procedure, the level of dilution was 50 (100 mg of callus extracted with 5 ml of MeOH–HCl). Color value index allows for the accurate and comparative quantification of the total anthocyanin produced from a mixture of different pigments, as is the case for many cells and callus cultures. Therefore, it is a preferred parameter to characterize and compare anthocyanin content results (Zhang et al. 2002). Hence in this study, CV was used to report the PC and PP.
Microscopic analysis
For microscopic study, fresh tissue of pigmented and non-pigmented callus were mounted on glass slides with a cover slip and viewed with a stereo microscope (Carl Zeiss Discovery.v8, Carl Zeiss MicroImaging, Germany). Digital images were captured with a digital camera (Carl Zeiss Axiovision, software version: Axiovision 4.8.2).
Data collection and statistical analysis
Four replicates were used for each of the treatments and each individual study was carried out using completely randomized design (CRD). The fresh cell biomass, PC and PP were determined for each sample after 15-day of culture. The data were analyzed using a one-way ANOVA followed by the post hoc test Tukey’s honestly significant difference (HSD) for mean comparison with the SPSS version 16.0 (SPSS Inc., USA). The correlation between frequency of color response, number of days taken for pigment initiation and intensification, fresh cell weight, PC and PP were computed and significance was assumed at P ≤ 0.05 or P ≤ 0.01.
Results and discussion
Effect of salicylic acid and/or methyl jasmonate on callus biomass
The evaluation of callus growth parameters cultured in the presence of SA showed that it did not significantly influenced the growth and biomass accumulation (Table 1). Compared to control, the growth of SA treated callus was non-significant, though marginally higher. An increase in SA concentrations from 100 to 400 μM in the culture medium slightly suppressed the growth of cultures, but these differences were statistically non-significant (P ≤ 0.05). Similarly, addition of MeJA resulted in a marginal improvement of growth compared to controls, but differed non-significantly (Table 1). To understand more about the callus biomass production, different combinations of SA and MeJA were supplemented in the culture medium. However, all the tried combinations failed to significantly enhance the callus biomass accumulation (Table 1).
Although information on the effect of jasmonates on the growth and development of callus is scanty, there is sufficient evidence suggesting that these compounds may up or down regulate callus biomass accumulation in the in vitro cultures. In general, SA elicitation has a negative effect on callus growth (Chaichana and Dheeranupattana 2012). In this study, higher concentration of SA also slightly decreased the callus growth, although it was non-significant (Table 1). In previous studies, it was found that SA slightly decreased the root growth in in vitro cultures of Stemona sp. (Chaichana and Dheeranupattana 2012) and also suppressed the growth of Rubia cordifolia callus cultures (Bulgakov et al. 2002). When SA (200 μM) was administered to the carrot callus cultures their growth was marginally higher than the control (Sudha and Ravishankar 2003). However, when hairy roots of Artemisia dubia were exposed to three different SA levels, 0.138 mg l−1 level showed a good growth rate response (Ali et al. 2012). Previously, similar results on the growth rate of hairy roots were also reported by Bais et al. (2002), while working on the effect of different SA levels on the growth rate of plants. In another study, it was reported that the cell growth of grape cultures was barely affected by SA treatment (Obinata et al. 2003). As regards jasmonates, these were found to be powerful inhibitors of cytokinin-induced soybean callus growth (Ueda and Kato 1982). The assumption that MeJA (1,000 μM) may inhibit cell growth was confirmed by Chen and Chen (1999) in S. miltiorrhiza cell cultures. A non-significant response of SA and MeJA addition on callus growth was found in this experiment (Table 1), which is conflicting with some previous studies. These results suggest that the callus growth in response to SA and MeJA treatment is dose- and plant species dependent response.
Effect of salicylic acid on anthocyanin production
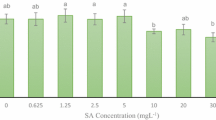
An elicitor in cell/callus cultures offer a novel approach for rapid accumulation of certain secondary metabolites by reducing time required to obtain the efficiency of product (Namdeo 2007). It has been observed that application of exogenous SA plays an important role in plant defense response through expression of defense related genes. However, little information is available about its effects on in vitro anthocyanin production especially in roses. Therefore, in this study effect of SA with different treatment doses was investigated. Table 1 shows the effect of SA on frequency of color response and days required for pigment initiation and intensification. Between the different SA levels, 200 μM level resulted in the maximum frequency of color response (83.50 ± 1.19 %) and this was significant (P ≤ 0.05) in comparison to control (EM and 204.5 mM sucrose). However, frequency of color response (77.75 ± 0.95 %) decreased due to higher SA level (400 μM) in the culture medium. Moreover, this frequency of color response did not differ significantly with 100 μM SA and control. Furthermore, all the tested levels of SA did not significantly affect the days required for pigment initiation and intensification (Table 1).
The effect of SA on PC and PP in callus cultures is shown in Table 1. Supplementation of SA in the culture medium increased the PP as compared to control and the maximum (1.285 ± 0.037 CV test-tube−1) response was with 200 μM SA. Furthermore, the PC was also highest (2.902 ± 0.078 CV g−1 FW) with 200 μM SA. High SA level (400 μM) resulted in a slight decrease in PC and PP alone in comparison to SA supplementation at lower concentrations.
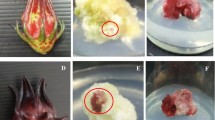
Stereo-microscopy was performed to understand more about the biosynthesis of anthocyanin in rose callus cultures (Fig. 1). At high magnification (up to 80×), the difference between the effects of different SA levels were visible and more red dots were observed in callus cultured on 200 μM SA (Fig. 1b) then controls (Fig. 1a). These observations, therefore suggest that the SA concentrations are decisive for the biosynthesis of anthocyanins in callus cultures.
Effect of different elicitors on anthocyanins accumulation in callus cultures of R. hybrida cv. Pusa Ajay. The cultures were cultured in EM medium (204.5 mM sucrose) supplemented with 2.45 μM IBA, 2.33 μM Kin, and different elicitors. a 204.5 mM sucrose (control), b 200 μM SA, c 0.5 μM MeJA, d 200, 0.5 μM SA and MeJA, respectively. The red dots in the images show the induced anthocyanins in response to different treatments. The images were taken after 14-day of culture. Numbers on the images indicate the frequency of color response values (%). The best images in their treatments were selected as representative image. Magnification bar = 100 μm. (Color figure online)
The present work has shown that SA elicitation resulted in a non-significant effect on callus biomass accumulation and days required for pigment initiation and intensification, while significantly affected the frequency of color response, PC and PP (Table 1; Fig. 1). SA plays a crucial role in the regulation of physiological and biochemical processes during the entire lifespan of a plant (Vicente and Plasencia 2011). Obinata et al. (2003) in cultured grape cells found that the production of procyanidin and anthocyanin was markedly increased when treated with SA. Moreover, Bulgakov et al. (2002) while examining the growth and production parameters of R. cordifolia callus cultures showed that SA inhibited growth of all cultures at 100 μM and stimulated anthraquinone production at all doses tested (1, 10, 100 μM). In addition, it has been demonstrated that SA plays an important role in the induction of systemic acquired resistance in plants (Hayat et al. 2010). The corresponding mechanism is that SA as a long distance mediator could move freely between the cells, tissues and organs (Klessig and Malamy 1994) and stimulate the activities of one or more antioxidative enzymes (Xu et al. 2012). Therefore, it is possible to postulate that the SA activated anthocyanin biosynthesis as a response to stress in callus cultures. The results of this study also supported the hypothesis that there is a relation between anthocyanin accumulation and stress responses in plants in accordance with the result obtained by other authors that used SA on plant species such as D. carota (Sudha and Ravishankar 2003), Catharanthus roseus (Xing et al. 2011) and Houttuynia cordata (Xu et al. 2012). However, further in-depth studies will throw more light on its involvement in anthocyanin biosynthesis and may help in getting higher recovery of the final product.
Effect of methyl jasmonate on anthocyanin production
For several plant systems, exogenously applied MeJA has had profound effects on secondary metabolite production in vitro, either alone or in combination with other elicitors (Curtin et al. 2003; Plata et al. 2003; Rudell and Mattheis 2008; Shimizu et al. 2010). Moreover, production of secondary metabolite can be enhanced in undifferentiated cells with elicitor treatment for example MeJA, SA and chitosan (Namdeo 2007).
As shown in Table 1, the frequency of color response significantly (P ≤ 0.05) varied with the supplementation of different MeJA levels in the culture medium. Significant rapid increase noticed in frequency of color response when the MeJA was supplemented at 0.50 μM and which was the maximum (97.25 ± 1.03). Higher concentration of MeJA (5.0 μM), however, showed a lower frequency of color response (89.75 ± 1.31) and this differed significantly even with the 0.05 μM MeJA (88.25 ± 1.11), but the difference was significant only with control (76.25 ± 2.14). This suggests that supplementation of 0.50 μM MeJA in the cultures medium was the best treatment to induce anthocyanins in rose callus.
In callus subjected to MeJA treatments, pigment initiation occurred approximately in 6-7 days after culture and continued to intensify approximately up to 14 days (Table 1). However, no significant variation was observed between treatments in the number of days required for pigment initiation and intensification. This implies that the MeJA did not play any role in hastening or delaying the pigment initiation and intensification while affecting the frequency of color response for anthocyanin biosynthesis in R. hybrida cv. Pusa Ajay callus cultures.
With regard to PC and PP cultures showed a significant difference with varying MeJA levels (Table 1). The callus cultured in EM medium supplemented with 204.5 mM sucrose (control) without MeJA exhibited the lowest PC (2.630 ± 0.083 CV g−1 FW) and PP (1.145 ± 0.046 CV test-tube−1). The callus subjected to 0.50 μM MeJA showed a significant increase in PC (3.482 ± 0.072 CV g−1 FW) and PP (1.556 ± 0.031 CV test-tube−1) as compared to 0.05 and 5.0 μM MeJA levels (Table 1) which still revealed PC and PP values higher than the control. The assay performed with the three MeJA levels, showed that the addition of 0.50 μM MeJA in EM medium supplemented with 204.5 μM sucrose could be used for the enhancement of pigmentation in rose callus cultures (Fig. 1).
To elucidate more about the biosynthesis of anthocyanin in response to MeJA treatments stereo-microscopic examinations were carried out (Fig. 1). It was revealed the appearance of more red dots and highly intensified anthocyanin pigmentation in the callus cultured on medium supplemented with 0.50 μM MeJA (Fig. 1c) compared to its other levels and control (Fig. 1a). The occurrence of more red regions in the cultured callus and production of anthocyanin was directly correlated. Therefore, the production of higher anthocyanin in response to 0.50 μM MeJA was also confirmed by the stereo-microscopic examination.
Statistical analysis and visual evaluation of cultures described in this study indicated that the PC and PP were increased with MeJA supplementation in the culture medium suggesting them as important supplement in the biosynthesis of anthocyanins in R. hybrida cv. Pusa Ajay callus cultures (Table 1; Fig. 1). Thus, MeJA acted as a positive inducer of anthocyanin biosynthesis in rose callus cultures. A similar magnitude of anthocyanin accumulation has been reported in D. carota callus cultures (Sudha and Ravishankar 2003). MeJA likely function as a stress messenger and in response to stress signals the cultures accumulated high amounts of anthocyanin. This hypothesis is supported by references that jasmonic acid was involved in induction of secondary metabolite accumulation, including anthocyanin or gene(s) expression of secondary metabolism biosynthesis (Curtin et al. 2003; Plata et al. 2003; Shimizu et al. 2010). Rudell and Mattheis (2002) found that the MeJA enhanced anthocyanin synthesis via stress response in plants which also support the idea in this experiment. The results of this study were also consistent with Fang et al. (1999) who reported that addition of MeJA influenced the anthocyanin accumulation depending on its concentrations in Vaccinium pahalae. Jasmonic acid and MeJA have been demonstrated as signal transducers in the intracellular signal cascade that begins with the interaction of an elicitor molecule with the plant cell surface and ultimately results in the accumulation of secondary compounds (Zhang and Furusaki 1999). Furthermore, MeJA stimulated the biosynthesis of anthocyanin production via induction of particular enzymes (catalyzes), which resulted in an increase in PP in M. malabathricum cell culture (See et al. 2011).
In addition, it has been revealed that MeJA and sucrose had a positive synergistic effect on anthocyanin accumulation in grapevine cell culture (Belhadj et al. 2008; Shimizu et al. 2010). The synergistic effect of jasmonic acid and sucrose for induction of anthocyanin biosynthesis were also observed in Arabidopsis thaliana (Loreti et al. 2008). The increased anthocyanin production in this study also corroborates with the findings of previous studies because the culture medium used in this experiment also had the high amount of sucrose (204.5 mM).
Effect of salicylic acid and methyl jasmonate combination on anthocyanin production
Having observed differential effects of SA and MeJA on anthocyanin production, the interaction effect of these two elicitors was also investigated to get the additional clues about their effect on anthocyanin biosynthesis. Among the different combinations of SA and MeJA, a combination of 200 μM SA and 0.50 μM MeJA caused a significant increase in frequency of color response but this increase was not as high as 0.50 μM MeJA alone in the culture medium (Table 1). The combination which had high levels of SA and MeJA (400, 5.0 μM, respectively) resulted in a decrease in frequency of color response regarding anthocyanin biosynthesis (Table 1; Fig. 1). Stereo microscopic observations revealed more anthocyanin biosynthesis by SA and MeJA combination compared to control (Fig. 1). Similar to SA and MeJA alone, all the combinations of these two elicitors did not affect the gain in FW and days taken for pigment initiation and intensification.
SA when added in combination with MeJA resulted in higher PC and PP than the control. However, the level of anthocyanin was still lower than 0.50 μM MeJA alone. This is evident from the color value index for the PC and PP for the best SA and MeJA combination (3.282 ± 0.075 CV g−1 FW and 1.469 ± 0.037 CV test-tube−1, respectively) (Table 1). However, higher level of SA and MeJA significantly decreased the PC and PP. Both elicitors induced a sharp increase in anthocyanin production after culture initiation, as compared to the control cultures when administered at optimum level but not as high as obtained with 0.50 μ MeJA alone. This implies that the combination of the best conditions of both the elicitors in the culture medium had a negative effect on anthocyanin production in comparison with the use of 0.5 μM MeJA alone suggesting negative crosstalk between the SA and MeJA-dependent defense pathways.
The results reported here are in accordance with the presence of negative crosstalk between the salicylate and jasmonate-dependent defense pathways in Arabidopsis (Traw and Bergelson 2003). Such response between different plant hormones was also reported by Loreti et al. (2008) in A. thaliana. Moreover, the antagonistic actions between SA and jasmonic acid have been shown on defense responses and inductions of secondary metabolism biosynthesis in other plants (Balbi and Devoto 2008). Results obtained by Shimizu et al. (2010) also substantiate the findings of this experiment, in which they indicated that SA inhibited anthocyanin accumulation in Gynura bicolor roots that were induced by MeJA treatment. Furthermore, a stimulating effect of MeJA alone and no additive effect with other elicitors on anthocyanin production were also reported in V. pahalae cell cultures (Fang et al. 1999). Moreover, some elicitors and signal compounds actually antagonize each other. Vidal et al. (1997) observed cross-interference between two distinct pathways for induction of plant defense genes by Erwinia elicitors and SA (a signal compound). Elicitation of anthocyanin from R. hybrida cv. Pusa Ajay callus cultures may similarly involve in two antagonistic signal transduction pathways, which may not account for the synergistic interaction between SA and MeJA elicitor.
Correlation between different parameters
To identify the relationship between different variables, Pearson’s correlation coefficient was computed. It was observed that there was a negative correlation between frequency of color response and days required for pigment initiation (r = −0.556) and intensification (r = −0.203). Correspondingly, PC, PP and days required to pigment initiation and intensification were also found to be negatively correlated (Table 2). This shows that the mechanism which controls these phenomena may be controlled by different processes. However, a statistically significant and positive correlation was found between frequency of color response and PC (r = 0.988) and PP (r = 0.990) (Table 2). Moreover, PC and PP were strongly correlated (r = 0.998) indicating the dependence of these two parameters on each other and in increasing the recovery of the final product. The strong correlation between these variables indicates that callus cultures with a high frequency of color response constitute a good index for ability to produce more anthocyanin.
The present study shows that anthocyanin production from R. hybrida cv. Pusa Ajay callus cultures was regulated by elicitors such as SA and MeJA. MeJA at 0.50 μM was found to be the most suitable elicitor to induce the anthocyanin in callus. Similarly, 200 μM SA was also found as a good elicitor for anthocyanin induction, however a combination of SA and MeJA, was not as effective as 0.50 μM MeJA alone. Finally, EM medium with 204.5 mM sucrose plus 0.50 μM MeJA may be adopted for the enhancement of anthocyanin production in R. hybrida cv. Pusa Ajay callus cultures. This protocol will be very useful in subsequent studies and in the future for getting enhanced recovery of the final product.
Abbreviations
- AdS:
-
Adenine sulphate
- CRD:
-
Completely randomized design
- CV:
-
Color value
- EM:
-
Euphorbia millii medium
- FW:
-
Fresh weight
- HSD:
-
Honestly significant difference
- IBA:
-
Indole-3-butyric acid
- Kin:
-
Kinetin
- MeJA:
-
Methyl jasmonate
- MS:
-
Murashige and Skoog’s medium
- PC:
-
Pigment content
- PP:
-
Pigment production
- SA:
-
Salicylic acid
References
Ali M, Kiani BH, Mannan A, Ismail T, Mirza B (2012) Enhanced production of artemisinin by hairy root cultures of Artemisia dubia. J Med Plants Res 6:1619–1622. doi:10.5897/JMPR11.1268
Bais HP, Walker TS, Schweizer HP, Vivanco JM (2002) Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiol Biochem 40:983–995
Balbi V, Devoto A (2008) Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol 177:301–318. doi:10.1111/j.1469-8137.2007.02292.x
Belhadj A, Telef N, Saigne C, Cluzet S, Barrieu F, Hamdi S, Mérillon JM (2008) Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol Biochem 46:493–499. doi:10.1016/j.plaphy.2007.12.001
Blechert RP, Brodshelm W, Hoylder S, Kammerer L, Kutchan TM, Mueller MJ, Xia ZQ, Zenk MH (1995) The octadecanoic pathway: signal molecules for the regulation of secondary pathways. Proc Natl Acad Sci 92:4099–4105
Bulgakov VP, Tchernoded GK, Mischenko NP, Khodakovskaya MV, Glazunov VP, Radchenko SV et al (2002) Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J Biotech 97:213–221. doi:10.1016/S0168-1656(02)00067-6
Chaichana N, Dheeranupattana S (2012) Effects of methyl jasmonate and salicylic acid on alkaloid production from in vitro culture of Stemona sp. Int J Biosci Biochem Bioinform 2:146–150
Chen H, Chen F (1999) Effects of methyl jasmonate and salicylic acid on cell growth and cryptotanshinone formation in Ti transformed Salvia miltiorrhiza cell suspension cultures. Biotechnol Lett 21:803–807
Curtin C, Zhang W, Franco C (2003) Manipulationg anthocyanin composition in Vitis vinifera suspension cultures by elicitation with jasmonic acid and light irradiation. Biotechnol Lett 25:1131–1135
de Pascual-Teresa S, Moreno DA, García-Viguera C (2010) Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci 11:1679–1703. doi:10.3390/ijms11041679
Downham A, Collins P (2000) Colouring our food in the last and next millennium. Int J Food Sci Technol 35:5–22
Fang Y, Smith MAL, Pépin M-F (1999) Effects of exogenous methyl jasmonate in elicited anthocyanin-producing cell cultures of ohelo (Vaccinum pahalae). In Vitro Cell Dev Biol Plant 35:106–113
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68:14–25
Jedinák A, Faragó J, Pšenáková I, Maliar T (2004) Approaches to flavonoid production in plant tissue cultures—review. Biologia Bratislava 59:697–710
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res 3:1222–1239
Klessig DF, Malamy J (1994) The salicylic acid signal in plants. Plant Mol Biol 26:1439–1458
Lila MA (2004) Anthocyanins and human health: an in vitro investigative approach. J Biomed Biotechnol 5:306–313
Loreti E, Povero G, Novi G, Solfanelli C, Alpi A, Perata P (2008) Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol 179:1004–1016. doi:10.1111/j.1469-8137.2008.02511.x
Mulabagal V, Tsay H-S (2004) Plant cell cultures—an alternative and efficient source for the production of biologically important secondary metabolites. Int J Appl Sci Eng 2:29–48
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Namdeo AG (2007) Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn Rev 1:69–79
Obinata N, Yamakawa T, Takamiya M, Tanaka N, Ishimaru K, Kodama T (2003) Effects of salicylic acid on the production of procyanidin and anthocyanin in cultured grape cells. Plant Biotechnol 20:105–111
Plata N, Konczak-Islam I, Jayram S, McCelland K, Woolford T, Franks P (2003) Effect of methyl jasmonate and p-coumaric acid on anthocyanin composition in a sweet potato cell suspension culture. Biochem Eng J 14:171–177
Ram M, Prasad KV, Kaur C, Singh SK, Arora A, Kumar S (2011) Induction of anthocyanin pigments in callus cultures of Rosa hybrida L. in response to sucrose and ammonical nitrogen levels. Plant Cell Tiss Org Cult 104:171–179. doi:10.1007/s11240-010-9814-5
Rudell DR, Mattheis JP (2002) Methyl jasmonate enhances anthocyanin accumulation and modifies production of phenolics and pigments in ‘Fuji’ apples. J Am Soc Hort Sci 127:435–441
Rudell DR, Mattheis JP (2008) Synergism exists between ethylene and methyl jasmonate in artificial light-induced pigment enhancement of ‘Fuji’ apple fruit peel. Postharvest Biol Technol 47:136–140
Schwinn KE, Davies KM (2004) Flavonoids. In: Davies KM (ed) Plant pigments and their manipulation, vol 14. CRC Press, NY
See KS, Bhatt A, Keng CL (2011) Effect of sucrose and methyl jasmonate on biomass and anthocyanin production in cell suspension culture of Melastoma malabathricum (Melastomaceae). Rev Biol Trop 59:597–606
Shimizu Y, Maeda K, Kato M, Shimomura K (2010) Methyl jasmonate induces anthocyanin accumulation in Gynura bicolor cultured roots. In Vitro Cell Dev Biol Plant 46:460–465. doi:10.1007/s11627-010-9294-7
Simões C, Bizarri CHB, da Silva Cordeiro L, de Castro TC, Coutada LCM, da Silva AJR et al (2009) Anthocyanin production in callus cultures of Cleome rosea: modulation by culture conditions and characterization of pigments by means of HPLC-DAD/ESIMS. Plant Physiol Biochem 47:895–903
Stintzing FC, Carle R (2004) Functional properties of anthocyanins and betalains in plants, food and in human nutrition. Trends Food Sci Technol 15:19–38
Sudha G, Ravishankar GA (2003) Elicitation of anthocyanin production in callus cultures of Daucus carota and the involvement of methyl jasmonate and salicylic acid. Acta Physiol Plant 25:249–256
Tanaka Y, Sasaki N, Ohmiya A (2008) Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J 54:733–749. doi:10.1111/j.1365-313X.2008.03447.x
Thanh NT, Murthy HN, Yu KW, Hahn EJ, Paek KY (2005) Methyl jasmonate elicitation enhanced synthesis of ginsenoside by cell suspension cultures of Panax ginseng in 5-l balloon type bubble bioreactors. Appl Microbiol Biotechnol 67:197–201
Traw MB, Bergelson J (2003) Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in arabidopsis. Plant Physiol 133:1367–1375. doi:10.1104/pp.103.027086
Ueda J, Kato J (1982) Inhibition of cytokinin-induced plant growth by jasmonic acid and its methyl ester. Physiol Plant 54:249–252
Vicente MR-S, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338. doi:10.1093/jxb/err031
Vidal S, Leon I, Denecke J, Palva ET (1997) Salicylic acid and the plant pathogen Erwinia carotovora induce defense genes via antagonistic pathways. Plant J 11:115–123
Vlot AC, Dempsey DMA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206. doi:10.1146/annurev.phyto.050908.135202
Xing S, Pan Q, Tian Y, Wang Q, Liu P, Zhao J et al (2011) Effect of plant growth regulator combinations on the biosynthesis of terpenoid indole alkaloids in Catharanthus roseus. J Med Plants Res 5:1692–1700
Xu YW, Lv SS, Zhao D, Chen JW, Yang WT, Wu W (2012) Effects of salicylic acid on monoterpene production and antioxidant systems in Houttuynia cordata. Afr J Biotech 11:1364–1372. doi:10.5897/AJB11.1524
Yamamoto Y, Kinoshita Y, Watanabe S, Yamada Y (1989) Anthocyanin production in suspension cultures of high producing cells of Euphorbia millii. Agric Biol Chem 53:417–423
Zhang W, Furusaki S (1999) Production of anthocyanins by plant cell cultures. Biotechnol Bioprocess Eng 4:231–252
Zhang W, Curtin C, Kikuchi M, Franco C (2002) Integration of jasmonic acid and light irradiation for enhancement of anthocyanin biosynthesis in Vitis vinifera suspension cultures. Plant Sci 162:459–468
Acknowledgments
M.R. gratefully acknowledges the Indian Agricultural Research Institute, New Delhi for awarding Senior Research Fellowship during the course of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ram, M., Prasad, K.V., Singh, S.K. et al. Influence of salicylic acid and methyl jasmonate elicitation on anthocyanin production in callus cultures of Rosa hybrida L.. Plant Cell Tiss Organ Cult 113, 459–467 (2013). https://doi.org/10.1007/s11240-013-0287-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-013-0287-1