Abstract
Background
Biosurfactants are amphipathic biological compounds with surface active potential and are produced by many microorganisms. Biosurfactant production by Lysinibacillus fusiformis MK559526 isolated from automobile-mechanic-shop soil was investigated with a view to assessing its potential for production and potential for optimization.
Materials and methods
Effects of carbon and nitrogen sources, pH, temperature and incubation periods on biosurfactant production were evaluated with a view to optimizing the processes. Fourier Transform Infra-Red absorption peaks and Gas chromatography mass spectrometry were used to determine the functional groups of the chemical make-up and the chemical profile of the biosurfactant respectively.
Results
Lysinibacillus fusiformis surfactant had emulsification index of 65.15 ± 0.35 %, oil displacement of 2.7 ± 0.26 mm, zone of haemolysis of 7.3 ± 0.16 mm and a positive drop collapse test. Optimized culture conditions for biosurfactant production: temperature, 35 ºC; pH, 7.0; starch solution, 40 g/L and urea, 1.5 g/L showed a reduction in surface tension to 28.46 ± 1.11 mN/m and increased emulsification index to 93.80 ± 0.41 %. Maximum biosurfactant production of 2.92 ± 0.04 g/L was obtained after 72 h. The biosurfactant contained peptides and fatty acids. The predominant fatty acid was 9-Octadecenoic acid (80.80%).
Conclusions
The above results showing high emulsification potential and remarkable reduction in the surface tension are good biosurfactant attributes. Consequently, Lysinibacillus fusiformis MK559526 is a good candidate for biosurfactant production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past years, interest has shifted to exploration of important biosurfactants for industrial, agricultural and environmental applications. Therefore, sourcing of biosurfactants from new environments is of essence. Lipopeptide which is a biosurfactant produced by Bacillus species is produced by many Bacilli found in different habitats [1]. Biosurfactants are composed of polar and non-polar moieties of glycolipids, phospholipids, lipopeptides and polymeric compounds intended to separate at interfaces and thus reduce the surface tension [2, 3].
The merits of biosurfactants over chemically synthesized surfactants are lower toxicity, specificity of action, simplicity of preparation and wide applicability [4]. Biosurfactants are used as dispersing agents, moistening agents, emulsifiers, foaming agents, important food compounds and detergents in many industrial regions [4]. Despite these uses, efforts geared towards commercialization of biosurfactants are failing due to the low yield obtained and high production cost. The need for commercial biosurfactant production arose in order to reduce the heavy waste pollutants generated by various oil companies [5].
Through biotechnological means, these biosurfactant-producing microorganisms can be identified and effectively studied. Molecular studies are required to identify the genes for the production of biosurfactants [6]. Among the genes needed for biosurfactant biosynthesis is a large operon of 25 kb, named srfA, which is also vital for sporulation and competence development [7]. The presence of srfA operon and sfp gene is required for the non-ribosomal biosynthesis of surfactin, a lipopeptide biosurfactant.
Apart from the nucleic acid manipulation of microorganisms, manipulation of the cultural environment remains a standard biotechnological means of optimization. Culture agitation, incubation time, oxygen level, pH and temperature had been studied in relation to their effects on either cell biomass or cellular product formation [2, 8, 9]. Whereas some environmental changes might not affect biomass or product formation, Wang et al. [10] recorded carbon and nitrogen concentrations as affecting biosurfactant production. Consequently, optimization of biosurfactant production by any microorganism is done based on the individual species requirement. To the best of our knowledge, the work presented here has not been reported by anybody or group.
The hypothesis of the study was that Lysinibacillus fusiformis isolated from automobile-mechanic-workshop soil was capable of producing biosurfactant. Biosurfactant production by Lysinibacillus fusiformis MK559526 isolated from automobile-mechanic-workshop soil and optimization of the cultural conditions to maximize the biosurfactant yield was the broad aim of the study.
Materials and methods
The organism
The organism was isolated from automobile-mechanic-workshop soil in Makurdi, Benue State, Nigeria (longitude 7° 47′ and 10° 0′ East, latitude 6° 25′ and 8° 8′ North), identified via microbiological, biochemical and molecular approaches [11]. The isolate with code MS1(3) C was identified and deposited in the GenBank with isolate Accession Number: MK559526. The isolate culture was stored in Nutrient Agar (Oxoid CM002, Hampshire, England) slants at 4 °C and was sub-cultured at intervals.
Screening of the isolate for biosurfactant production
The isolate was screened for biosurfactant production using 30-mL nutrient broth in 100-mL flask inoculated with 3-mL (approximately 4.5 × 108 cells) McFarland 0.5 standardized pure culture grown on nutrient broth for 24 h. This was incubated at 30 °C on a rotary shaker (Orbital Shaker, Series F200, England) at 150 rpm for 72 h. The cultures were centrifuged (Centrifuge Machine, Model 80–213, 2000) at 3000 rpm for 30 min to obtain cell-free supernatants. The supernatants were collected and cells discarded. The various supernatants were applied for subsequent determination of emulsification index test, drop collapse and oil spread capacity test.
Emulsification stability test (E24)
Emulsification index test was measured according to the method described by Balogun and Fagade [12]. Two millilitres (2 mL) of kerosene was added to 2 mL of the culture supernatant, vortex-mixed for 2 min (electronic vortex mixing machine Model XH-B, 2012), and allowed to stand for 24 h. The E24 was given as the height of emulsified layer (mm) divided by total height of the liquid column (mm) expressed as percentage and was determined at interval of 24 h.
Drop collapse assay
To determine whether the culture supernatant was positive for drop collapse test, 10 μL of cell-free broth was dropped in the centre of a vegetable oil drop on a clean glass slide. This was examined after 1 min for visible destabilizations of supernatant which confirmed result as positive. In the test, distilled water was used to replace supernatant as a control as described by Seema and Nakuleshwar [13].
Oil spreading technique
Oil spreading was determined by adding 20-mL distilled water into a Petri plate followed by 1 mL of crude oil dropped at the centre of the plate. Thereafter, 20 μL of the supernatant was added to the centre of the crude oil. Displacement of crude oil led to the formation of a ring which was measured with a meter rule. The control experiment was obtained using same volume of distilled water in place of culture supernatant [14].
Determination of blood haemolysis
Blood haemolysis test was determined using sterilized Blood Agar Base and fresh goat blood. The blood agar base was autoclaved and allowed to cool to about 45–50 °C and 20 mL aseptically collected goat blood was added and gently mixed before pouring into Petri dishes. A young culture of the isolate (24 h freshly grown culture) was point-inoculated at the centre of the plate and incubated at 30 °C for 24 h. As recommended by El-Shahawy [15], zones of clearing around the colonies were measured using a meter rule.
Determination of surface tension
Determination of surface tension was carried out by the use of a tensiometer (KSV Sigma 702 tensiometer). After centrifuging the culture broth (10,000 rpm for 15 min, centrifuge model: 80–213, 2000), 10 mL of the supernatant was transferred into a clean 20-mL beaker and placed onto the tensiometer platform. This was followed by submerging a platinum wire ring into the solution followed by pulling through the liquid-air interface, to measure the surface tension (mN/m). Using a Bunsen burner flame, the platinum wire was sterilized intermittently after each determination.
Determination of dry cell biomass
Dry cell biomass or cell dry weight was measured by initially weighing a clean glass Petri dish. This was followed by finally weighing the Petri dish containing residue from centrifuged culture broth which was poured into the Petri dish, and dried in a hot air oven for 30 min at 100 °C. After obtaining the final weight, dry cell weight was calculated:
Determination of effects of environmental parameters on biosurfactant production
Biosurfactant production potential of the isolate was studied using mineral salt medium (MSM) and sterile 40 g/L glucose carbon substrate. The composition of MSM adopted by Elazzazy et al. [5] was used. The MSM contained KH2PO4, 1.4; Na2HPO4, 2.2; (NH4)2SO4, 3; MgSO47H2O, 0.6; NaCl, 0.05; FeSO4 7H2O, 0.01; and CaCl2 7H2O, 0.02 g/L. The medium was supplemented with 2 mL of trace element solution. The trace element solution contained ZnSO4, 0.29; CaCl2, 0.24; CuSO4, 0.25; and MnSO4, 0.17 g/L. Sterilized MSM was inoculated with 3-mL (4.5 × 108 CFU/mL) McFarland 0.5 standardized pure culture grown on nutrient broth for 24 h.
Effect of different carbon sources on biosurfactant production
Determination of the effect of glucose, lactose, dextrose and soluble starch on growth and on biosurfactant production of the isolate was done by varying the concentrations of the carbohydrate substrate contents, 10, 20, 30, 40 and 50 g/L of the MSM. The MSM (1000 mL) was amended with the carbon sources individually and the initial pH adjusted to 7.0. This was followed by inoculation with 3 mL of the overnight culture of the isolate (McFarland 0.5 standardized pure culture grown on nutrient broth for 24 h to obtain 1 × 108 CFU/mL), followed by incubation at 150 rpm (Orbital Shaker, Series F200, England) at 30 °C for 72 h under shaking condition.
Effect of different nitrogen sources on biosurfactant production
Yeast extract, urea and peptone each at concentrations 0.5, 1.0, 1.5, 2.0, and 2.5 g/L in the MSM were the formulated media for the production of biosurfactant. To a sterile 1 L MSM, the nitrogen sources were added individually and the medium pH adjusted to 7. The medium was inoculated with 3-mL overnight culture of the isolate (McFarland 0.5 standardized pure culture grown on nutrient broth for 24 h to obtain 1 × 108 CFU/mL) The medium was incubated under shaking condition at 150 rpm as described above.
Determination of the effect of pH on biosurfactant production
This was done using 1000 mL of sterile 40 g/L glucose MSM. The fermentation medium pH was adjusted (pH 6 to 10) using 5 M HCl and 1% NaOH and inoculated with 3-mL overnight culture of the isolate (McFarland 0.5 standardized pure culture grown on Nutrient broth for 24 h to obtain 1 × 108 CFU/mL). This was thereafter incubated at 30 °C for 72 h with shaking at 150 rpm.
Effect of incubation temperature on biosurfactant production
This was carried out using 1000 mL of sterile 40 g/L glucose MSM which pH was adjusted to 7.0 using 5 M HCl and 1% NaOH. The cultures were incubated under shaking condition as described above. The rotary shaker temperature was varied from 25~45 °C at 5 °C intervals for 72 h.
Determination of the effect of incubation time on biosurfactant production
In 250-mL Erlenmeyer flasks, 50 mL of 40 g/L glucose MSM were dispensed. The media were inoculated with 3-mL overnight culture of the isolate. The fermentation media were previously adjusted to pH 7 before inoculation. The flasks were incubated at 30 °C under shaking condition as described previously. Samples were taken twenty-four hourly for analyses.
Biosurfactant extraction
For biosurfactant extraction, the culture broth was centrifuged (10,000 rpm, at 4 °C for 20 min) to get a cell-free supernatant. Thereafter, the supernatant pH was adjusted to 2.0 with 0.5 M HCl. This was stood for 24 h for precipitate formation and then equal volume of chloroform: methanol (2:1) mix was added to the tube, shaken vigorously and left to stand overnight. Following Anitha et al.’s [16] protocol, white-coloured sediments were collected as the crude biosurfactant after 24 h.
Column chromatography purification
A 50 g of slurry of silica gel was loaded on the column. An aliquot of fractionated biosurfactant 13:20, 20:15, 15:25, 20:30 and 10:15 v/v was each introduced into the solvent system (chloroform and methanol). Two millilitres (2 mL) of eluent was collected after every 10 min and 32 different fractions were collected.
Partial purification of the fractions by thin layer chromatography (TLC)
A commercially prepared aluminium TLC sheet covered with silica gel was used for the partial purification of the fractions. The plates were cut to fit 5 × 5 cm size. Eluents were placed at distance of 0.5 cm from the bottom of the TLC plate followed by placing the plate in a chromatographic tank containing mixture of chloroform and methanol (15:25).
Structural identification of biosurfactant
Structural classification of the biosurfactant was carried out using FTIR and GC-MS. An FTIR machine (Buck scientific M530 USA FTIR) equipped with a detector of deuterated triglycine sulphate and beam splitter of potassium bromide was used for the analysis. Gram A1 software was used to obtain the spectra and to manipulate them. One milligramme of the biosurfactant sample was mixed thoroughly with 100 mg of homogenized porcelain-milled KBr. FTIR spectra was obtained at the frequency regions of 4000–600 cm−1 and co-added at 32 scans and at 4 cm−1 resolution. The values of the FTIR spectra were shown as transmitter.
The GC-MS analysis was done using GC-MS (Agilent Technologies, Agilent GCMSD 7890 B, USA) chromatograph. Carrier gas used was helium, the flow rate was set as 1.5 mL min−1 and the working temperature of the GC injector ranged between 240 and 260 °C.
Statistical analysis of data
The results obtained were presented in graphs, tables and charts. Results were statistically analyzed using analysis of variance (ANOVA) at 99% confidence level. Means were separated using Duncan’s Multiple Range Test.
Results
Preliminary biosurfactant production screening of the isolates
The isolate with code MS1(3) C which was identified as Lysinibacillus fusiformis MK559526 showed high biosurfactant production activity. The zone of β-haemolysis, oil spreading and emulsification index of 7.30 ± 0.16 mm, 7.20 ± 0.26 mm and 65.15 ± 0.35% respectively wereobtained. The crude oil displacement and kerosene emulsification by Lysinibacillus fusiformis were presented in Figs. 1 and 2, respectively.
Effects of pH, temperature and incubation period on Lysinibacillus fusiformis MS1(3) C biosurfactant production
The optimum pH for the Lysinibacillus fusiformis was 7.0. Subsequent increase in pH level showed a considerable decline in biosurfactant production (Table 1). Similarly, the effect of temperature on biosurfactant production is presented in Table 1. The optimum temperature was confirmed as 35 °C. The effect of different incubation periods on biosurfactant production potential of Lysinibacillus fusiformis are also shown Table 1. The results showed that an increase in incubation period increased biosurfactant production (Table 1). Lysinibacillus fusiformis showed maximum biosurfactant production of E24 68.08 ± 2.00%, surface tension 35.99 ± 1.21 mN/m and cell dry weight 1.04 ± 0.05 g/L at 120 h.
Biosurfactant activity of Lysinibacillus fusiformis under different concentrations of carbon and nitrogen
Table 2 showed the biosurfactant activity of Lysinibacillus fusiformis on different concentrations of carbon sources. Among the carbon sources, 40-g soluble starch was the most suitable for Lysinibacillus fusiformis (E24 77.70 ± 0.50%, surface tension 30.99 ± 0.18 mN/m and cell dry weight 1.06 ± 0.05 g/L). Similarly, the effect of the different nitrogen source concentrations on biosurfactant production by Lysinibacillus fusiformis is presented in Table 3. Among the 3 nitrogen sources, 1.5-g urea showed the highest value (E24 78.31 ± 0.87%, surface tension 29.07 ± 1.42 mN/m and cell dry weight 0.95 ± 0.06 g/L), followed by yeast extract (Table 3).
Biosurfactant production at optimum medium concentration
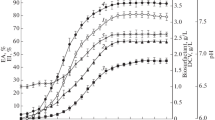
As indicated in Figs. 3, 40 g of soluble starch and 1.5 g of urea were the optimum concentrations of carbon and nitrogen sources for biosurfactant production by Lysinibacillus fusiformis. Due to the application of optimized conditions including pH, temperature and carbon and nitrogen sources, emulsification index (93.80 ± 0.41%) and surface tension (28.46 ± 1.11 mN/m) values were gradually increased to maximum level in the substrates concentration optimization compared to preliminary assay values. Biosurfactant produced by Lysinibacillus fusiformis was measured as 2.92 ± 0.04 g/L at 72 h.
FTIR profile of surfactant isolated from Lysinibacillus fusiformis
FTIR analysis of the surfactant isolated from Lysinibacillus fusiformis revealed 12 absorption peaks (Table 4). The absorption peaks demonstrated the presence functional groups. The functional groups identified were carbonyl, amine, aliphatic, alkyl chain, peptides and esters (Table 4). FTIR analysis of the biosurfactant obtained from Lysinibacillus fusiformis showed the presence of 12 absorption peaks (Fig. 4). Each peak represented a functional group of compounds. There were bands that depicted peptides at 3000–3500 cm−1 and others showing (1500–1800 cm−1) CO–N bond.
GC-MS profile of biosurfactant produced by Lysinibacillus fusiformis
The GC-MS profile of biosurfactant produced by Lysinibacillus fusiformis shown in Table 5 has 9-Octadecenoic acid (80.80%) as the predominant compound. Other major compounds included: n-Hexadecanoic acid (4.50%), trimyristin (3.94%), cis-vaccenic acid (1.81%), 3-Heptafluorobutyroxydodecane (1.74%) and oleic acid. Esters and alkanol groups were also eluted. Total ion chromatogram of biosurfactant produced by Lysinibacillus fusiformis showing the peaks of the individual compounds is presented in Fig. 5. 9-Octadecenoic acid was the predominant compound in the biosurfactant.
Discussion
Ample reports suggest that biosurfactant-producing Bacillus species can be obtained from hydrocarbon-contaminated habitats [17, 18]. In the present study, Lysinibacillus fusiformis obtained from automobile-mechanic-workshop oil contaminated sites, screened for potential for biosurfactant production, showed high emulsification index, oil displacement and zone of haemolysis (65.15 ± 0.35%, 0.26 ± 7.20 mm and 0.16 ± 7.30 mm, respectively). It showed positive drop collapse test and had a β-haemolytic action. These attributes showed Lysinibacillus fusiformis as a good biosurfactant producer. El-Sersy [19] obtained an emulsification index of 70.3% and a positive drop collapse result for the Bacillus subtilis N10. Similarly, Elazzazya et al. [5] demonstrated that a marine organism, Virgibacillus salaries gave E24 value of 80%, confirmed positive results for drop collapse, oil displacement and blood haemolysis.
In this study, pH, temperature, incubation period, carbon and nitrogen sources influenced biosurfactant production by Lysinibacillus fusiformis. The result of the effect of pH on growth and biosurfactant production showed maximum production of biosurfactant at pH 7. There was a reduced biosurfactant production as the pH of the medium increased. The study corroborated that of Jagtap et al. [20] and Husam and Ahmed [21] which showed maximum biosurfactant production at pH 7. Similarly, the role of temperature on biosurfactant production showed that the Lysinibacillus fusiformis gave the highest emulsification index, surface tension and cell dry weight at 35 °C indicating the isolate is a mesophilic bacterium. In a related study, El-Sersy [19] observed that the temperature 35 °C resulted in a high yield of biosurfactant production by a Bacillus species.
Increasing the incubation period of Lysinibacillus fusiformis led to a corresponding increase in biosurfactant production. The organism gave the maximum cell dry weight of 1.22 ± 0.04 g/L after 72 h of growth. However, the highest reduction in surface tension of the culture broth (35.99 ± 1.21 mN/m), and the highest emulsification index (68.08 ± 2.00%) were both obtained after 120 h. The result therefore supported the report of Sonali et al. [22] that the produced biosurfactant in the culture broth is a secondary metabolite.
Effects of four carbon sources screened for biosurfactant production by Lysinibacillus fusiformis showed maximum production at 40 g/L soluble starch. Jain et al. [23] reported highest production of biosurfactant at 30 g/L of starch by Klebsiella sp. RJ-03. The choice of carbon source plays a significant role on the composition of emulsifiers produced by microbes. The chemical structure of biosurfactants, particularly the hydrophobic tail is often determined by the carbon source [24]. Similarly, the effect of the different nitrogen sources on biosurfactant production by Lysinibacillus fusiformis showed that 1.5 g/L urea had optimal yield. Zhang et al. [25] obtained highest biosurfactant production at 3.0 g/L urea for a related species Bacillus atrophaeus. Nitrogen is required for microbial growth and production of certain primary and secondary metabolites [26]. The type of nitrogen source found in the production medium has an effect on the biosurfactant production by microbes [27].
Optimized conditions were used for final biosurfactant production including pH (7), temperature (35 °C), incubation period (120 h), carbon (40 g/L soluble starch) and nitrogen (1.5 g/L urea) sources. Biosurfactant yield reached its maximum (2.92 ± 0.04 g/L) at the stationary growth phase (72 h). This is contrary to the results of Joshi et al. [28] where they reported highest biosurfactant production of 1.83 g/L after 72 h. A significant reduction in the surface tension of Lysinibacillus fusiformis supernatant (39.84 ± 1.65 mN/m) was obtained after 72 h of incubation, then reaching its minimal value (28.46 ± 1.11 mN/m) after about 96 h. Thereafter, a slight increase in the surface tension was up to the end of cultivation (120 h). This may be attributed to the critical micelle concentration (CMC) value, in which the surface tension remains stable (32.99 ± 1.21 mN/m).
In the present study, the emulsification index of broth supernatant mainly fluctuated between 50 and 70%. The surface tension of broth supernatant mainly fluctuated between 30 and 50 mN/m. This result suggests a possibility of the presence of a bioemulsifier in the fermentation broth in addition to the biosurfactant. High emulsification indices show better emulsion formation and combined with reduced surface tension of the fermentation broth show high quality biosurfactant production. Ren [29] noted that glycolipid and lipopeptide biosurfactants can be produced from microbial fermentations with similar functionalities as emulsifiers and antimicrobial agents. And as will be discussed later, the present biosurfactant is lipopeptide in nature, having lipids and peptides in the GC-MS and FTIR assays. Viramontes-Ramos et al. [30] had previously reported on the interchangeability of the usage of the terms biosurfactants and bioemulsifiers and noted that whereas all bioemulsifiers are considered biosurfactants, not all biosurfactants can emulsify. Further, the molecular structure of biosurfactants is well defined.
In order to determine biosurfactant production by microorganisms, many researchers have utilized emulsification index (E24) as a measure of biosurfactant activity. Emulsification index of the biosurfactant produced by Lysinibacillus fusiformis was initially 65.15 ± 0.35%. However, as the culture conditions were optimized, the biosurfactant reduced surface tension to 28.46 ± 1.11 mN/m and increased emulsification of kerosene to 93.80 ± 0.41%. Similar to this, Viramontes-Ramos [30] obtained E24 range of 0 to 100% for both diesel and kerosene and from 76.2 to 92.8% for motor oil. Maia [31] reported E24 for the following substances (%): soybean oil 50, corn oil 65, canola oil 50, olive oil 90, waste soybean oil 50, kerosene 40 and burnt engine oil, 95 for biosurfactant produced by a bioemulsifier-Producing Bacillus subtilis UCP 0146. Further, testing the effects of different parameters on biosurfactant production, Elazzazy et al. [5] obtained an E24 values within the range 74.2 to 100% with Virgibacillus salarius surfactant.
On the other hand, lower values of E24 were also reported by some researchers. For instance, Phulpoto et al. [32] using biosurfactant produced by Bacillus nealsonii obtained 55% E24 with kerosene. Araújo et al. [33] reported E24 values of 56.7%, 51.9%, 49% and 49% for toluene, xylene, hexadecane and hexane, respectively, while Barakat et al [34] obtained 57% and 65% E24 for Bacillus amyloliquefaciens SH20- and Bacillus thuringiensis SH24-derived surfactants, respectively, using paraffin oil. Also, the E24 obtained by Gudiña et al. [35] working with biosurfactant produced by Paenibacillus strain were as follows: chloroform 63.8%, crude oil 75.1%, dichloromethane 66.1%, ethyl acetate 52.7, gas oil 15.9, heating oil 62.7, n-hexadecane 59.3, n-hexane 50.9 and paraffin 63.1%.
Although the surface tension reduction obtained by Zhang et al. [25] was lower than what was obtained in the present study, the E24 of the present study was higher than E24 reported for some carbohydrate and lipid sources. For brown sugar, they reported E24 of 61.81% with a surface tension of 26.12 mNm−1. Others included sucrose, glucose, maltose, starch, mannitol, glycerol and paraffin with the respective E24 and surface tension of 56.76%, 26.32 mNm−1; 58.34%, 26.38 mNm−1; 54.80%, 26.11 mNm−1; 56.85%, 26.39 mNm−1; 54.11%, 25.82 mNm−1; 57.43, 26.32 mNm−1; and 0.00%, 40.49 mNm−1. Much lower emulsion formation (E24 values between 7.81 and 21.73%) had been reported by Sohail and Jamil [36]. With light crude oil, Purwasena et al. [37] working with Bacillus licheniformis DSI obtained E24 of 65.19%, whereas Astuti et al. [38] obtained E24 of 72.90% while working with Pseudoxanthomonas sp. G3.
The FTIR result of the biosurfactant isolated from Lysinibacillus fusiformis showed the presence of aliphatic groups as well as peptides and esters. The present FTIR result supported that obtained by Faria et al. [39] who reported the presence of aliphatic hydrocarbons joined with a peptide moiety that is characteristic of lipopeptide-type biosurfactants. The GC-MS result showed that the compound produced by Lysinibacillus fusiformis was a lipopeptide type also. The result is similar to the work of Seghal et al. [40] and Anitha et al. [16].
Even though the chemical composition of the biosurfactant of the present study suggests that it is a lipopeptide with the specific compounds as 9-Octadecenoic acid, n-Hexadecanoic acid, trimyristin, cis-vaccenic acid, 3-Heptafluorobutyroxydodecane and oleic acid, Pradhan et al. [41] obtained a glycolipid-type biosurfactant from Lysinibacillus fusiformis S9. Using the biosurfactant, they demonstrated the inhibition of pathogenic bacterial biofilm from Escherichia coli and Streptococcus mutans. Also, Kim et al. [42] demonstrated the production of 10-hydroxystearic acid from oleic acid and olive oil hydrolysate by an oleate hydratase from Lysinibacillus fusiformis. These reports therefore show that depending on substrate, biosynthetic pathways, enzymes and the cultivation condition, Lysinibacillus fusiformis of the present study can produce a chemically diverse, different structural or functional type biosurfactant including glycolipids and lipopeptides. Further, Li et al. [43] reported on petroleum hydrocarbon utilization by Lysinibacillus fusiformis strain 15–4. They presented many specific genes responsible for the oxidation of hydrocarbon compounds to include alkane monooxygenase genes, flavin-utilizing monooxygenase genes and alkane sulfonate monooxygenase genes.
The Lysinibacillus fusiformis isolated from automobile mechanic workshop soil of the present study was capable of producing biosurfactant. The biosurfactant produced was optimized via changes in cultivation conditions leading to the production of a biosurfactant with reduced surface tension and increased emulsion formation.
Conclusions
Lysinibacillus fusiformis produced biosurfactant with initial emulsification index of 65.15 ± 0.35%. However, when the culture conditions such as temperature, pH, glucose, and urea were optimized, the biosurfactant reduced surface tension to 28.46 ± 1.11 mN/m and increased emulsification index to 93.80 ± 0.41%. At optimal condition, maximum biosurfactant production of 2.92 ± 0.04 g/L was obtained after 72 h. The biosurfactant is made of peptides and fatty acids predominantly 9-Octadecenoic acid (80.80%). Consequently, Lysinibacillus fusiformis is a good candidate for biosurfactant production.
Change history
15 March 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42770-021-00435-0
References
Parthipan P, Preetham E, Machuca LL, Rahman PKSM, Murugan K, Rajasekar A (2017) Biosurfactant and degradative enzymes mediated crude oil degradation by bacterium Bacillus subtilis A1. Front Microbiol 8:1–14
Mulligan CN, Sharma SK, Mudhoo A (2014) Biosurfactants. research trends and applications. CRC Press Taylor and Francis Group, Boca Raton, London, New York
Abdul HN, Mohamed SM, Lai Y (2018) Culture medium development for microbial-derived surfactants production—an overview. Molecules 23(5):1049. https://doi.org/10.3390/molecules23051049
Perfumo A, Smyth TJP, Marchant R, Banat IM (2010) Production and roles of biosurfactants and bioemulsifiers in accessing hydrophobic substrates. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin, pp 1501–1512
Elazzazya AM, Abdelmoneima TS, Almaghrabi OA (2015) Isolation and characterization of biosurfactant production under extreme environmental conditions by alkali-halo-thermophilic bacteria from Saudi Arabia. Saudi J Biol Sci 22(4):466–475
Shoeb E, Faiza A, Uzma B, Jameela A, Samina I (2013) Classification and industrial applications of biosurfactants. Acad Res Int 4(3):243–252
Nakano MM, Corbell N, Besson J, Zuber P (1992) Isolation and characterization of Sfp: a gene that functions in the production of the lipopeptide biosurfactant, surfactin, in Bacillus subtilis. Mol Gen Genet 232:313–321
Makkar RS, Cameotra SS, Banat IM (2011) Advances in utilization of renewable substrates for biosurfactant production. J Appl Microbiol Biotechnol 1:1–19. https://doi.org/10.1186/2191-0855-1-5
Sidkey NM, Mohamed HF, Elkhouly IH (2016) Evaluation of different screening methods for biosurfactant producers isolated from contaminated Egyptian samples grown on industrial olive oil processing waste. Br Microbiol Res J 17(4):1–19. https://doi.org/10.8734/BMRI/2016/28437
Wang XB, Nie Y, Tang YQ, Wu G, Wu XL (2013) N-alkane chain length alters Dietzia Sp. strain DQ12-45-1b biosurfactant production and cell surface activity. Appl Environ Microbiol 79:400–402. https://doi.org/10.1128/AEM.02497-12
John WC (2019) Detection of surfactin gene in biosurfactant producing Bacillus species from different contaminated soil in Makurdi metropolis. PhD Thesis, Federal University of Agriculture, Makurdi, Nigeria
Balogun SA, Fagade OE (2010) Emulsifying bacteria in produce water from Niger Delta, Nigeria. Afr J Microbiol Res 4(9):730–734
Seema D, Nakuleshwar DJ (2012) Isolation of biosurfactant-producing marine bacteria. Afr J Environ Sci Technol 6(6):263–266
Hasham MM, Mohamed AF, Mohamed NH (2012) Production of biosurfactant from certain Candida strains under special conditions. Researcher 4(7):39–55
El-Shahawy MR (2014) Biosynthesis of biosurfactant by Egyptian local bacterial isolates using different agricultural wastes. J Nucl Technol Appl Sci 2(4):409–417
Anitha J, Jeyanthi V, Ganesh P (2015) Production and characterization of biosurfactant by Bacillus and its applicability in enhanced oil recovery. Int J Adv Res Biol Sci 2(5):7–16
Putri M, Hertad R (2015) Effect of glycerol as carbon source for biosurfactant production by halophilic bacteria Pseudomonas stutzeri BK-AB12. Procedia Chem 16:321–327
Barakat KM, Sahar WM, Hassan O, Darwesh M (2017) Biosurfactant production by haloalkaliphilic Bacillus strains isolated from Red Sea, Egypt. Egypt J Aquat Res 3(5):1–7
El-Sersy N (2012) A Plackett-Burman design to optimize biosurfactant production by marine Bacillus subtilis N10. Rom Biotechnol Lett 17(2):7049–7064
Jagtap S, Yavankar S, Pardesi K, Chopade P (2010) Production of bioemulsifier by Acinetobacter species isolated form healthy human skin. Indian J Exp Biol 48:70–76
Husam SA, Ahmed IM (2013) Effect of different environmental and nutritional factors on biosurfactant production from Azotobacter chrococcum. Int J Adv Pharm Biol Chem 2(3):477–481
Sonali S, Sriparna D, Dipa B (2011) Optimization of culture conditions for biosurfactant production from Pseudomonas aeruginosa OCD. J Adv Sci Res 2(3):32–36
Jain RM, Mody K, Mishra A, Jha B (2013) Colloids and surfaces. Bio-interfaces 108:199–204
Youssef NH, Duncan KE, Mcinerney MJ (2005) Importance of 3-hydroxy fatty acid composition of lipopeptides for biosurfactant activity. Appl Environ Microbiol 71:7690–7695
Zhang J, Xue Q, Gao H, Lai H, Wang P (2016) Production of lipopeptide biosurfactants by Bacillus atrophaeus 5-2a and their potential use in microbial enhanced oil recovery. Microb Cell Factories 15:168. https://doi.org/10.1186/s12934-016-0574-8
Saharan BS, Sahu RK, Sharma DA (2012) Review on biosurfactants: fermentation, current developments and perspectives. Genet Eng Biotechnol J 1:1–14
Desai AJ, Patel RM, Desai JD (1994) Advances in production of biosurfactant and their commercial applications. J Sci Ind Res 53:619–629
Joshi I, Sanket J, Yahya M, Al-Wahaibi Saif N, Al-Bahry A, Abdulkadir E, Elshafie C, Ali S, Al-Bemani B, Asma Al-Bahri A, Musallam S, Al-Mandhari R (2016) Production, characterization and application of Bacillus licheniformis W16 biosurfactant in enhancing oil recovery. Front Microbiol 7:1–14
Ren K (2018) Synthesis of some biobased surfactants, and their functionalities as emulsifiers and antimicrobial agents. Graduate Theses and Dissertations. Iowa State University, Capstones. https://lib.dr.iastste.edu/etd
Viramontes-Ramos S, Ballinas-Casarrubias Portillo-Ruiz MCML, Muñoz JVT, Rivera-Chavira BE, Nevárez-Moorillón GV (2010) Selection of biosurfactant / bioemulsifier-producing bacteria from hydrocarbon contaminated soil. Braz J Microbiol 41:668–675
Maia PCVS, Santos VP, Fereira AS, Luna MAC, Silva TAL, Andrade RFS, Campos-Takaki GM (2018) An efficient bioemulsifier-producing Bacillus subtilis UCP 0146 isolated from mangrove sediments. Colloids Interfaces 2:58. https://doi.org/10.3390/colloids2040058
Phulpoto IA, Yu Z, Hu B, Wang Y, Ndayisenga F, Li J, Liang H, Qazi MA (2020) Production and characterization of surfactin-like biosurfactant produced by novel strain Bacillus nealsonii S2MT and it’s potential for oil contaminated soil remediation. Microb Cell Factories 19:145. https://doi.org/10.1186/s12934-020-01402-4
Araújo SCS, Silva-portela RCB, de Lima DC, da fonsêca MMB, Araújo WJ, da Silva UB, Napp AP, Pereira E, Vainstein MH, Agnez-Lima LF (2020) MBSP1: a biosurfactant protein derived from a metagenomic library with activity in oil degradation. Sci Rep 10:1340
Barakat KM, Hassan SWM, Darwesh OM (2017) Biosurfactant production by haloalkaliphilic Bacillus strains isolated from Red Sea Egypt. Egypt J Aquat Res 43(2):205–211
Gudiña EJ, Pereira JFB, Costa R, Evtuguin DV, Coutinho JAP, Teixeira JA, Rodrigues LR (2015) Novel bioemulsifier produced by a Paenibacillus strain isolated from crude oil. Microb Cell Factories 14:14. https://doi.org/10.1186/s12934-015-0197-5
Sohail R, Jamil N (2020) Isolation of biosurfactant producing bacteria from Potwar oil fields: effect of non-fossil fuel based carbon sources. Green Process Synth 9:77–86
Purwasena IA, Astuti D, Syukron M, Amaniyah M, Sugai Y (2019) Stability test of biosurfactant produced by Bacillus licheniformis DS1 using experimental design and its application for MEOR. J Pet Sci Eng 183:106383
Astuti DI, Purwasena IA, Putri RE, Amaniyah M, Sugai Y (2019) Screening and characterization of biosurfactant produced by Pseudoxanthomonas sp. G3 and its applicability for enhanced oil recovery. J Pet Explor Prod Technol 9:2279–2289
Faria AF, Teodoro-Martinez DS, Barbosa GNO, Vaz BG, Silva IS, Garcia JS (2011) Production and structural characterization of Surfactin (C14/Leu7) produced by Bacillus subtilis isolate LSFM-05 grown on raw glycerol from the biodiesel industry. Process Biochem 46:1951–1957
Seghal KG, Anto TT, Selvin J, Sabarathnam B, Lipton AP (2010) Optimization and characterization of a new lipopeptide biosurfactant produced by marine Brevibacterium aureum MSA13 in solid state culture. Bioresour Technol 101:2389–2396
Pradhan AK, Pradhan N, Sukla LB, Panda PK, Mishra BK (2014) Inhibition of pathogenic bacterial biofilm by biosurfactant produced by Lysinibacillus fusiformis S9[J]. Bioprocess Biosyst Eng 37(2):139–149
Kim BN, Joo YC, Kim YS, Kim KR, Oh DK (2012) Production of 10-hydroxystearic acid from oleic acid and olive oil hydrolyzate by an oleate hydratase from Lysinibacillus fusiformis. Appl Microbiol Biotechnol 95(4):929–937
Li SW, Huang YX, Liu MY (2020) Transcriptome profiling reveals the molecular processes for survival of Lysinibacillus fusiformis strain 15-4 in petroleum environments. Ecotoxicol Environ Saf 192:110250
Author information
Authors and Affiliations
Contributions
The author has gained full consent from the responsible authorities at the institution where the research has been carried out.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Responsible Editor: Inês Conceição Roberto
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: in the original publication of the article, the first author’s name was incorrect. The correct name is Walter Chinaka John , not Walter Chika John.
Rights and permissions
About this article
Cite this article
John, W.C., Ogbonna, I.O., Gberikon, G.M. et al. Evaluation of biosurfactant production potential of Lysinibacillus fusiformis MK559526 isolated from automobile-mechanic-workshop soil. Braz J Microbiol 52, 663–674 (2021). https://doi.org/10.1007/s42770-021-00432-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00432-3