Abstract
The chitosanase from Bacillus sp. TS (CsnTS) is an enzyme belonging to the glycoside hydrolase family 8. The sequence of CsnTS shares 98 % identity with the chitosanase from Bacillus sp. K17. Crystallography analysis and site-direct mutagenesis of the chitosanase from Bacillus sp. K17 identified the important residues involved in the catalytic interaction and substrate binding. However, despite progress in understanding the catalytic mechanism of the chitosanase from the family GH8, the functional roles of some residues that are highly conserved throughout this family have not been fully elucidated. This study focused on one of these residues, i.e., the aspartic acid residue at position 318. We found that apart from asparagine, mutation of Asp318 resulted in significant loss of enzyme activity. In-depth investigations showed that mutation of this residue not only impaired enzymatic activity but also affected substrate binding. Taken together, our results showed that Asp318 plays an important role in CsnTS activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chitosan oligosaccharides attract considerable interest due to their biological activities and commercial applications in multiple fields, including biomedical, food, and chemical industries (10, 28). Chitosanases (EC 3.2.1.132) are glycoside hydrolases that catalyze the hydrolysis of β-1, 4-glycosidic linkages of chitosan and can be used to produce chitosan oligosaccharides. They represent a diverse group of enzymes classified as members of several different families on the basis of amino-acid sequence homology (1, 5, 6, 9, 20, 21, 24, 25); generally, enzymes from the same family share a similar catalytic mechanism. Over the past decade, chitosanases from the glycoside hydrolase family 46 (GH46) have been well characterized (4, 7, 14, 18–20); however, chitosanases from other families remain poorly understood to date.
Recently, several chitosanases from the glycoside hydrolase family 8 (GH8) have been discovered and characterized (1, 12, 16), and the three-dimensional structure of GH8 chitosanase from Bacillus sp. K17 determined (1). Structural biology studies revealed that its catalytic domain folds into a regular (α/α)6 barrel with a tunnel-shaped substrate-binding region. By site-directed mutagenesis studies and crystallography data analysis, several residues, such as Glu122 and Glu309 in the chitosanase from Bacillus sp. K17 were found to possess catalytic functions (1).
GH8 and GH46 enzymes employ the same inverting catalytic mechanism (1, 19, 27), with two acidic residues of Asp and Glu oriented at the cleavage site within a reasonable distance. A number of conserved residues, especially aromatic residues, interact with the −2 substrate subsite via intermolecular interactions and hydrogen bonds. Nevertheless, there exist several notable differences between GH8 and GH46 enzymes (17). In particular, GH46 enzymes display higher frequencies of Asp and Glu residues and contain more conserved nonpolar residues (e.g., Gly and Ala). Although both families can catalyze the substrates of six sugar rings, the GH46 enzymes possess more residues than GH8 enzymes bound to the subsites. Moreover, even within the GH8 families, sequence analysis indicates that GH8 chitosanases constitute a relatively independent branch of the molecular evolutionary tree (17). Thus, it is necessary to characterize the catalytic mechanism of the GH8 chitosanases. Previous studies also recognized that some residues in proximity of the catalytic residues might also play an essential role in enzyme function (4, 14, 23, 26).
In previous work, we successfully cloned the chitosanase gene from Bacillus sp. TS and characterized the enzymatic properties of the recombinant enzyme (29). Our results indicated that CsnTS belongs to the family 8 of glycoside hydrolases, which are endohydrolase enzymes that act through an inverting mechanism. Moreover, this enzyme shares 98 % sequence identity with the chitosanase from Bacillus sp. K17 (29). In this study, in order to gain a better understanding of the enzymatic characteristics of the chitosanase from Bacillus sp. TS, we investigated the functional roles of the residues surrounding the csnTS catalytic and substrate-binding sites based on the previous study of the chitossanase from Bacillus sp. K17. The residue Asp318 is in the vicinity of the active site and substrate-binding domain and is highly conserved throughout the GH8 family, especially in chitosanases. Our results indicated that, although the D318N mutant did not affect enzyme activity, other Asp318 mutations resulted in significant loss of catalytic activity. Our investigations suggested that Asp318 might play a dual role in regulating csnTS functions specifically involved in both catalytic activity and substrate-binding capability.
Materials and Methods
Strains, Plasmids, and Materials
The chitosanase gene (GenBank accession number: KU363821) was cloned from Bacillus sp. TS. The recombinant plasmid pET22b (+)-csn was constructed as described previously (29). The Escherichia coli DH5a strain was used as the host for the cloning experiments, while E. coli BL21 (DE3) competent cells were used for expressing the recombinant proteins. The strains were purchased from TransGen Biotech (Beijing, China). The chitosan substrates were purchased from Weihai Disha Marine Biological Products Co., LTD, with the degree of deacetylation of >90 %.
Site-Directed Mutagenesis
The mutants were produced by site-directed mutagenesis PCR-amplification with mutagenic oligonucleotides using TransStart FastPfu DNA Polymerase (Transgen Biotech). The PCR template was the CsnTS gene cloned into the pET29a (+) vector between the NdeI and XhoI restriction sites. The PCR procedure was performed as follows:
The first round of amplification was performed by using a common forward primer, NdeI-csn_5’ (5′- ACGCACATATGGCTGCTGCAAAGGAAATG −3′), and the reverse primer for each specific mutation. A parallel series of amplifications was performed by using a common reverse primer, XhoI-csn_3’ (5′- TTACTCTCGAGATTATCGTATCCTTCATAAATT −3′), and the forward primer specific for each mutation.
The second round of PCR amplification was performed by mixing corresponding PCR products from the first round of amplification with these products used as templates in subsequent rounds.
The third round of PCR amplification was performed using the common forward and reverse primers. The resulting 1228-bp mutated fragments were inserted between the pET29a(+) NdeI and XhoI restriction sites for expression in E. coli. The mutated DNA sequences were confirmed by DNA sequencing.
Protein Expression, Purification, and Assay
E. coli BL21 (DE3) cells were employed for expression of the wild-type Bacillus sp. TS chitosanase and its mutants. A single fresh colony was grown in Luria-Bertani (LB) broth containing 50 μg mL−1 of kanamycin overnight at 37 °C, then 1 % of the overnight culture was transferred into 500 mL LB with 50 μg mL−1 kanamycin and cultured at 37 °C with vigorous shaking (200 rpm). Isopropyl-β-D-thiogalactopyranoside (IPTG) was added to the culture broth to a final concentration of 0.1 mM upon the OD600 reaching 0.6–0.9. The induction was done at 37 °C for 4 h with vigorous shaking (200 rpm). The cells were harvested by centrifugation at 6000×g for 15 min at 4 °C. The cell pellets were washed and suspended in 20 mL of loading buffer (50 mM HEPES-KOH [pH 7.6], 1 M NH4Cl, 10 mM MgCl2, and 7 mM β-mercaptoethanol). Cell disruption was carried out by sonication and cell debris removed by centrifugation at 12,000×g for 45 min at 4 °C. The supernatant contained the crude enzyme extract.
Chitosanase and mutants were expressed as recombinant fusion proteins containing a C-terminal His tag, enabling the purification by one-step Ni-NTA affinity chromatography under the native condition. The proteins were purified and assayed using a previously described method (29). The substrate-inhibition data were quantified using a nonlinear regression to fit the experimental data to the enzyme kinetic-substrate inhibition equation using Prism software (GraphPad Prism, version 5.0 for Windows, San Diego, CA, USA).
The kinetic parameters of wild-type and mutated enzymes were determined in 100 mM sodium acetate buffer (pH 5.0) at 50 °C. In all assays, the concentration of the enzyme was maintained constant at 1 U/mL and chitosan was added to a concentration of 0.2–2.5 mg/mL.
Computer-Aided Modeling of the Wild-Type and Mutants
In this study, the Swiss-Model program (http://swissmodel.expasy.org/) was used to identify structural homologs and model the structures. The theoretical structure of CsnTS was obtained through homology modeling with the Swiss-Model server using the known crystal structure of the ChoK (1v5c) from Bacillus sp. k17 as the template. The sequence of CsnTS had the highest identity of 98 % with the template. The final model displayed an excellent geometry, with less than 1 % of residues disallowed.
The Discovery Studio 4.1 software was used to calculate intramolecular interactions (including hydrogen and cation-pi interactions) in both the wild-type CsnTS and mutants.
Thermal Unfolding Experiments
Differential scanning calorimetry (DSC) measurements were performed using a Nano DSC scanning microcalorimeter Model 5100 (Calorimetry Science Corporation, Utah, USA) at a heating rate of 1 °C per minute and an excess pressure of 3.0 atm. Protein samples were measured in the stock buffer (50 mM HEPES-KOH [pH 7.6], 10 mM MgCl2, 100 mM KCl and 30 % glycerol) and protein concentration was 2 mg/ml. The T m values were calculated by analyzing the spectrum with NanoAnalyze software (TA Instruments, New Castle, DE, USA).
Circular Dichroism (CD) Measurements
Ultraviolet CD spectra of the chitosanases and mutants were obtained using the Chirascan CD spectropolarimeter (Applied Photophysics, Leatherhead, UK). The proteins were dissolved in 10 mM sodium phosphate buffer (pH 7.0) at a concentration of 0.2–0.3 mg/mL. The scan wavelength was 0.1 cm, and measurements were undertaken at room temperature at ranges of 320–200 nm through a computer interface. The spectrum was analyzed using the software Pro-data (Applied Photophysics).
Results
Design of Mutations, Construction, and Purification of Mutant Enzymes
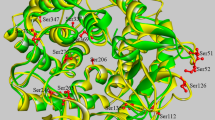
The chitosanase sequence from Bacillus sp. TS in this study shared 98 % identity with ChoK from Bacillus sp. K17. The ChoK model proposed by Adachi et al. (1) suggested that the Asp318 (i.e., Asp363 in ChoK) residue occupied a position close to the active site cleft and substrate-binding site of chitosanase (Fig. 1).
Structure view of the substrate binding site of chitosanase from Bacillus sp. K17 (PDB ID: 1V5D). a Overall view of all residues in ChoK. The residues in active site are colored by yellow (E122 in ChoK vs E77 in CsnTS; E309 in ChoK vs E264 in CsnTS). The residues in substrate binding site are colored by cyan (Y318 in ChoK vs Y273 in CsnTS; D363 in ChoK vs E318 in CsnTS; Y375 in ChoK vs Y330 in CsnTS). b View of interaction of Asp318 with residues around. The image represents a portion of the chain from the structure file in Protein Data Bank (1). Relative interactions between atoms are indicated by dashed yellow lines. The model was drawn using the PyMOL software (PyMol version 1.6, Schrödinger, LLC, New York, NY, USA)
This close proximity indicated that there might be a possible interaction between residues in the substrate-binding site and Asp318. The multiple-sequence alignment of CsnTS with other GH8 glycoside hydrolases (Fig. 2 and Table 1) revealed that Asp318 was conserved in all chitosanases and also highly conserved in most other glycoside hydrolases that belong to the GH8 family. It was, therefore, of particular interest to investigate the potential functional role of csnTS Asp318 in detail.
Multiple sequence alignment of the proteins of chitosanase members of the GH8 family. The numbering refers to the distance of the first residue from the N-terminus of the mature protein or of the precursor protein as shown in GenBank. The name and organism of origin for each sequence are listed in Table 1. a The characterized chitosanase sequences from GH8 family. b Several Glycoside hydrolase sequences from GH 8 family. All the sequences were cited from CAZy database (www.cazy.org)
Enzymatic Activity Assay of Asp318 Mutants
To assess the effect of Asp318 on the hydrolytic activity of chitosanase from Bacillus sp. TS, this residue was mutated to other amino acids, including alanine, asparagine, glutamic acid, arginine, and lysine. All mutated genes were expressed and purified using the same methods as those for wild-type chitosanase. The activities of chitosanase mutants showed that several mutants (except D318N) severely impaired the enzymatic activity (Fig. 3). In contrast, the activity displayed by the D318N mutation was comparable to that observed in wild-type chitosanase.
To better understand the functional role of Asp318 in the chitosanase catalytic mechanism, we measured the specific activity of Asp318 mutants in terms of chitosan substrate (Fig. 4). The obtained specific activities and other kinetic parameters are listed in Table 2.
Substitution of Asp318 with arginine or lysine dramatically affected catalytic activity. As shown in Table 2, the specific activities of the D318K and D318R mutants retained only 2.95 and 4.68 % of wild-type activity, respectively. This change suggested that Asp318 is important for maintaining csnTS catalytic activity. The D318A and D318E mutants also exhibited decreased specific activities, retaining only 29.6 and 16.4 % of wild-type activity, respectively. Surprisingly, the D318N mutant exhibited an enhanced specific activity relative to wild-type.
The kinetic parameters of mutated enzymes were also determined using chitosan as the substrate. We found that the K m value for all of the mutated enzymes increased (Table 2), indicating that the mutated enzyme exhibited lower affinity to the substrate as compared to wild-type. Therefore, the Asp318 residue also exhibited an important effect on chitosanase substrate binding. For the D318N mutant, although the K m value slightly increased, the k cat value was higher than that observed in the wild-type variant, suggesting that the increase in specific activity was mainly caused by changes in the rate of the catalytic reaction.
For the D318A, D318E, D318R, and D318K mutants, the results indicated that catalytic activity decreased with increases in substrate concentration (Table 2). Therefore, data were analyzed according to the substrate-inhibition model. This method was also applied to other chitosanases using both chitosan and glucosamine oligosaccharides as substrates (3, 8, 13, 30).
We also determined the optimum pH for the D318R and D318K mutants and found that both mutants had the same optimum value (pH 5.0) as the wild-type chitosanase from Bacillus sp. TS (data not shown).
Thermal Unfolding Assay for Wild-Type and Mutant Variants
The thermal unfolding processes associated with the wild-type and mutant chitosanases were monitored using DSC (Fig. 5a). For wild-type chitosanase, the T m value obtained from the peak heat capacity was 67.1 °C, and the T m value for D318N was 67.5 °C. The T m value for the D318A mutant was 63.6 °C, while those for the D318K and D318R mutants dramatically decreased to 57 °C. These results suggested that these mutations might affect protein conformation.
Mutation of Asp318 Does not Change the Global Conformation of Chitosanase
To investigate conformational changes induced by mutation, we compared the CD spectra of mutant chitosanases with those of the wild-type variant. As shown in Fig. 5b, although some spectra were slightly different from the others, the spectra of the mutated chitosanases appeared unchanged relative to those observed for the wild-type enzyme. This suggested that there were no global conformational changes between the mutated chitosanases and the wild-type.
Intermolecular Interaction of CsnTS and Mutants
To characterize the effect of Asp318 on the catalytic activity, we compared computer-simulated 3D structural models between CsnTS and the mutants (Fig. 6). The intermolecular interaction of Glu77 and Glu264 did not change in all Asp318 mutants. The results indicate that Asp318 did not affect the intermolecular interaction of catalytic residues; rather, it might affect the catalytic activity via changing the electrostatic environment. Asp318 interacts with Tyr273 via hydrogen-bonding. Except for the D318N mutant and the wild-type, the hydrogen-bonding between these two residues disappeared in all other mutants (Fig. 6 and Table S1). In the wild-type CsnTS, Asp318 interacts with four other residues: Tyr273, Gly328, Ser329, and Tyr330, while in D318N mutant, Asn318 interacts with three other residues: Tyr273, Ser329, and Tyr330. In D318A and D318E mutants, this residue interacts with two other residues: Ala318 interacts with Gly328 and Tyr330 in the D318A mutant, while E318 interacts with Ser329 and Tyr330 in the D318E mutant. However, in D318K and D318R mutants, the residue interacts with only one residue, namely Tyr330. Compared to the wild-type CsnTS and D318N mutant, the other mutants lost the interaction with Tyr273. Especially for the two basic-residue mutants, only the interaction with Tyr330 remained. Previous work indicates that these interactions could stabilize substrate binding (17). As a result, the mutation occurring on this residue might lead to the destabilizing substrate binding.
Changes in intermolecular interactions of CsnTS following the single-site mutation of Asp318. The interactions are displayed using the Discovery Studio 4.1 progarm. The residues in the active site and substrate binding site are colored by yellow and cyan, respectively. The residues are shown using the stick representation scheme. The green dash lines indicate hydrogen-bonding interactions. a Wild-type, b D318N, c D318A, d D318E, e D318K, and f D318R
Discussion
The 3D structure of the GH8-family chitosanase revealed a regular (α/α)6 barrel formed by six inner and six outer α helices (1). Previous studies identified important residues believed to act as proton donors and acceptors during catalysis, including formation of hydrogen bonds with a catalytic water molecule, to stabilize the protein structure and participate in substrate binding (1). The residue Asp318 (i.e., Asp373 in ChoK) is located on the loop between α9 and α10 helices. It interacts with the Tyr273 (i.e., Tyr318 in Chok) in the substrate-binding residue (1) via hydrogen bonding. Asp318 is highly conserved in the GH8 family (Fig. 2) and participates in hydrogen-bonding interactions with protein and water molecules in the endoglucanase CelA from Clostridium thermocellum (2). In this study, we demonstrated that Asp318 is also important for chitosanase catalysis.
The substitution of Asp318 with alanine, glutamic acid, arginine, or lysine resulted in decreased catalytic and specific activity. Specifically, significant decreases in activity associated with the D318R and D318K mutations suggested that Asp318 cannot be replaced by basic residues for maintaining the catalytic activity of the enzyme. From the structural perspective, Asp318 is not located in the catalytic site (1). We also analyzed the intermolecular interactions of catalytic residues of Glu77 (i.e., Glu122 in ChoK) and Glu264 (i.e., Glu309 in ChoK) of CsnTS and mutants (Fig. 6). The results suggest that the intermolecular interaction of catalytic residues remain. Given that Asp318 does not directly participate in this interaction, the residue might be involved in forming the suitable electrostatic environment that is required by neighboring residues. The electrostatic potential of acidic aspartic acid differs from basic residues, such as arginine and lysine. This residue change would also affect the pK a value of residues in the vicinity of the active site. It was reported that inclusion of an arginine residue lowered the pK a value of an adjacent glutamate residue in the active site of the Bacillus circulans xylanase (GH11 family) (22).
The Asp318 interacts with the substrate by indirect hydrogen-bonding (2). Therefore, Asp318 mutants might affect these interactions through losses or changes in hydrogen bonding with the substrate. Our data also suggest that Asp318 has an important functional role in the enzyme mechanism. The increased k m value of Asp318 mutants indicates that this residue participate in substrate binding (Table 2). With the exceptions of the wild-type and D318N chitosanases, chitosan hydrolysis by other Asp318 mutants was inhibited by the substrate. These data combined with characterization of the hydrolysis products from the wild-type and mutated chitosanases indicated that Asp318 appears to influence the binding of the chitosan substrate. The intermolecular interaction analysis showed that several hydrogen-bonding interactions disappeared in other mutants except for the D318N mutant. These intermolecular interactions supposedly stabilize the substrate the binding during catalytic process (17). Our results here suggest Asp318 is likely to participate in the catalytic process through hydrogen-bonding with the Tyr273 residue to stabilize the substrate-enzyme complex.
Protein thermostability depends upon a number of important structural features (11), including hydrogen bonds, salt bridges (15), aromatic π-π interactions, and cation-π interactions. In the case of chitosanase, the T m value of mutant D318A was 63.6 °C, 5 °C lower than that observed for wild-type chitosanase (Fig. 5 and Table 3). The T m values of mutants D318R and D318K were 57.5 and 57.7 °C, respectively, 10 °C lower relative to wild-type (Fig. 5 and Table 3). The Asp318 residue forms hydrogen bonds with surrounding residues in the endoglucanase CelA (2). Our structure analysis also suggested that Asp318 interacts with four residues via hydrogen bonds. Asp318 mutation would possibly eliminate these interactions and, as a consequence, decrease protein thermostability. These results suggested that Asp318 is important in maintaining protein thermostability.
In conclusion, we demonstrated that the residue Asp318, highly conserved in GH8 family, contributes to the enzymatic function of the Bacillus sp. TS chitosanase. The specific function of Asp318 could be determined from the structure of the enzyme-substrate complex. The experimental data suggested that the interaction of Asp318 and other residues should cause the conformational change of the enzyme in substrate binding. These changes remain to be resolved in new information based on the enzyme-substrate complex structure.
References
Adachi, W., Sakihama, Y., Shimizu, S., Sunami, T., Fukazawa, T., Suzuki, M., et al. (2004). Crystal structure of family GH-8 chitosanase with subclass II specificity from Bacillus sp K17. Journal of Molecular Biology, 343, 785–795.
Alzari, P. M., Souchon, H., & Dominguez, R. (1996). The crystal structure of endoglucanase CelA, a family 8 glycosyl hydrolase from Clostridium thermocellum. Structure, 4, 265–275.
Boucher, I., Dupuy, A., Vidal, P., Neugebauer, W. A., & Brzezinski, R. (1992). Purification and characterization of a chitosanase from Streptomyces N174. Applied Microbiology and Biotechnology, 38, 188–193.
Fukamizo, T., Honda, Y., Goto, S., Boucher, I., & Brzezinski, R. (1995). Reaction-mechanism of chitosanase from Streptomyces sp N174. The Biochemical Journal, 311, 377–383.
Gupta, V., Prasanna, R., Natarajan, C., Srivastava, A. K., & Sharma, J. (2010). Identification, characterization, and regulation of a novel antifungal chitosanase Gene (cho) in Anabaena spp. Applied and Environmental Microbiology, 76, 2769–2777.
Gupta, V., Prasanna, R., Srivastava, A. K., & Sharma, J. (2012). Purification and characterization of a novel antifungal endo-type chitosanase from Anabaena fertilissima. Annales de Microbiologie, 62, 1089–1098.
Honda, Y., Fukamizo, T., Boucher, I., & Brzezinski, R. (1997). Substrate binding to the inactive mutants of Streptomyces sp. N174 chitosanase: indirect evaluation from the thermal unfolding experiments. FEBS Letters, 411, 346–350.
Honda, Y., Kirihata, M., Fukamizo, T., Kaneko, S., Tokuyasu, K., & Brzezinski, R. (1999). Chitosanase-catalyzed hydrolysis of 4-methylumbelliferyl beta-chitotrioside. The Journal of Biochemistry-Tokyo, 126, 470–474.
Ike, M., Ko, Y., Yokoyama, K., Sumitani, J. I., Kawaguchi, T., Ogasawara, W., et al. (2007). Cellobiohydrolase I (Ce17a) from Trichoderma reesei has chitosanase activity. Journal of Molecular Catalysis B: Enzymatic, 47, 159–163.
Jung, W. J., & Park, R. D. (2014). Bioproduction of chitooligosaccharides: present and perspectives. Marine Drugs, 12, 5328–5356.
Karshikoff, A., Nilsson, L., & Ladenstein, R. (2015). Rigidity versus flexibility: the dilemma of understanding protein thermal stability. The FEBS Journal, 282, 3899–3917.
Kim, P. I., Kang, T. H., Chung, K. J., Kim, I. S., & Chung, K. C. (2004). Purification of a constitutive chitosanase produced by Bacillus sp. MET 1299 with cloning and expression of the gene. FEMS Microbiology Letters, 240, 31–39.
Lacombe-Harvey, M. E., Fortin, M., Ohnuma, T., Fukamizo, T., Letzel, T. and Brzezinski, R. (2013) A highly conserved arginine residue of the chitosanase from Streptomyces sp N174 is involved both in catalysis and substrate binding. BMC Biochemistry, 14.
Lacombe-Harvey, M. E., Fukamizo, T., Gagnon, J., Ghinet, M. G., Dennhart, N., Letzel, T., et al. (2009). Accessory active site residues of Streptomyces sp N174 chitosanase. The FEBS Journal, 276, 857–869.
Lebbink, J. H., Consalvi, V., Chiaraluce, R., Berndt, K. D., & Ladenstein, R. (2002). Structural and thermodynamic studies on a salt-bridge triad in the NADP-binding domain of glutamate dehydrogenase from Thermotoga maritima: cooperativity and electrostatic contribution to stability. Biochemistry, 41, 15524–15535.
Lee, H. S., Jang, J. S., Choi, S. K., Lee, D. W., Kim, E. J., Jung, H. C., et al. (2007). Identification and expression of GH-8 family chitosanases from several Bacillus thuringiensis subspecies. FEMS Microbiology Letters, 277, 133–141.
Liu, S., Shao, S., Li, L., Cheng, Z., Tian, L., Gao, P., et al. (2015). Substrate-binding specificity of chitinase and chitosanase as revealed by active-site architecture analysis. Carbohydrate Research, 418, 50–56.
Lyu, Q., Shi, Y., Wang, S., Yang, Y., Han, B., Liu, W., et al. (2015). Structural and biochemical insights into the degradation mechanism of chitosan by chitosanase OU01. Biochimica et Biophysica Acta, 1850, 1953–1961.
Lyu, Q., Wang, S., Xu, W., Han, B., Liu, W., Jones, D. N., et al. (2014). Structural insights into the substrate-binding mechanism for a novel chitosanase. The Biochemical Journal, 461, 335–345.
Marcotte, E. M., Monzingo, A. F., Ernst, S. R., Brzezinski, R., & Robertus, J. D. (1996). X-ray structure of an anti-fungal chitosanase from Streptomyces N174. Nature Structural Biology, 3, 155–162.
Park, J. K., Shimono, K., Ochiai, N., Shigeru, K., Kurita, M., Ohta, Y., et al. (1999). Purification, characterization, and gene analysis of a chitosanase (ChoA) from Matsuebacter chitosanotabidus 3001. Journal of Bacteriology, 181, 6642–6649.
Pokhrel, S., Joo, J. C., & Yoo, Y. J. (2013). Shifting the optimum pH of Bacillus circulans xylanase towards acidic side by introducing arginine. Biotechnology and Bioprocess Engineering, 18, 35–42.
Rye, C. S., & Withers, S. G. (2000). Glycosidase mechanisms. Current Opinion in Chemical Biology, 4, 573–580.
Shimosaka, M., Kumehara, M., Zhang, X. Y., Nogawa, M., & Okazaki, M. (1996). Cloning and characterization of a chitosanase gene from the plant pathogenic fungus Fusarium solani. Journal of Fermentation and Bioengineering, 82, 426–431.
Tanabe, T., Morinaga, K., Fukamizo, T., & Mitsutomi, M. (2003). Novel chitosanase from Streptomyces griseus HUT 6037 with transglycosylation activity. Bioscience, Biotechnology, and Biochemistry, 67, 354–364.
Tremblay, H., Yamaguchi, T., Fukamizo, T., & Brzezinski, R. (2001). Mechanism of chitosanase-oligosaccharide interaction: subsite structure of Streptomyces sp N174 chitosanase and the role of Asp57 carboxylate. The Journal of Biochemistry-Tokyo, 130, 679–686.
Viens, P., Lacombe-Harvey, M. E., & Brzezinski, R. (2015). Chitosanases from family 46 of glycoside hydrolases: from proteins to phenotypes. Marine Drugs, 13, 6566–6587.
Xia, W. S., Liu, P., Zhang, J. L., & Chen, J. (2011). Biological activities of chitosan and chitooligosaccharides. Food Hydrocolloids, 25, 170–179.
Zhou, Z. P., Zhao, S. Z., Wang, S. Q., Li, X. M., Su, L., Ma, Y. H., et al. (2015). Extracellular overexpression of chitosanase from Bacillus sp TS in Escherichia coli. Applied Biochemistry and Biotechnology, 175, 3271–3286.
Zitouni, M., Fortin, M., Scheerle, R. K., Letzel, T., Matteau, D., Rodrigue, S., et al. (2013). Biochemical and molecular characterization of a thermostable chitosanase produced by the strain Paenibacillus sp 1794 newly isolated from compost. Applied Microbiology and Biotechnology, 97, 5801–5813.
Acknowledgments
This work was supported, in part, by grants from the Hundred Talents Program of the Chinese Academy of Sciences (CAS), the Knowledge Innovation Program of CAS (KSCX2-EW-G-8), and the Tianjin Municipal Science and Technology Commission (10ZCKFSY05600). J.L. is an Australian National Health and Medical Council (NHMRC) Senior Research Fellow. J.S. is a recipient of the Hundred Talents Program of CAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors and the authors declare that they have no competing interest.
Electronic supplementary material
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Zhou, Z., Zhao, S., Liu, Y. et al. A highly Conserved Aspartic Acid Residue of the Chitosanase from Bacillus Sp. TS Is Involved in the Substrate Binding. Appl Biochem Biotechnol 180, 1167–1179 (2016). https://doi.org/10.1007/s12010-016-2159-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2159-8