Abstract
Waste wheat straw (WWS) is the waste product from feedstock preparation process in a straw pulp mill. It has a significant annual production rate and no commercial value has been explored on this material. In this study, waste wheat straw was pretreated using an autohydrolysis process followed by mechanical refining, and the pretreated materials were further enzymatically hydrolyzed to evaluate the total sugar recovery for bioethanol production. Results show that autohydrolysis at 170 °C for 40 min followed by 6000 revolution PFI refining provided the best result in this study, where a total sugar recovery (total sugars in autohydrolysis filtrate and enzymatic hydrolyzate over total carbohydrates on raw WWS) of 70 % at 4 filter paper unit per oven dry gram (FPU/OD g) substrate enzyme charge could be obtained. The economic evaluation of this biorefinery process indicates that cellulosic ethanol production from autohydrolysis of WWS is a very profitable business, with 28.4 % of internal rate of return can be achieved based on current ethanol wholesale price in China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growing concern for energy security and greenhouse gas emissions, combined with the need for finding better strategies to handle municipal, industrial, and agricultural residues, continues to motivate a great deal of interest in conversion of lignocellulosic biomass to fuels and chemicals. Wheat straw is one of the most abundant agriculture residues produced in large amounts all over the world. Like many other lignocellulosic biomass, wheat straw is mainly composed of cellulose (33–40 % by dry weight), hemicellulose (20–25 %), and lignin (15–20 %) [30]. The abundance of carbohydrates in wheat straw renders it a promising candidate for bioethanol production.
The enzymatic conversion of lignocellulosic biomass to ethanol comprises two major processes: hydrolysis of cellulose and hemicellulose to fermentable reductive sugars and fermentation of the free sugars to ethanol. The efficiency of this technology is limited by the complex chemical and physical structure of lignocellulosic biomass. Thus, pretreatment is essential to open up the matrix structure of biomass and provide an enzyme-accessible substrate. Various pretreatment technologies, including physical, chemical, and biological pretreatment have been extensively investigated, but few of them have been successfully scaled up for commercialization due to high capital cost coupled with low conversion yield, high enzyme loading, low chemical recovery efficiency, or environmental pollutions [2, 5, 19].
Autohydrolysis, also known as liquid hot water pretreatment, is a hydrothermal process which treats the lignocellulosic biomass in a water-only media at elevated temperature (160–240 °C). This chemical-free process provides a simple, low-cost, and environmental-friendly way for generation of ethanol from bagasse, agriculture residue, energy crops, and hardwood [9, 10, 15, 21, 22, 38]. The objective of autohydrolysis is to solubilize mainly hemicellulose, provoke disruption of lignocellulosic structure, and thus making the cellulose more accessible to enzymatic attack. During the autohydrolysis, hemicelluloses are depolymerized and converted into soluble sugars and lignin is partially depolymerized and solubilized as well but the recondensation of lignin-soluble components onto biomass surface makes complete delignification impossible [1]. It is revealed that the removal of hemicellulose and change in lignin structure dramatically open up the cell wall matrix structure and expose more cellulose-accessible surface to enzyme attack [8]. In addition, the formation of degradation byproducts (organic acids, furan derivatives, and phenolic compounds) from monomeric sugars at an elevated temperature can lower the yield of fermentable sugars and may inhibit the yeast activity during the fermentation. The amount of degradation products generated in autohydrolysis is largely driven by the severity of the reaction.
Mechanical refining has been widely used in the pulp and paper industry to promote fibrillation of fiber and increase paper strength. Refining of pretreated biomass has been shown to improve significantly the efficiency of enzymatic hydrolysis [39]. It has been found to be able to shorten fiber length, create microfibrils on fiber surface, promote fiber swelling, and loosen fiber internal structure [16]. It has been reported that mechanical refining could generate substantial improvements on enzymatic digestibility of corn stover [7, 36], hardwood [20], and recovered office printing paper [6].
In this study, autohydrolysis combined with mechanical refining of a special wheat straw (refers to waste wheat straw), which is generated from raw material preparation process in a straw pulp mill, was investigated. If the biorefinery mill can be co-located with the pulping mill, the zero cost of waste wheat straw will render this waste material very attractive as a bioethanol feedstock candidate. Therefore, this study is aimed at (a) identifying the best autohydrolysis condition for waste wheat straw to maximize the fermentable sugars yield and minimize the degradation byproducts; (b) determining the financial value of bioethanol production from autohydrolysis of waste wheat straw in China.
Methods
Raw Materials
Wheat straw is one type of widely used raw materials for pulping and paper making in China. To keep the good quality of the pulp, wheat straw is usually cut, crushed, and screened to remove dirt, sand, ash, and leaf debris. The reject comprises a fair amount of carbohydrates but have no commercial application yet. The reject used in this study was provided by a Chinese pulp and paper company, which has a pulp production rate of 400,000 metric tons per year. The mill has a plan to expand its annual production to 800,000 metric tons in the near future. For every ton of pulp produced, 0.6 tons of reject is generated. The reject has a very high ash content of 58 % and contains largely of dirt and sand. The reject is screened in a laboratory 20-mesh screen. Most of the dirt and sand passed through the screen. The reject retained by the 20-mesh screen (about 50 % of the original reject) is used for this study and is referred as waste wheat straw (WWS). The screening of the reject was kindly done by Prof. Yongcan Jin of Nanjing Forestry University, and only WWS was shipped to our laboratory for this study. The raw WWS with an initial moisture content of 10 % was stored in an air-tight plastic bag at room temperature without any physical treatment prior to composition analysis and treatment.
Autohydrolysis
Autohydrolysis was carried out in a 300-ml Alloy C276 Parr reactor (Parr Instrument Company, Moline, IL, USA). WWS and water were mixed in the vessel at a ratio of 4:1 (w/w) (72 g water and 18 dry g WWS). The residence time at target temperature was set at 10, 20, 40, and 60 min for 170 °C and 10, 20, and 40 min for 180 °C. Only 10-min residence time was investigated for 190 and 200 °C. The vessel was vacuumed before heating up to minimize the effect of false pressure generated by air. The ramping time to reach the target temperature varies from 18 to 23 min depending on the target temperature. The vessel was equipped with a stirrer operated at a speed of 30 revolutions/min. Triplicate runs were performed. After reaction, the whole reactor was quenched in a cold water bath and then pretreated samples were taken out and squeezed using cheese cloth. The filtrate was collected for solids content, pH measurement, sugar analysis, and byproduct measurements. The remaining solids were washed with excess water, centrifuged, and stored in a sealed plastic bag in a 4 °C cold room for solids yield measurement, composition analysis, PFI refining, and enzymatic hydrolysis. The moisture content of the pretreated samples was measured in a 105 °C convection oven by weight difference of wet sample and oven dry (OD) sample. The total solids yield of pretreated samples was calculated by Eq. (1).
For integrated process, the pretreated samples were directly collected in sealed plastic bags for PFI refining and enzymatic hydrolysis without solids and liquor separation.
PFI Refining
Part of the pretreated samples was subjected to a 6000 revolution refining in a PFI mill (Hamjern Maskin A/S, Hamar, Norway) using 24 g of dry biomass at 10 % consistency according to Technical Association of the Pulp and Paper Industry (TAPPI) method T248 sp-00. The samples were then centrifuged with cheese cloth, fluffed, and sealed in plastic bags in a cold room. For integrated process, the refined samples were directly stored in sealed bags for future use without centrifuging.
Enzymatic Hydrolysis
Enzymatic hydrolysis was performed in 50-ml centrifuge tubes in an environmental incubator shaker (New Brunswick Scientific, Edison, NJ, USA) set at 50 °C, 180 rpm for 96 h. Two oven dry grams of pretreated samples were immersed into 50-mM acetate buffer (pH 4.8) to achieve a 5 % (w/v) solids loading. Sodium azide (0.1 % w/v) was added in the mixture to inhibit microbial growth during the hydrolysis [42]. A commercial cellulase complex, including a cellulase mixture (C-tec2) and a xylanase (H-tec2) was generously provided by Novozymes (Franklinton, NC). The activity of the cellulase (C-tec2) was determined to be 139 filter paper unit (FPU)/mL according to a method described by Ghose [13]. A dosage of 4 FPU/OD g substrate (5.2 mg enzyme protein per gram of substrate) was added into the mixture supplemented with H-tec2 at a dosage of a ninth of the C-tec2 which was recommended by Novozymes. In addition, a high enzyme dosage of 10 FPU/OD g substrate was also investigated. The enzymatic hydrolysis was stopped by soaking the samples into the boiling water for 10 min and then centrifuged. The supernatants were filtered through 0.2-μm filter for sugar analysis. All the measurements were duplicated.
Analytical Methods
The determination of extractives was carried out according to TAPPI method T 204 cm-97 [37]. The total solids, ash content, acid-soluble lignin, acid-insoluble lignin of untreated and pretreated straw, and sugar analysis were measured according to National Renewable Energy Laboratory’s (NREL) analytical procedures [31, 33, 34]. The elemental analysis of WWS ash was carried out by the Department of Soil Science at North Carolina State University using a PerkinElmer 2400 analyzer. The concentration of sugars (glucose, xylose, galactose, arabinose, and mannose) was quantified by a high-performance liquid chromatography (HPLC) system (Agilent 1200, Agilent, Santa Clara, CA, USA). The sugar samples were filtered by 0.2-μm filter and passed through Shodex SP0810 column (8 × 300 mm, Showa Denko, Tokyo, Japan) at the temperature of 80 °C. The mobile phase for the column was Milli-Q water at a flow rate of 0.5 mL/min. A refractive index detector was used to quantify all the sugar concentrations at the temperature of 50 °C. The byproducts (acetic acid, formic acid, furfural, and HMF (5-hydroxymethyl furfural)) were determined using a HPLC system (Dionex UltiMate 3000, Dionex Corporation, Sunnyvale, CA, USA), equipped with a Bio-Rad Aminex HPX-87H column (300 mm × 7.8 mm), a Bio-Rad Micro-Guard column, and a refractive index detector. The analytical column was operated at 65 °C with 0.005 M H2SO4 as the mobile phase at a flow rate of 0.6 mL/min. The acid hydrolysis of the prehydrolyzate was carried out to break down all the oligo-sugars released in prehydrolyzate according to the NREL’s analytical procedure [32]. The prehydrolyzate was hydrolyzed with 4 % w/v sulfuric acid at 121 °C for 1 h. The yields of total sugars, monomeric sugars, and sugar degradation products were calculated based on per gram of raw biomass.
Process Simulation
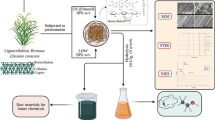
A complete process model was developed using WinGEMS V.5.3, which is widely used in the pulp and paper industry for mass and energy balance. The proposed process for cellulosic ethanol production from WWS is illustrated in Fig. 1. WWS is subject to autohydrolysis at 170 °C for 40 min. After blowing, the blow heat is recovered to preheat the make-up water in the digester, and the slurry is diluted to 4 % and sent to mechanical refining. The refined pulp goes through a screw press, where liquor-solid separation takes place. The liquor is heat exchanged and detoxified by an ion exchange resin and recycled back as dilution liquor for mechanical refining feed. The solids are diluted to 20 % consistency and sent to a multiple-stage enzymatic hydrolysis system [41]. A five-carbon and six-carbon separated fermentation is carried out, where a 95 % conversion efficiency for hexoses and 80 % for pentoses is assumed. After distillation, the beer column tops are further dehydrated to 99.5 % cellulosic ethanol as a final product and the beer column bottoms pass through a clarifier to separate the liquor and solids. The liquor is recycled back as dilution water, while the solids are sent to the fertilizer plant as raw material for fertilizer production because of high potassium and lignin content in the residues [17]. It is noted that due to the high ash content, the residues are not considered a good fuel for biomass boiler.
Economic Analysis
Economic model was built based on the outputs of simulation results. Major assumptions applied in the financial analysis are listed in Table 1. Briefly, the project life was set at 15 years with a feedstock supply of 200,000 dry metric tons per year. Ten-year straight line depreciation schedule was applied and the discount rate was set at 12 %. The tax rate was set at 25 % with enterprise income tax only because of the tax subsidy on cellulosic ethanol in China. A terminal value in year 15 of five times of year-15 earnings before interest, taxes, depreciation, and amortization (EBITDA) was assumed. The maintenance expense was estimated as a function of the replacement asset value (RAV), where the investment cost to replace the original asset escalates annually in cost at 2 %. Capital reinvestment and other mill fixed costs were estimated at 1 and 3 % of replacement asset value, respectively. The overhead was assumed to be 3 % of annual sales. The enzyme price is assumed at US$1 per kilogram enzyme product which equals to US$5.7 per kilogram enzyme protein according to the methodology developed by Phillips [29]. All the other prices were set according to 2013 Chinese market prices.
Results and Discussion
Chemical Composition of Waste Wheat Straw
The composition analysis of WWS is summarized in Table 2. The total carbohydrates of WWS are 47.3 % of the total weight, including glucan 28.2 %, xylan 13.1 %, and other minor sugars 6.0 %. It is noted that the ash content of WWS is very high, reaching up to 25 % of the total weight. The elemental analysis on ash content (Table 3) shows that 19.6 % of ash comes from silicon and no heavy metal contamination was detected. It is of interest to know that the high amount of potassium, which is essential for cell metabolism, was found in WWS ash, making the final residue a potential raw material for fertilizer production. It is obvious that the WWS has a distinct composition difference compared to that of regular wheat straw. The value of WWS is not as high as regular wheat straw in terms of lower carbohydrates content and high ash content. However, considering the zero cost of the WWS feedstock, the production of bioethanol from WWS would be economically feasible if an efficient biorefinery process is applied.
Effect of Autohydrolysis Conditions on pH and Solids Yield
Autohydrolysis is catalyzed by hydronium ions from the water autoionization and acetic acid released from acetyl groups in hemicellulose [12, 24]. During the autohydrolysis, the cleavage of acetyl groups in raw material lowers the pH of the filtrate to between 3 and 4. To some extent, the pH value of the autohydrolysis filtrate is related to the reaction of the hemicellulose and indicates the severity of the treatment. As shown in Fig. 2, the acidity of the filtrate is higher with an increase of the autohydrolysis temperature and time, indicating a higher temperature and a longer time to promote the cleavage of acetyl groups in hemicellulose. Nevertheless, the pH of the WWS filtrate is relatively higher than that of other feedstock in similar treatment condition [10, 14, 22, 27]. This is probably due to the high ash content in WWS which neutralizes the acid generated during autohydrolysis. It is noteworthy that the high pH of the prehydrolyzate contributes to minimizing the formation of degradation byproducts and potentially eliminating the need for conditioning chemicals before enzymatic hydrolysis.
As illustrated in Fig. 2, elevated temperature and residence time contributed to lower solids recovery yield. The solids yield decreased from 74.0/100 g raw WWS after 10-min pretreatment at 170 °C to 46.6/100 g raw WWS after 10-min autohydrolysis at 200 °C. The decrease of solids recovery yield is in agreement with the drop of pH in the prehydrolyzate, indicating an increased depolymerization of WWS under severe autohydrolysis conditions.
Effect of Autohydrolysis on Composition of Waste Wheat Straw
The components of autohydrolysis residues at different conditions are shown in Table 4. The solubilization of ashes dominated the reduction of solid residues, buffering the acidity of the prehydrolyzate. Increased amount of extractives under higher temperature indicates more extractable small molecular substances were produced during the treatment, showing a notable influence of temperature on the depolymerization of biomass. Higher temperature and longer residence time promoted the removal of lignin during autohydrolysis. It is observed that nearly 40 % of lignin in the raw WWS was removed after autohydrolysis at 200 °C for 10 min.
Xylan is the major hemicellulose in waste wheat straw. Higher temperature and longer residence time helps to solubilize hemicellulose. Both 40-min autohydrolysis at 180 °C and 10-min autohydrolysis at 200 °C reduced the xylan content from 13.1/100 g (in untreated WWS) to 2.0/100 g raw WWS. The minor sugars such as galactan, arabinan, and mannan showed a greater reduction as well under harsh condition and no significant minor sugars were detected in the residue. The cellulose was nearly untouched during the autohydrolysis, resulting in a cellulose-enriched substrate. It is observed that the glucan content of 200 °C and 10-min pretreated WWS residue accounted for 50.9 % of the total solids, which provided an excellent cellulose enriched substrate for subsequent enzymatic hydrolysis.
Sugars and Byproducts in Autohydrolysis Filtrate
Autohydrolysis contributes to the recovery of mainly hemicellulose in the prehydrolyzate. In this study, autohydrolysis filtrates were hydrolyzed using 4 % sulfuric acid to decompose all oligomeric sugars into monomeric forms. The total sugar released into prehydrolyzate showed a pronounced increase from 3.4/100 g raw WWS in untreated WWS to a maximum of 8.5/100 g raw WWS when WWS was treated at 180 °C for 20 min or 190 °C for 10 min (Fig. 3). When biomass was treated at 170 °C, the longer residence time helps to release more sugars, especially xylan into the prehydrolyzate. However, at 180 °C, the sugars released in treated prehydrolyzate started to decrease after the maximum sugar amount was reached at 20 min. It is probably due to the degradation of monomeric sugars to furfural, HMF, and other byproducts under severe conditions.
Sugar degradation products from autohydrolysis, such as organic acids, furfural, and HMF (5-hydroxymethyl furfural), which are known to inhibit microorganisms during fermentation process [18, 26], were investigated as well. Figure 4 shows that higher temperatures and longer residence times increased the total amount of byproducts. However, the total amount of byproducts generated in this study is substantially less than that in another study at similar conditions [10]. It is probably because high pH value in the filtrate can help minimize the degradation of monomeric sugars to various byproducts. The autohydrolysis at 180 °C for 40 min generated the highest amount of byproducts, which is in agreement with the decline in the sugar content in the prehydrolyzate as discussed above.
Effect of Autohydrolysis Conditions on Enzymatic Hydrolysis
Tables 5 and 6 display the material balance from autohydrolysis followed by enzymatic hydrolysis at different enzyme dosages. The autohydrolysis-treated WWS exhibited a substantial increase by 2–3-folds of total enzymatic hydrolysis sugar yield in contrast to untreated raw material (Table 5). The enzymatic digestibility of cellulose increased progressively with elevated temperatures and longer residence times. The highest glucan yield 21.1/100 g raw WWS after enzymatic hydrolysis is observed when WWS was pretreated at 200 °C for 10 min without mechanical refining. In contrast, the enzymatic efficiency of xylan displayed a notable reduction at harsh conditions. A large amount of xylan was released to the prehydrolyzate and some were degraded to form byproducts under harsh condition, leaving a xylan-deficient substrate for enzymatic hydrolysis.
It is widely acknowledged that the recalcitrance of the lignocellulosic biomass originates from the lignin and hemicelluloses’ strong molecular framework which protects the cellulose from the attack of cellulolytic enzymes [4, 28]. In this study, a couple of reasons can be speculated to explain the improvement of digestibility of autohydrolysis-treated WWS. Firstly, the solubilization of hemicellulose and acetyl groups may remove the physical barriers and increase the accessibility of the cellulose to enzyme. This is consistent with results obtained in this work where the residue xylan amount in pretreated pulp is proportional to the enzymatic digestibility (Fig. 5). Secondly, the partially solubility of lignin after autohydrolysis facilitated the digestibility of the biomass by removing the physical barrier caused by lignin [25] or reducing the non-productive adsorption of cellulase to lignin [3, 11]. It is noted that the autohydrolysis does not feature largely delignification effects, but the redistribution of lignin has been reported on maize cell walls, which opens up the structure of the cell wall and therefore improves the accessibility of the cellulose microfibrils [8].
Effect of Refining on Enzymatic Digestibility of Autohydrolysis-treated WWS
The PFI refining significantly improved the cellulose conversion during enzymatic hydrolysis. As shown in Table 5, an average increase of 20 % glucan conversion was achieved when autohydrolysis pretreated WWS was refined. The major effects of refining on the substrate can be summarized as fiber shortening, fiber fibrillation, swelling (penetration of water into the cell wall) and hydration (breaking of some intra-fiber bonds and replaced with water-fiber hydrogen bonds) [35]. It is speculated that refining improved the enzymatic digestibility via swelling of the fiber and internal fibrillation. It is noted that almost no improvement was made by refining for 200 °C autohydrolysis-treated biomass, indicating that a severe disruption of biomass structure under this condition allows enzymes to attack cellulose easily without additional post-treatment.
Effect of Enzyme Charge on Enzymatic Digestibility
Effect of higher enzyme dosage on enzymatic hydrolysis was also evaluated to see the maximum sugar recovery yield that can be achieved from autohydrolysis pretreated substrate (Table 6). The 10 FPU/OD g substrate enzyme dosages improves enzymatic sugar yield by 1.4 to 5.8/100 g raw WWS compared to that at 4 FPU/OD g substrate enzyme charge. No significant improvement has been observed with higher enzyme charge for severely treated substrate such as 180 °C for 40 min and 200 °C for 10 min combined with refining. This might be due to the limited amount of sugar content remains in the pretreated biomass after a significant amount of sugar released to the prehydrolyzate. It is observed that autohydrolysis pretreated WWS at 170 °C for 40 min followed by refining showed a maximum enzymatic sugar yield of 30.0/100 g raw WWS when 10 FPU/OD g substrate enzyme was charged compared to 25.3/100 g raw WWS enzymatic sugar yield at 4 FPU/OD g substrate enzyme dosage. It is speculated that either increasing enzyme loading or applying efficient autohydrolysis pretreatment can help improving enzymatic digestibility.
Total Sugar Recovery
The total sugar recovery was calculated by the sum of sugars released in autohydrolysis prehydrolyzate and enzymatic hydrolyzate over total carbohydrates in the raw WWS. Table 5 shows that higher temperature and longer residence time enhanced the enzymatic sugar yield but hampered the sugar yield in the filtrate through sugar degradation. The results indicate that refining improved the total sugar recovery by an average of 8 %. The highest total sugar recovery around 70 % could be achieved when WWS was pretreated at 170 °C for 40 min, 180 °C for 20 min, or 190 °C for 10 min followed by refining. But autohydrolysis at 170 °C for 40 min was recommended for bioethanol production because of its lower steam pressure requirement for reactor feeding system.
Effect of Solids and Liquor Separation After Autohydrolysis on Total Sugar Recovery
After autohydrolysis, pretreated pulp and prehydrolyzate were separated and pulp was further washed to remove all the non-structure sugar attached to the fiber surface. The major purpose of this operation is to acquire a better understanding of biomass characteristics after autohydrolysis and the ratio of sugar yield from autohydrolysis filtrate and enzymatic hydrolyzate. However, in a commercial process, all the slurry after autohydrolysis would be subjected to enzymatic hydrolysis to reduce complexity of the process and maintaining a high sugar concentration. Therefore, the effect of solids and liquor separation after autohydrolysis on total sugar recovery was investigated. Figure 6 displays that the solid and liquor separation after autohydrolysis has a slight increase of total sugar recovery for most cases. The improvement may come from the removal of inhibitions like unbounded lignin and other impurities generated during autohydrolysis [23]. However, considering the complexity of adding wash process and increased capacity for enzyme saccharification, fermentation, and ethanol purification, the integrated process seems to be more economically feasible; though a small amount of ethanol yield might be sacrificed.
Economic Evaluation
Economic evaluation was performed on the scenario of autohydrolysis at 170 °C for 40 min followed by refining and enzymatic hydrolysis at 4 FPU/OD g substrate enzyme loading, which yields the highest sugar recovery and relatively lower byproducts. The following economic indicators were evaluated including net present value (NPV), internal rate of return (IRR), and minimum ethanol revenue (MER). The NPV is defined as the sum of discounted free cash flow at target rate of return (12 % in this study) at each project year. The IRR corresponds to the discount rate that gives a zero NPV and it is as widely used as a metric to evaluate the return on investment for each project. The MER is defined as the required minimum wholesale price of ethanol to achieve a specific internal rate of return.
Table 7 displays that the total capital investment on this project is around US$37 million, corresponding to US$0.86 capital expenditure (CAPEX) per liter ethanol product. It is noted that the equipment cost and installation cost are much cheaper in China than those in the USA, resulting in a relatively low capital investment. The total cash cost is US$0.45 per liter ethanol, amid which energy cost accounts for the highest portion, followed by enzyme cost. This biorefinery process presents a net present value of approximately US$83 million at 12 % discount rate and 28.4 % of internal rate of return based on Chinese ethanol wholesale price, indicating a very profitable business. The MER at 12 % internal rate of return is US$0.56 per liter ethanol, much lower compared to US$0.8 per liter ethanol wholesale price in China. Overall, the cellulosic ethanol production from autohydrolysis of WWS displays a very attractive approach for commercialization of cellulosic ethanol. The major reasons can be attributed to the zero cost of raw material and low capital cost due to process simplicity.
Conclusion
Waste wheat straw from feedstock preparation process in a straw pulp mill was subjected to an autohydrolysis pretreatment with different temperatures and residence time. The pretreated materials were further refined and enzyme hydrolyzed. Results showed that during autohydrolysis, 3.4 to 8.5/100 g raw WWS sugar can be recovered from prehydrolyzate, and 0.5 to 2.9/100 g raw WWS byproducts can be generated as well under different pretreatment conditions. For the maximum enzymatic sugar yield at 4 FPU/OD g substrate enzyme dosage, 23.4/100 g raw WWS enzymatic sugar was achieved when WWS was pretreated at 200 °C for 10 min without mechanical refining, and 25.8/100 g raw WWS enzymatic sugar was obtained when WWS was pretreated at 180 °C for 40 min followed by mechanical refining. The highest total sugar recovery including sugar recovered from autohydrolysis filtrate and enzymatic hydrolyzate based on total carbohydrates in raw WWS can be approximately 70 % at affordable enzyme dosage when WWS was pretreated at 170 °C for 40 min or 190 °C at 10 min. But considering the feed system limitations at the mill, autohydrolysis at 170 °C at 40 min followed by 6000 revolution PFI refining was recommended for bioethanol production. The economic evaluation based on the optimal condition in this study indicates that cellulosic ethanol production from autohydrolysis of WWS is a very attractive business, which can generate 28.4 % internal rate of return based on current ethanol wholesale price in China.
References
Alvira, P., Tomás-Pejó, E., Ballesteros, M., & Negro, M. (2010). Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresource Technology, 101, 4851–4861.
Banerjee, S., Mudliar, S., Sen, R., Giri, B., Satpute, D., Chakrabarti, T., & Pandey, R. A. (2010). Commercializing lignocellulosic bioethanol: technology bottlenecks and possible remedies. Biofuels, Bioproducts and Biorefining, 4, 77–93.
Berlin, A., Gilkes, N., Kurabi, A., Bura, R., Tu, M., Kilburn, D., & Saddler, J. (2005). Weak lignin-binding enzymes. Applied Biochemistry and Biotechnology, 121, 163–170.
Bidlack, J., Malone, M., & Benson, R. (1992). Molecular structure and component integration of secondary cell walls in plants. Proceedings of the Oklahoma Academy of Science, 72, 51–56.
Brown, T. R., & Brown, R. C. (2013). A review of cellulosic biofuel commercial—scale projects in the United States. Biofuels, Bioproducts and Biorefining, 7, 235–245.
Chen, H., Venditti, R. A., Jameel, H., & Park, S. (2012). Enzymatic hydrolysis of recovered office printing paper with low enzyme dosages to produce fermentable sugars. Applied Biochemistry and Biotechnology, 166, 1121–1136.
Chen, X., Tao, L., Shekiro, J., Mohaghaghi, A., Decker, S., Wang, W., Smith, H., Park, S., Himmel, M. E., & Tucker, M. (2012). Improved ethanol yield and reduced minimum ethanol selling price (MESP) by modifying low severity dilute acid pretreatment with deacetylation and mechanical refining: 1) experimental. Biotechnology for Biofuels, 5, 60.
Donohoe, B. S., Decker, S. R., Tucker, M. P., Himmel, M. E., & Vinzant, T. B. (2008). Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnology and Bioengineering, 101, 913–925.
Ertas, M., Han, Q., & Jameel, H. (2014). Acid-catalyzed autohydrolysis of wheat straw to improve sugar recovery. Bioresource Technology, 169, 1–8.
Ertas, M., Han, Q., Jameel, H., & Chang, H.-M. (2013). Enzymatic hydrolysis of autohydrolyzed wheat straw followed by refining to produce fermentable sugars. Bioresource Technology, 152, 259–266.
Esteghlalian, A. R., Srivastava, V., Gilkes, N., Gregg, D. J, Saddler, J. N. (2000). An overview of factors influencing the enzymatic hydrolysis of lignocellulosic feedstocks. In: Glycosyl Hydrolases for Biomass Conversion, American Chemical Society, 769, 100–111.
Garrote, G., Domı́nguez, H., & Parajó, J. C. (2001). Generation of xylose solutions from Eucalyptus globulus wood by autohydrolysis—posthydrolysis processes: posthydrolysis kinetics. Bioresource Technology, 79, 155–164.
Ghose, T. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59, 257–268.
Huijgen, W., Smit, A., de Wild, P., & den Uil, H. (2012). Fractionation of wheat straw by prehydrolysis, organosolv delignification and enzymatic hydrolysis for production of sugars and lignin. Bioresource Technology, 114, 389–398.
Jing, L., Jin, Y.-C., Chang, H.-M., Han, Q., Hasan, J., & Richard, P. (2010). Effects of auto-hydrolysis of rice straw on its chemical composition and enzymatic hydrolysis. Journal of Cellulose Science and Technology, 2, 002.
Jones, B. W., Venditti, R., Park, S., Jameel, H., & Koo, B. (2013). Enhancement in enzymatic hydrolysis by mechanical refining for pretreated hardwood lignocellulosics. Bioresource Technology, 147, 353–360.
Jun-he, L. (2004). Physical and chemical properties of lignin and its study as carrier of fertilizers. Journal of Cellulose Science and Technology, 1, 008.
Kim, Y., Mosier, N. S., & Ladisch, M. R. (2009). Enzymatic digestion of liquid hot water pretreated hybrid poplar. Biotechnology Progress, 25, 340–348.
Klein-Marcuschamer, D., Oleskowicz-Popiel, P., Simmons, B. A., & Blanch, H. W. (2012). The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnology and Bioengineering, 109, 1083–1087.
Koo, B.-W., Treasure, T. H., Jameel, H., Phillips, R. B., Chang, H.-M., & Park, S. (2011). Reduction of enzyme dosage by oxygen delignification and mechanical refining for enzymatic hydrolysis of green liquor-pretreated hardwood. Applied Biochemistry and Biotechnology, 165, 832–844.
Laser, M., Schulman, D., Allen, S. G., Lichwa, J., Antal, M. J., & Lynd, L. R. (2002). A comparison of liquid hot water and steam pretreatments of sugar cane bagasse for bioconversion to ethanol. Bioresource Technology, 81, 33–44.
Lee, J. M., Shi, J., Venditti, R. A., & Jameel, H. (2009). Autohydrolysis pretreatment of coastal Bermuda grass for increased enzyme hydrolysis. Bioresource Technology, 100, 6434–6441.
Liu, H., & Zhu, J. (2010). Eliminating inhibition of enzymatic hydrolysis by lignosulfonate in unwashed sulfite-pretreated aspen using metal salts. Bioresource Technology, 101, 9120–9127.
Mittal, A., Chatterjee, S. G., Scott, G. M., & Amidon, T. E. (2009). Modeling xylan solubilization during autohydrolysis of sugar maple wood meal: reaction kinetics. Holzforschung, 63, 307–314.
Öhgren, K., Bura, R., Saddler, J., & Zacchi, G. (2007). Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover. Bioresource Technology, 98, 2503–2510.
Palmqvist, E., & Hahn-Hägerdal, B. (2000). Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresource Technology, 74, 25–33.
Pérez, J., Ballesteros, I., Ballesteros, M., Sáez, F., Negro, M., & Manzanares, P. (2008). Optimizing liquid hot water pretreatment conditions to enhance sugar recovery from wheat straw for fuel-ethanol production. Fuel, 87, 3640–3647.
Pérez, J. A., González, A., Oliva, J. M., Ballesteros, I., & Manzanares, P. (2007). Effect of process variables on liquid hot water pretreatment of wheat straw for bioconversion to fuel‐ethanol in a batch reactor. Journal of Chemical Technology and Biotechnology, 82, 929–938.
Phillips, R. B., Jameel, H., & Chang, H. M. (2013). Integration of pulp and paper technology with bioethanol production. Biotechnology for Biofuels, 6, 13.
Prasad, S., Singh, A., & Joshi, H. (2007). Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resources, Conservation and Recycling, 50, 1–39.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J. and Templeton, D. (2005). Determination of total solids in biomass. Laboratory Analytical Procedure (LAP).
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D. (2008). Determination of sugars, byproducts, and degradation products in liquid fraction process samples. NREL/TP-510-42623.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D. (2008). Determination of ash in biomass. NREL/TP-510-42622.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Crocker, D. (2011). Determination of structural carbohydrates and lignin in biomass. NREL/TP-510-42618.
Smook, G. A. (1992). Handbook for pulp & paper technologists (2nd ed.). Vancouver: Angus Wilde Publications.
Tao, L., Chen, X., Aden, A., Kuhn, E., Himmel, M. E., Tucker, M., Franden, M. A. A., Zhang, M., Johnson, D. K., & Dowe, N. (2012). Improved ethanol yield and reduced minimum ethanol selling price (MESP) by modifying low severity dilute acid pretreatment with deacetylation and mechanical refining: 2) Techno-economic analysis. Biotechnology for Biofuels, 5, 1–11.
TAPPI, T. (2007). 204 cm-97, Solvent extractives of wood and pulp. TAPPI test methods.
Treasure, T., Gonzalez, R., Venditti, R., Pu, Y., Jameel, H., Kelley, S., & Prestemon, J. (2012). Co-production of electricity and ethanol, process economics of value prior combustion. Energy Conversion and Management, 62, 141–153.
Wu, S.-F., Chang, H.-M., Jameel, H., & Philips, R. (2010). Novel green liquor pretreatment of loblolly pine chips to facilitate enzymatic hydrolysis into fermentable sugars for ethanol production. Journal of Wood Chemistry and Technology, 30, 205–218.
Wyman, C. (1996). Handbook on bioethanol: production and utilization. 1st ed. CRC.
Xue, Y. (2011). Process modifications on enzymatic saccharification for improved conversion and concentration of sugars for bioethanol. PhD thesis. North Carolina State University, Raleigh.
Zhou, X., Xu, J., Wang, Z., Cheng, J. J., Li, R., & Qu, R. (2012). Dilute sulfuric acid pretreatment of transgenic switchgrass for sugar production. Bioresource Technology, 104, 823–827.
Acknowledgments
This work was funded by the Wood-to-Ethanol Research Consortium II (WERCII). Thanks to Novozymes North America Inc. for providing enzyme samples. And also thanks to Dr. Yongcan Jin for screening and shipping the waste wheat straw.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, Q., Jin, Y., Jameel, H. et al. Autohydrolysis Pretreatment of Waste Wheat Straw for Cellulosic Ethanol Production in a Co-located Straw Pulp Mill. Appl Biochem Biotechnol 175, 1193–1210 (2015). https://doi.org/10.1007/s12010-014-1349-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1349-5