Abstract
In this study, molecular imprinted polymer (MIP)-based impedimetric sensor has been developed to detect dengue infection at an early stage. Screen-printed carbon electrode (SPCE) was modified with electrospun nanofibers of polysulfone (PS) and then, coated with dopamine while using NS1 (non-structural protein 1—a specific and sensitive biomarker for dengue virus infection) as template during polymerization. The self-polymerization of dopamine at room temperature helps to retain exact structure of template (NS1) which results in generating geometrically fit imprinted sites for specific detection of target analyte. The electrochemical properties of MIP-modified SPCEs were studied by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) at every step of modification. Under optimal conditions, impedimetric measurements showed linear response in the range from 1 to 200 ng/mL. The developed sensor can selectively detect NS1 concentrations as low as 0.3 ng/mL. Moreover, impedimetric sensor system was also employed for NS1 determination in real human serum samples and satisfying recoveries varying from 95 to 97.14% were obtained with standard deviations of less than 5%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dengue virus is a member of the genus Flavivirus of Flaviviridae family, affecting more than 100 countries, including Europe and USA. Southeast Asia is considered as an area with the highest prevalence of this disease [28]. It is one of the most significant causes of arthropod-borne diseases on earth, with 400 million infections annually, affecting particularly children [14]. Dengue virus exists as four distinct antigenically related viral serotypes (DEN-1, DEN-2, DEN-3, and DEN-4), producing a wide spectrum of illness ranging from asymptomatic to serious complications [4]. Dengue can be manifested according to three distinct pathologies: dengue fever (DF) and its more serious forms; dengue hemorrhagic fever (DHF), or dengue shock syndrome (DSS). Dengue-related diseases have emerged as a major public health problem and are considered by the World Health Organization (WHO) as a health challenge in tropic and subtropic nations [21, 22]. The prevention of this disease is largely focused on rapid and reliable diagnosis [2]. Due to the limited resources of affected countries, WHO suggests tourniquet test as a vital parameter in dengue diagnosis [23]. However, immunological-based tests, mainly hemagglutination inhibition and MAC-EIA (monoclonal antibody capture enzyme-linked immunosorbent assay), are more applicable, because of their accuracy and sensitivity [10, 12]. A peptide-based chemiluminescence enzyme immunoassay has also been developed for diagnosis of dengue virus infection in short time [32]. Polymerase chain reaction (PCR) and related techniques have also been used for early diagnosis of dengue infection [16]. Despite their effectiveness, these techniques are laborious, time-consuming, and expensive and require sophisticated equipment and highly qualified staff for point-of-care testing in resource-limited settings [31]. Biosensors offer a simple, sensitive, and low-cost analysis for early diagnosis of dengue diseases in comparison with these routine laboratory tests.
The principle of biosensing is based on target binding by a bioreceptor followed by transduction of this biological response into a measurable signal. In the literature, different bioreceptors and transducers have been used for dengue virus detection [9, 17, 20]. Electrochemistry is one of the most sensitive and simple transduction techniques allowing device miniaturization [3]. Apart from transducers, the recognition element plays a key role in the specificity and sensitivity of diagnostic method, where antibodies are the most commonly employed [18]. However, these are costly, lack stability, and their use is limited to physiological conditions. Therefore, artificial receptors such as aptamers and MIPs have received significant attention, in recent years [25]. MIPs are synthetic intelligent materials that mimic biological recognition through specific imprinted cavities. In MIP sensing, the selective recognition of target analyte is based on its geometrical as well as chemical fitting in imprinted cavities of polymer matrix [7]. MIP-based electrochemical sensors are characterized by a mechanical/thermal stability, excellent specificity, and sensitivity and provide a greater prospect for high-quality sensing applications, over other types of sensors [13]. In the recent advancement in MIP formation techniques, a better selectivity of the MIP systems for large molecules can be achieved. The MIP-based sensors have been reported for a number of bioanalytes in the literature [8, 26].
Electrospun polysulfone nanofibrous membrane has proven an excellent material for rapid and on-site visual detection of hydrogen peroxide by decorating these nanofibers with gold nanocluster [27]. Moreover, polysulfone membranes were also employed as a supporting material for polyaniline to develop optical pH sensor [1].
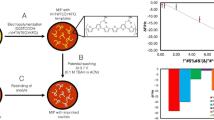
In the present work, advantages of MIPs and electrochemical transduction have been employed to develop a sensor system for NS1 protein. Herein, NS1 was selected as a target for early diagnosis of dengue viral infection, since it is considered as a common biomarker for all the serotypes, and it is present at high concentrations even in early stages of infection (DHF, DSS) [19]. In order to fabricate this sensor system, functional monomer dopamine was polymerized (in presence of NS1 protein) directly on polysulfone nanofiber-modified screen-printed carbon electrodes. Finally, the modified SPCE was used for electrochemical detection of dengue viral infection. Fabrication principle of MIP-based sensor system and its application for NS1 detection is shown in Fig. 1.
Furthermore, the selectivity of our MIP-based impedimetric sensor has been demonstrated in the presence of other interfering species. The results highlight that the sensor system is selective towards its imprinted analyte in comparison with other interfering species.
Materials and Methods
Reagents
Dengue NS1 protein (recombinant) was purchased from Enzo Lifescience New York. Polysulfone was purchased from Scientific polymer products, Inc., whereas N,N-dimethylformamide (DMF) and tetrahydrofuran (THF) were purchased from Merck-Schuchardt, Germany. Dopamine hydrochloride (DA) was purchased from Thermofisher, Germany. Proteinase K was purchased from Bio Plus Fine Research Chemicals. Chitosan was purchased from MP Biomedical, France. Acetic acid, potassium ferrocyanide, and ferricyanide were purchased from Uni-Chem Chemical Reagents. Sulfuric acid was obtained from AnalaR. Phosphate buffer saline (PBS) was purchased from Oxoid Limited, England. Aqueous solutions were prepared in deionized water.
Instrumentation
An AMEL Potentiostat/Galvanostat (model 2553, AMEL Electrochemistry, Italy) was used for electrochemical characterization of SPCE and its different modification stages. SPCEs were fabricated by employing screen printing system DEK 248 (DEK, Weymouth, UK) [15] using graphite for printing working (diameter 4 mm) as well as counter electrode and Ag/AgCl for reference electrode. Scanning electron microscope (TESCAN VEGA 3) was used for surface profiling of SPCE at each modification step.
SPCE Modification with Polysulfone Nanofibers
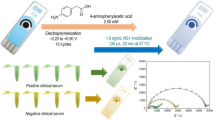
The SPCE was cleaned electrochemically in order to remove impurities through continuous CV scans in 0.5 M H2SO4 within potential range of − 1.5 to 1.5 V. Afterwards, a homogeneous solution of polysulfone, 24 wt.% in tetrahydrofuran (THF)/dimethylformamide (DMF) (3:1), was electrospun at an applied potential of 17 kV in order to modify SPCE surface with polysulfone nanofibers. These nanofibers were anchored on SPCE surface by using 10 μL of 0.5% chitosan solution.
Preparation of Molecularly Imprinted SPCEs
At first, a freshly prepared solution of dopamine hydrochloride (2 mg/mL in 10 mM TRIS buffer, pH 8.5) was mixed with 5 μg/mL NS1 solution. Then, 20 μL of NS1/dopamine mixture was dropped on polysulfone-modified SPCEs and allowed to proceed until complete polymerization [24]. After self-polymerization of dopamine, the resulting electrode (SPCE/PS/NS1/DA) was thoroughly washed with buffer. Afterwards, it was treated with 500 μg/mL of proteinase K for 2 h in the dark, to remove protein template. Finally, it was washed with PBS to remove proteinase K and remaining fragments of protein. The regeneration of MIP-modified SPCE was performed to detect different concentrations of NS1 protein as well as for selectivity studies, as shown in Figs. 4 and 5, respectively. The polydopamine was employed to retain the shape of imprinted cavity structure after proteinase treatment.
Non-imprinted polymer (NIP)-based electrode was prepared by following similar procedure but without adding template (NS1 protein) and used as control.
Selectivity and Applicability Analysis
The specificity of sensor system was analyzed by exposing it to interfering proteins: FBS and lysozyme. Afterwards, analytical applicability of the proposed method was verified by determining NS1 protein in serum samples. At first, human serum was diluted with PBS buffer for specific working concentrations and then spiked with different concentrations of NS1 ranging from (5–200 ng/mL), to study matrix effect and NS1 recovery.
Results and Discussion
Morphological and Electrochemical Characterization of Sensor System
Surface morphology of SPEC was analyzed by SEM at every modification step and results are shown in Fig. 2. SEM image of bare electrode is shown in Fig. 2a, whereas Fig. 2b represents SPCE modification with polysulfone electrospun nanofibers. Afterwards, nanofiber-modified SPCE was covered with dopamine in the presence of NS1 protein. The dopamine engulfs NS1 protein during self-polymerization and subsequent removal of protein generates imprinted sites on the surface of nanofiber-modified SPCE for NS1 re-inclusion, as shown in Fig. 2c.
Electrospun nanofibers of polysulfone were used as a support for preparing imprinting material on the basis of its large surface area and mechanical strength. The NS1-imprinted polymer is in the possession of large surface area because polymerization of dopamine occurs on PS nanofibers surface. In this way, recognition sites were generated on the surface of imprinted layer which results in reducing template removal and binding time as well as improves accessibility to target analyte. Moreover, self-polymerization of dopamine at room temperature helps to retain exact structure of template protein which results in generating geometrically fit imprinted sites for specific detection of target analyte. All these factors such as large surface area, accessibility to binding sites, and template structure preservation during polymerization help to achieve higher sensitivity and excellent performance of developed sensor system. Proteinase K was used to remove protein template and subsequently excess of PBS was used to remove proteinase K and remaining fragments of protein. The electrochemical properties of MIP-modified SPCE were studied by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) at every step of modification. As can be seen in Fig. 3, a significant increase in peak current was observed which is attributed to the formation of imprinted sites after extraction of template protein.
The electrochemical measurements were performed in PBS (pH 7.4) containing 0.5 mM of ferricyanide/ferrocyanide couple [Fe(CN)6]4−/3− as electroactive probe. Figure 3A shows the voltammograms recorded by varying the potential range from − 1 to + 1 mV at a scan rate of 50 mV/s. Scan rate in the range of 10–100 mV/s was employed. A better peak to peak separation with well-defined redox peak was observed at 50 mV/s. Furthermore, the objective of CV studies was employed to validate the EIS characterization of transducer/substrate surface at different fabrication and modification steps. As can be seen from voltammograms, a significant decrease in redox peak was observed after the modification of SPCE with polysulfone nanofibers (SPCE/PS) (curve b). The decrease in the current is attributed to non-conductive behavior of polysulfone fibers [1]. The oxidation and reduction current values were decreased to 5.7 × 105− and − 5.0 × 105−, in comparison with 9.2 × 105− and − 8.0 × 105− for bare electrodes. A further decrease in redox peak was observed when dopamine and NS1 were coated on nanofiber-modified SPCE represented as SPCE/PS/DA(NS1), as shown in curve c. The observed redox current was 5 × 105− and − 4.8 × 105− for oxidation and reduction peak, respectively. Furthermore, a slight shift to higher potential was observed for redox peaks. This shift to higher potential can be related to formation of NS1 insulating, leading to a slow diffusion process for flow of electrons. In the next step, corresponding to the removal of template protein represented by SPCE/PS/DA(−) in curve d, a significant increase was observed in the peak current. The observed current values were 6.8 × 105− and − 5.0 × 105− for oxidation and reduction peak, respectively. This is, certainly, due to the formation of imprinted sites after the extraction of template protein NS1.
EIS is also a very sensitive method to investigate the characteristics of a sensing platform. Therefore, it was used to characterize different steps of SPCE surface modification. The corresponding impedance spectra (Nyquist plots) were obtained within a frequency range of 100–0.2 Hz, at AC amplitude of 100 mV and a sampling rate of 10 points (Fig. 3B). The interface is modeled using the Randles equivalent circuit with ohmic electrolyte resistance (Rs), the electron transfer resistance (Ret), the Warburg impedance element (Zw) resulting from the diffusion of ions from electrolyte bulk to interface and the constant phase element (3C). The electron transfer resistance (Ret) is measured with respect to different frequency levels for impedance data. Breaks in the impedance spectra represent the frequency pulse range and each point of impedance data in the spectra corresponds to a specific frequency value. Electrochemical analyzer has built-in software for frequency measurement, with tunable parameters as per experimental requirements. EIS characterization was based on Ret, because it is directly related to the facial properties of the surface. Before deposition of polysulfone fibers, the Ret value of bare electrode was 7.5 Ω (curve a). Afterwards, an increased resistance can be observed in curve b (13 Ω) corresponding to SPCE/PS. This phenomenon can be attributed to the polysulfone layer which hampers electron transfer between electrolyte and the interface. This increase was more significant (22 Ω), in curve c, when NS1-imprinted polydopamine was formed on the polysulfone-modified surface. The entrapped NS1 protein blocked the permeating channel of probe. This can be confirmed by curve d, where the Ret value decreased to 16 Ω after template removal showing that cavities were successfully formed for specific recognition of NS1 protein.
Impedimetric Detection of NS1 Protein Using the Designed MIP Sensor
Following the principle shown in Fig. 1, the detection of different concentrations of NS1 protein was carried out using MIP-modified SPCE electrode. The corresponding electrochemical responses were recorded by EIS using a frequency range of 100–0.2 Hz, at AC amplitude of 100 mV and a sampling rate of 10 points. As shown in Fig. 4a, presenting the obtained impedimetric signals, sensor resistance increases with increasing NS1 concentrations.
Interaction sites formed by imprinting technique bind with target protein NS1 and sensor signals increase with increasing NS1 concentrations. This can be attributed to rebinding of NS1 into the imprinted sites of sensor, thus hindering electron transfer of redox probes to electrode surface. These results were used to construct calibration curve by plotting the Δratio as a function of protein concentration, whereas Δratio was calculated on the basis of Ret values of MIP and NIP, presented in Fig. 4b. A linear relationship was observed over NS1 concentrations within the wide range of (1–200 ng/mL). The limit of detection (LOD) was calculated as 0.3 ng/mL (S/N = 3). Each point of calibration graph is the average of three replications. The comparison of imprinted sensor performance with previously reported electrochemical methods for NS1 detection is listed in Table 1.
Selectivity Studies
In order to analyze the specificity of this MIP-based impedimetric sensor for NS1 protein, lysozyme and FBS were selected as interfering proteins. This validation step is crucial since false positive readings and interference of cross reactivity is the main problems encountered in disease diagnosis [9]. Selectivity of MIP-based impedimetric sensor for NS1 was analyzed by exposing it to different proteins such as FBS and lysozyme. However, applicability and reliability of proposed sensor system was confirmed by determining NS1 in spiked human serum samples which contains all the interfering species (including albumin, globulins, and fibrinogen) and satisfying recoveries were obtained. To perform this study, the MIP sensor was exposed to 50 ng/mL concentrations of interfering species. ΔRatio values were then calculated from obtained impedimetric signals of MIP and NIP, as presented in Fig. 5. It can be seen that ΔR value of the modified sensor towards its templated analyte is very high as compared with that of interfering proteins. These results show that the selectivity of our MIP-based sensor is sufficient to identify the target protein in the presence of other interfering analytes.
Detection of NS1 Protein in Serum Samples
Aiming to confirm the applicability and reliability of the proposed method, the imprinted sensor was employed for NS1 determination in real human serum samples. The absence of infection was first verified in non-spiked samples, by measuring the corresponding resistance and observing no related response. Then, serum samples were spiked with three concentrations of NS1 protein within the linear range of MIP sensor. Afterwards, the modified electrodes were exposed to spiked samples and corresponding EIS spectra were recorded for determination purpose according to calibration curve, previously described. Table 2 summarizes the obtained recovery percentages for three tested concentrations: 5, 50, 70, 100, and 200 ng/mL. As can be seen, satisfying recoveries varying from 95 to 97.14% were obtained with standard deviations less than 5%. These results confirm the accuracy and reproducibility of developed sensor for clinical diagnosis in complex biological samples.
Conclusion
In summary, an impedimetric MIP-based sensor has been successfully developed for early diagnosis of dengue viral infection. The NS1-imprinted sensor was constructed by polymerizing dopamine (having NS1 protein as template) on polysulfone nanofiber-modified screen-printed carbon electrodes. The removal of template creates imprinted sites for detecting dengue virus biomarker specifically. Afterwards, the resulting sensing system was employed for impedimetric determination of NS1 protein. The developed sensor system showed excellent analytical performances in terms of sensitivity and selectivity. Moreover, applicability of the proposed method for clinical diagnosis of dengue virus was demonstrated with high accuracy. Finally, the sensor simplicity and low cost allow its application for other analytes detection in different domains.
References
Abu-Thabit, N., Umar, Y., Ratemi, E., Ahmad, A., & Ahmad Abuilaiwi, F. (2016). A flexible optical pH sensor based on polysulfone membranes coated with pH-responsive polyaniline nanofibers. Sensors, 16, 986.
Baeumner, A. J., Schlesinger, N. A., Slutzki, N. S., Romano, J., Lee, E. M., & Montagna, R. A. (2002). Biosensor for dengue virus detection: Sensitive, rapid, and serotype specific. Analytical Chemistry, 74, 1442–1448.
Bandodkar, A. J., & Wang, J. (2014). Non-invasive wearable electrochemical sensors: A review. Trends in Biotechnology, 32, 363–371.
Bharaj, P., Chahar, H. S., Pandey, A., Diddi, K., Dar, L., Guleria, R., Kabra, S. K., & Broor, S. (2008). Concurrent infections by all four dengue virus serotypes during an outbreak of dengue in 2006 in Delhi, India. Virology Journal, 5, 1.
Cavalcanti, I., Silva, B., Peres, N., Moura, P., Sotomayor, M. D. P. T., Guedes, M., & Dutra, R. (2012). A disposable chitosan-modified carbon fiber electrode for dengue virus envelope protein detection. Talanta, 91, 41–46.
Cavalcanti, I. T., Guedes, M. I., Sotomayor, M. D., Yamanaka, H., & Dutra, R. F. (2012). A label-free immunosensor based on recordable compact disk chip for early diagnostic of the dengue virus infection. Biochemical Engineering Journal, 67, 225–230.
Cieplak, M., & Kutner, W. (2016). Artificial biosensors: How can molecular imprinting mimic biorecognition? Trends in Biotechnology, 34, 922–941.
Crapnell, R. D., Hudson, A., Foster, C. W., Eersels, K., Grinsven, B. v., Cleij, T. J., Banks, C. E., & Peeters, M. (2019). Recent advances in electrosynthesized molecularly imprinted polymer sensing platforms for bioanalyte detection. Sensors, 19, 1204.
Darwish, N. T., Alrawi, A. H., Sekaran, S. D., Alias, Y., & Khor, S. M. (2016). Electrochemical immunosensor based on antibody-nanoparticle hybrid for specific detection of the dengue virus NS1 biomarker. Journal of the Electrochemical Society, 163, B19–B25.
de Souza, V. A. U. F., Tateno, A. F., Oliveira, R. R., Domingues, R. B., Araújo, E. S., Kuster, G. W., & Pannuti, C. S. (2007). Sensitivity and specificity of three ELISA-based assays for discriminating primary from secondary acute dengue virus infection. Journal of Clinical Virology, 39, 230–233.
Dias, A. C. M., Gomes-Filho, S. L., Silva, M. M., & Dutra, R. F. (2013). A sensor tip based on carbon nanotube-ink printed electrode for the dengue virus NS1 protein. Biosensors and Bioelectronics, 44, 216–221.
Fernandez, R., & Vazquez, S. (1990). Serological diagnosis of dengue by an ELISA inhibition method (EIM). Memorias do Instituto Oswaldo Cruz, 85(3), 347–351.
Gui, R., Jin, H., Guo, H., & Wang, Z. (2018). Recent advances and future prospects in molecularly imprinted polymers-based electrochemical biosensors. Biosensors and Bioelectronics, 100, 56–70.
Hasan, S., Jamdar, S. F., Alalowi, M., & Al Beaiji, S. M. A. A. (2016). Dengue virus: A global human threat: Review of literature. Journal of International Society of Preventive & Community Dentistry, 6, 1.
Hayat, A., Barthelmebs, L., & Marty, J.-L. (2012). Electrochemical impedimetric immunosensor for the detection of okadaic acid in mussel sample. Sensors and Actuators B: Chemical, 171, 810–815.
Hirayama, T., Mizuno, Y., Takeshita, N., Kotaki, A., Tajima, S., Omatsu, T., Sano, K., Kurane, I., & Takasaki, T. (2012). Detection of dengue virus genome in urine by real-time reverse transcriptase PCR: A laboratory diagnostic method useful after disappearance of the genome in serum. Journal of Clinical Microbiology, 50(6), 2047–2052.
Jahanshahi, P., Zalnezhad, E., Sekaran, S. D., & Adikan, F. R. M. (2014). Rapid immunoglobulin M-based dengue diagnostic test using surface plasmon resonance biosensor. Scientific Reports, 4, 3851.
Justino, C. I., Freitas, A. C., Pereira, R., Duarte, A. C., & Santos, T. A. R. (2015). Recent developments in recognition elements for chemical sensors and biosensors. TrAC Trends in Analytical Chemistry, 68, 2–17.
Libraty, D. H., Young, P. R., Pickering, D., Endy, T. P., Kalayanarooj, S., Green, S., Vaughn, D. W., Nisalak, A., Ennis, F. A., & Rothman, A. L. (2002). High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. The Journal of Infectious Diseases, 186(8), 1165–1168.
Nawaz, M. H., Hayat, A., Catanante, G., Latif, U., & Marty, J. L. (2018). Development of a portable and disposable NS1 based electrochemical immunosensor for early diagnosis of dengue virus. Analytica Chimica Acta, 1026, 1–7.
World Health Organization. (2009). Dengue guidelines for diagnosis, treatment, prevention and control: New edition. Geneva: World Health Organization.
Parida, M., Horioke, K., Ishida, H., Dash, P. K., Saxena, P., Jana, A. M., Islam, M. A., Inoue, S., Hosaka, N., & Morita, K. (2005). Rapid detection and differentiation of dengue virus serotypes by a real-time reverse transcription-loop-mediated isothermal amplification assay. Journal of Clinical Microbiology, 43(6), 2895–2903.
Phuong, C. X. T., Nhan, N. T., Wills, B., Kneen, R., Ha, N. T. T., Mai, T. T. T., Huynh, T. T. T., Lien, D. T. K., Solomon, T., & Simpson, J. A. (2002). Evaluation of the World Health Organization standard tourniquet test and a modified tourniquet test in the diagnosis of dengue infection in Viet Nam. Tropical Medicine & International Health, 7, 125–132.
Postma, A., Yan, Y., Wang, Y., Zelikin, A. N., Tjipto, E., & Caruso, F. (2009). Self-polymerization of dopamine as a versatile and robust technique to prepare polymer capsules. Chemistry of Materials, 21, 3042–3044.
Rhouati, A., Bakas, I. and Marty, J. L. (2019) MIPs and aptamers as artificial receptors in advanced separation techniques: Application in food analysis. Handbook of Smart Materials in Analytical Chemistry, pp 825–857.
Saylan, Y., Akgönüllü, S., Yavuz, H., Ünal, S. and Denizli, A. (2019). Molecularly imprinted polymer based sensors for medical applications. Sensors, 19.
Senthamizhan, A., Balusamy, B., Aytac, Z., & Uyar, T. (2016). Ultrasensitive electrospun fluorescent nanofibrous membrane for rapid visual colorimetric detection of H 2 O 2. Analytical and Bioanalytical Chemistry, 408(5), 1347–1355.
Shepard, D. S., Undurraga, E. A., & Halasa, Y. A. (2013). Economic and disease burden of dengue in Southeast Asia. PLoS Neglected Tropical Diseases, 7, e2055.
Silva, M. M., Dias, A. C., Silva, B. V., Gomes-Filho, S. L., Kubota, L. T., Goulart, M. O., & Dutra, R. F. (2015). Electrochemical detection of dengue virus NS1 protein with a poly (allylamine)/carbon nanotube layered immunoelectrode. Journal of Chemical Technology & Biotechnology, 90, 194–200.
Sinawang, P. D., Rai, V., Ionescu, R. E., & Marks, R. S. (2016). Electrochemical lateral flow immunosensor for detection and quantification of dengue NS1 protein. Biosensors and Bioelectronics, 77, 400–408.
Teles, F. S. R. R. (2011). Biosensors and rapid diagnostic tests on the frontier between analytical and clinical chemistry for biomolecular diagnosis of dengue disease: A review. Analytica Chimica Acta, 687(1), 28–42.
Zhu, T., He, J. a., Chen, W., Ho, H. P., Kong, S. K., Wang, C., Long, J., Fong-Chuen Loo, J., & Gu, D. (2018). Development of peptide-based chemiluminescence enzyme immunoassay (CLEIA) for diagnosis of dengue virus infection in human. Analytical Biochemistry, 556, 112–118.
Funding
The authors are thankful to the Higher Education Commission (HEC) of Pakistan for financial support under project no. 5411.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arshad, R., Rhouati, A., Hayat, A. et al. MIP-Based Impedimetric Sensor for Detecting Dengue Fever Biomarker. Appl Biochem Biotechnol 191, 1384–1394 (2020). https://doi.org/10.1007/s12010-020-03285-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03285-y