Abstract
The effects of hydraulic retention time (HRT), pH, and operating temperature (T OP) on the degradation of food waste-recycling wastewater (FRW) were investigated in laboratory-scale hydrolysis/acidogenesis reactors. Response surface analysis was used to approximate the production of volatile organic acids and degradation of volatile suspended solids (VSS), carbohydrate, protein, and lipid with regard to the independent variables (1 ≤ HRT ≤ 3 days, 4 ≤ pH ≤ 6, 25 ≤ T OP ≤ 45 °C). Partial cubic models adequately approximated the corresponding response surfaces at α < 5 %. The physiological conditions for maximum acidification (0.4 g TVFA + EtOH/g VSadded) and the maximal degradation of VSS (47.5 %), carbohydrate (92.0 %), protein (17.7 %), and lipid (73.7 %) were different. Analysis of variance suggested that pH had a great effect on the responses in most cases, while T OP and HRT, and their interaction, were significant in some cases. Denaturing gradient gel electrophoresis analysis revealed that Sporanaerobacter acetigenes, Lactobacillus sp., and Eubacterium pyruvivorans-like microorganisms might be main contributors to the hydrolysis and acidogenesis of FRW. Biochemical methane potential test confirmed higher methane yield (538.2 mL CH4/g VSadded) from an acidogenic effluent than from raw FRW.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In South Korea, 4 million tonnes of food waste is generated annually, and 91 % of it is collected separately from other solid wastes. Of the food waste collected, 86 % is recycled to animal feed or fertilizer at public or private facilities [36]. The recycling plants discharge process water, called food waste-recycling wastewater (FRW), primarily from storage pits (i.e., leachate), facility washing, and drying units. In 2011, 3.4 × 106 m3 of FRW was generated [28]. The large volume of FRW and its high content of organics present serious impediments to its disposal.
From both energy conservation and environmental perspectives, anaerobic digestion has long been regarded as a promising method to treat high-strength organic wastewater. Anaerobic digestion consists of multistep biochemical processes that are broadly grouped into four stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. The complex organic macromolecules in the substrate are first hydrolyzed to monomers and fermented to intermediate products, such as volatile fatty acids (VFAs), by hydrolytic/acidogenic bacteria. The intermediates are then converted to methane-rich biogas by methanogenic archaea, in many cases in close partnership with syntrophic bacteria [4].
FRW contains a high proportion (>50 %) of volatile suspended solids (VSS) to volatile solids (VS) [25, 46]. When a substrate is rich in particulate organic matters (POMs) like FRW, hydrolysis is usually considered the primary rate-limiting step [51]. High loading of POMs to anaerobic filters or upflow anaerobic sludge blankets can also cause clogging of the media and undesirable effluent quality [6, 14]. Although two-stage anaerobic digestion of FRW was previously reported [46], a detailed investigation of the hydrolysis and acidogenesis of FRW has not yet been conducted. The overall efficiency of an anaerobic process treating FRW would be increased if favorable growth conditions for the hydrolyzers/acidogens are maintained [57]. Therefore, operation of an acidogenic reactor at an optimized condition is desirable to improve the overall process efficiency and to minimize operational problems for the anaerobic digestion of FRW [18].
Many environmental factors affect anaerobic digestion, either by inhibiting or enhancing substrate utilization and microbial growth. Changing hydraulic retention time (HRT) controls the specific growth rate and, thus, the physiological state of the microorganisms especially in a continuous stirred tank reactor (CSTR) system [20, 32]. pH and the operating temperature (T OP) greatly affect enzymatic activities, which in turn influence the overall process efficiency of a biological system [51, 55]. Therefore, significant efforts have been made to optimize anaerobic hydrolysis and acidogenesis by estimating the effects of HRT, pH, and T OP. Maximal solubilization of an organic fraction of municipal solid waste in a CSTR occurred when it was operated at HRT = 1.9 days [43]. Maximal VFA production was estimated at 2.7 days HRT for acidogenic fermentation of waste sludge [35]. High substrate solubilization rate was obtained in an acidogenic reactor treating fruit and vegetable waste at mildly acidic pH conditions (5.5–6.2) [15]. In contrast, hydrolysis of food waste was maximized at pH ≤ 4.0 [55]. Veeken and Hamelers [51] and Mahmoud et al. [32] showed that the hydrolysis constant below 40 °C increased with increasing temperature. Although a few studies estimated the simultaneous effect of multiple parameters [25], most studies examined the effect of a single parameter without considering potential interactions among variables [43, 46, 55]. Assessing the simultaneous effects of HRT, pH, and T OP should improve our understanding of the hydrolysis and acidogenesis of FRW in continuous systems.
Raw organic materials that constitute anaerobic feedstock mostly consist of carbohydrates, proteins, and lipids. The hydrolysis and acidification profiles of carbohydrates, proteins, and lipids differ widely due to the heterogeneity of the physicochemical properties of these materials and corresponding enzymes [12, 50]. Thus, monitoring the biodegradation profiles of the individual organic components, with regard to the variations of the process parameters, should provide better understanding of the anaerobic hydrolysis/acidogenesis of complex substrates. Mahmoud et al. [32] investigated the effects of sludge retention time on the hydrolysis and acidogenesis of carbohydrates, proteins, and lipids in sewage sludge. However, most studies have only tested the destruction of crude solids and the production of VFAs during anaerobic hydrolysis and acidogenesis [25, 63].
Thus, the aim of this study was to investigate the simultaneous effects of HRT, pH, and T OP on the hydrolysis/acidogenesis of POMs in FRW. Profiles of the crude organics (i.e., VSS) degradation and of the VFAs and ethanol production were analyzed with respect to independent variables. Biodegradation of the individual organic components (i.e., carbohydrates, proteins, and lipids) were monitored to provide more in-depth discussion. Response surface analysis (RSA) was used to quantitatively estimate the simultaneous effects of different operating conditions on the hydrolysis/acidogenesis of FRW. Denaturing gradient gel electrophoresis (DGGE) was also conducted at the maximal hydrolysis condition to identify major hydrolyzer/acidogens. Improved understanding of how operational parameters affect the degradation of POMs and the production of VFAs can guide design and improvement of anaerobic processes to treat FRW.
Material and Methods
Preparation of Substrate and Reactor Operation
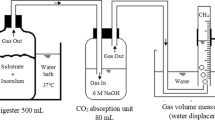
A 320-L batch of FRW was collected from a storage pit at a local composting facility. The raw FRW was slurry that contained small chunks of suspended particles. The FRW was mixed to homogeneity, filtered using an 850-μm-pore sieve to prevent clogging of the inlet tube, subdivided into portions, and frozen at −25 °C for later use. Anaerobic sludge, taken from a local full-scale anaerobic digester that treats a mixture of sewage sludge and food waste, was used as seed inoculum (5 %, v/v).
Three identical anaerobic CSTRs equipped with pH and temperature controllers with working volumes of 2.5 L were used in continuous mode for all trials. The pH of each reactor was adjusted using 6.0 N NaOH. Steady state at assigned HRTs was assumed when the residual concentrations of VS and total VFAs in the effluent did not vary by >5 % over four volume changes. 2-Bromoethanesulfonate (1 mM) was added to inhibit growth of methanogens.
Experimental Design and Evaluation of Model
RSA is useful to quantify the simultaneous effects of various factors and their interactions [57]. The central composite in cube design, which consists of an orthogonal 23 factorial design augmented by a center and 2 × 3 axial points, was used in this study.
The experimental ranges of HRT and pH were determined based on a survey from six full-scale acidogenic digesters in Korea (Supplementary Table 1) and a literature review. The survey showed that the acidogenic bioreactors (i.e., the first stage of the two-stage anaerobic digester) were operated at 1.5 ≤ HRT ≤ 3.0 days and 3.9 ≤ pH ≤ 6.3. The optimum HRT and pH for the hydrolysis and acidogenesis vary greatly in the literature, but many of them fall within these ranges [21, 34, 35, 43, 55, 63]. However, due to the economic burden of adding an alkali reagent to compensate for the low pH of the FRW (average 4.0) [45], most first-stage tanks in full-scale facilities were operated without pH control. Two facilities returned a part of the effluent from the methanogenic digester; in these, the pH of the acidogenic reactor remained around 6. Therefore, the ranges of operational parameters were 1.0 ≤ HRT ≤ 3.0 days, 4 ≤ pH ≤ 6, and 25 ≤ T OP ≤ 45 °C (i.e., mesophilic ± 10 °C), respectively. Experiments at the center point (i.e., HRT = 2 days, pH = 5, and T OP = 35 °C) were replicated three times to estimate the error to assess model adequacy.
A sequential procedure of collecting data, estimating polynomials, and checking the adequacy of the model (i.e., the coefficient of determination (R 2), p value of regression, and lack of fit) was used to find the best polynomials and estimate the response surface.
Parameters of the polynomials were estimated using the least squares method. Statistical results were considered significant at p ≤ 0.05. Analysis of variance using the corresponding models was performed to investigate possible two-way interactions among the variables (Supplementary Table 2). One additional trial was conducted for the validation of the model using the conditions (i.e., HRT = 3 days, pH = 6, and T OP = 45 °C) at which the removal efficiency of VSS was maximal.
DNA Extraction and Bacterial DGGE Analysis
Pelleted samples for molecular biological analysis were taken from the anaerobic seed sludge, FRW, and reactor run under HRT = 3 days, pH = 6, and T OP = 45 °C (i.e., trial 8) at steady state. DNA was extracted using an automated nucleic acid extractor (Magtration, System 6GC, PSS, Chiba, Japan). Bacterial 16S ribosomal RNA (rRNA) gene was amplified by polymerase chain reaction (PCR) using the bacterial universal primers BAC 338F (5′-ACTCCTACGGGAGGCAG-3′) and BAC 805R (5′-GACTACCAGGGTATCTAATCC-3′) [59]. A 40-bp GC clamp (5′-CGCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCACGGGGGG-3′) was added at the 5′ end of BAC 338F to stabilize the PCR products during DGGE. Touchdown PCR was conducted according to the following procedures: initial denaturation at 94 °C for 10 min; 20 cycles of touchdown PCR (denaturation at 94 °C for 30 s, annealing for 30 s with an 0.5 °C/cycle decrement at 65–55 °C and extension at 72 °C for 1 min); and 15 additional cycles of regular PCR (94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min and a final extension step for 7 min at 72 °C). For DGGE, 20 μL of PCR products was loaded onto 8 % acrylamide gel containing a linear gradient ranging from 40 to 60 % denaturant (100 % denaturants is a mixture of 7 M urea and 40 % [v/v] formamide). Electrophoresis was performed for 13 h at 80 V in 1× TAE electrophoresis buffer in a D-code system (Bio-Rad, Inc., Hercules, CA, USA). After electrophoresis, the gel was stained with ethidium bromide for 20 min, rinsed for 20 min with deionized distilled water (DDW), and then scanned under UV illumination. The DGGE bands were excised from the gel and eluted into 40 μL DDW and incubated overnight at 4 °C. Then, 2 μL of each band elution solution was further amplified using the same primers without a GC clamp. The PCR products were purified from a 1 % (w/v) low-melting-point agarose gel and cloned into the pGEM-T Easy vector (Promega, Mannheim, Germany). The cloned 16S rRNA gene fragments were sequenced using a 3730XL DNA analyzer (Applied Biosystems, Foster City, CA, USA) using the T7 primer. Data homology searches to identify the sequences were performed using the BLAST program in the National Center for Biotechnology Information database (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Biochemical Methane Potential Test
To investigate the effect of hydrolysis and acidification of FRW on methane production, two sets of biochemical methane potential (BMP) tests were performed using raw FRW and effluent from an acidogenic reactor where maximum removal of VSS in the FRW occurred (i.e., HRT = 3 days, pH = 6, and T OP = 45 °C). A blank reactor was also operated to account for endogenous methane production. The food-to-microorganism ratio at start-up was adjusted to 4.0 by adding 1.6 g VS substrate and 0.4 g VS seed inoculum to 100 mL working volume in a serum bottle filled with deionized distilled water. All reactors were purged with N2 gas for 5 min to remove dissolved O2 and then sealed tightly using rubber septa and aluminum caps. The reactors were kept in a shaking incubator (120 rpm) at 35 °C until methane production ceased. Methane content was measured by gas chromatography whenever gas samples were taken. Each reactor was run in duplicate and the average value was reported.
Analytical Methods
Chemical oxygen demand (COD), total solids (TS), VS, VSS, and volatile dissolved solids (VDS) were measured using the procedures in Standard Methods [2]. The carbohydrate concentration was measured using the phenol–sulfuric acid method [11]. Protein concentration and total ammonia nitrogen were determined using the Kjeldahl method [2]. Lipid concentrations were measured using gravimetric analysis after extraction of lipids using chloroform/methanol (1:2, v/v) [5]. Gas composition (i.e., CH4 and CO2) was analyzed using a gas chromatograph (6890 Plus, Agilent, Palo Alto, CA, USA) equipped with an HP-5 capillary column and a thermal conductivity detector. C2–C6 VFAs and ethanol were measured using a gas chromatograph (6890 Plus, Agilent) equipped with an Innowax capillary column and a flame ionization detector. The carrier gas was helium at a flow rate of 2.5 mL/min with a split ratio of 10:1.
Results
Characteristics of FRW
The physicochemical characteristics of the FRW used in this study were fairly typical of those reported in the literature [45] (Table 1). The ratio of VS to TS was 86.3 %, which means that most of the solids in the FRW were organic and potentially biodegradable. The total amount of carbohydrate, protein, and lipid was 83.4 g/L, i.e., 105 % of VS, which means that almost all of the organic material in the FRW was composed of these three subgroups. Of these three organics, 45.7 % was carbohydrate, 30.0 % was protein, and 24.3 % was lipid. More than 67 % of carbohydrate was in soluble form (i.e., 25.6 g/L), which accounted for 89.8 % of VDS (i.e., 28.5 g/L). The 12.5 g/L of particulate carbohydrate constituted 24.6 % of the VSS content (i.e., 50.9 g/L) in the FRW; considering the low proportion of soluble protein and lipid to VDS (collectively up to 10.2 %), the remaining 75.4 % was presumably protein and lipid. Of the total organics in the FRW, 64.1 % were POMs, primarily carbohydrate, protein, and lipid, which must be converted to a soluble form before they can be utilized by microorganisms.
Response Surface Analysis of VFA Production and Degradation of Organics
Seventeen sets of experimental trials (Table 2) were run to approximate the responses of VFA and ethanol formation and degradation of carbohydrate, protein, lipid, and VSS. Influent substrate concentration was maintained at 79.2 ± 1.9 g VS/L for all trials. None of the trials produced methane; therefore, methanogenesis had been suppressed completely.
Overall, the regressions for each response surface were significant (p ≤ 0.05), with R 2 ≥ 0.93 (Supplementary Table 3). Lack of fit was not significant, and the residual plots of the models showed random patterns with low variances (data not shown). Additionally, the results of the validation trial closely corresponded to the predicted values of VSS removal efficiency and total VFA and ethanol (TVFA + EtOH) production (Supplementary Data 2). Therefore, the partial cubic models represented the responses adequately. pH had the strongest influence on most response surfaces.
Hydrolysis and Acidification
VSS degradation efficiency ranged from 6.9 to 47.5 % within the investigated regions (Fig. 1 and Table 2). The partial cubic model to approximate the response surfaces was
where η VSS represents VSS removal efficiency (in percent) and X i is the corresponding variable term (i = HRT, pH, and T OP in order). The estimated maximal VSS removal efficiency was 47.2 % at HRT = 3 days, pH = 6, and T OP = 45 °C; at these conditions (trial 8), the observed degradation efficiency was 47.5 %. The two-way interactions of HRT and pH (i.e., HRT × pH) and HRT and T OP (i.e., HRT × T OP) were statistically significant, which means that they were interdependent (Supplementary Table 2).
The two- and three-dimensional response surfaces of the TVFA + EtOH production were normalized by input VS concentration (Fig. 2). The TVFA + EtOH production ranged from 0.01 to 0.33 g TVFA + EtOH/g VSadded within the investigated regions and can be described as
where \( {\eta}_{\frac{\mathrm{TVFA}+\mathrm{EtOH}}{VS_{added}}} \) represents the normalized yield of TVFA and ethanol (in grams of TVFA + EtOH per gram VSadded). The model estimated a maximal TVFA + EtOH production of 0.4 g TVFA + EtOH/g VSadded at HRT = 3 days, pH = 6, and T OP = 36.6 °C. The interaction of HRT × pH was statistically significant (Supplementary Table 2). TVFA + EtOH production per input VS content (i.e., each contour line) increased significantly as pH increased at almost all HRTs and T OP, but HRT had nearly no effect on the response when HRT was >2 days and at 4.3 ≤ pH ≤ 5.4 (Fig. 2a).
Degradation of Carbohydrate, Protein, and Lipid
Carbohydrate utilization efficiency ranged from 12.2 to 83.0 % within the design boundary (Fig. 3) and was described as
where η carbohydrate represents carbohydrate removal efficiency (in percent). The estimated maximum efficiency was 92.0 % at HRT = 3 days, pH = 6, and T OP = 34.0 °C, indicating higher biodegradability than other macromolecules composing FRW. The interactions of HRT × pH and HRT × T OP were statistically significant. In the HRT × pH response, carbohydrate removal efficiency increased rapidly from 29 to 80 % as HRT and pH increased to 2 days and 5.0 (i.e., the steepest ascent at the lower left region in Fig. 2a), respectively. A saddle point also existed at HRT = 2 days and T OP = 35.6 °C with high carbohydrate removal efficiency ≥80 % (Fig. 3b).
Protein degradation efficiency ranged from 0.1 to 17.7 % (Fig. 4) and was described as
where η protein represents protein removal efficiency (in percent). The calculated maximum using Eq. 4 was 17.6 % at HRT = 2.4 days, pH = 6, and T OP = 39.4 °C, indicating a much lower removal efficiency than other macromolecules. None of the possible two-way interactions was significant at p = 0.05 (Supplementary Table 2); this result indicates that the effects of the three independent variables are additive, so the protein degradation response surface was simpler than the other response surfaces. The effect of HRT on protein removal gradually decreased as HRT and pH increased and was minimal at HRT > 2 days and pH > 5 (Fig. 4a). The effect of T OP on the response also decreased as pH increased (Fig. 4b).
Lipid degradation efficiency ranged from 13.1 to 73.7 % (Fig. 5) and was described as
where η lipid represents lipid removal efficiency (in percent). The estimated maximum efficiency was 74.2 % at HRT = 1 day, pH = 6, and T OP = 45 °C (i.e., the same condition as trial 3), which was very close to the observed value of 73.7 % in trial 3. The interactions of HRT × pH and pH × T OP were statistically significant. The effect of HRT was significant at pH < 5.0, but relatively insignificant at pH > 5.0.
Bacterial Community Structure Contributed to Hydrolysis and Acidogenesis
Thirteen DGGE bands were detected from seed inoculum (B1–B5), FRW (B6–B8), and the trial with the maximal VSS degradation (B6 and B9–B14; Fig. 6). The sequences obtained from the bands were compared with the GenBank database to determine their affiliations (Table 3).
All bands visualized using DGGE were affiliated with one of two phyla: Firmicutes and Proteobacteria. Bands B10, B11, and B12 were closely related to Lactobacillus sp. with >99 % similarity and showed high intensity (B12), implying their dominance at the maximal VSS degradation condition (Table 3). Lactobacillus spp. are facultative anaerobes that grow at a wide range of temperature (2 ≤ T OP ≤ 53 °C); their optimal pH is 5.5–6.2 [52]. They are obligately saccharoclastic with complex nutritional requirements for peptides, amino acid, vitamins, salts, fatty acids or fatty acid esters, and fermentable carbohydrates. B9 was related to Sporanaerobacter acetigenes with 95.0 % similarity. S. acetigenes, a strict anaerobe, can grow at 25 ≤ T OP ≤ 50 °C and 5.5 ≤ pH ≤ 8.5 [19]. B13 was associated with Eubacterium pyruvivorans with 92.8 % similarity. E. pyruvivorans is a non-saccharolytic amino acid-fermenting anaerobe that is found in the rumen [53]. Pyruvate, vinyl acetate, and, to a less extent, lactate and crotonate support the growth of E. pyruvivorans. B6 showed 100 % similarity to Psychrobacter maritimus which grows at 4 ≤ T OP ≤ 37 °C (optimal growth temperature, 25–28 °C) and 5.0 ≤ pH ≤ 10.0 (optimal pH 6.0–8.5); this species does not produce acid from carbohydrates [42]. B7 was closely related to Pseudomonas fluorescens with 99.8 % similarity. P. fluorescens is commonly related to spoilage of foods (eggs, cured meats, fish, and milk); it grows best at 25–28 °C and can use various sugars as a substrate [16]. B8 was 99.4 % similar to Clostridium novyi, a strict anaerobe which grows well at 37 °C and moderately at 30 °C. C. novyi produces major amounts of butyric and propionic acids, small amounts of acetic, valeric acid, and abundant H2 gas [52]. Five other bands (B1, B2, B3, B4, and B5) obtained from seed sludge were closely related to uncultured clones with 99.3–100 % similarities (Table 3).
Biochemical Methane Potential Test
In the BMP tests, cumulative methane production was 40.4 % greater when using acidogenic effluent (538.2 mL CH4/g VSadded) than when using raw FRW (383.3 mL CH4/g VSadded; Supplementary Fig. 1). This difference indicates that hydrolysis–acidification of FRW increased the potential for subsequent methane production. The presence of pre-acidified VFAs and ethanol in the FRW likely contributed to the utilization of the acidogenic effluent by methanogens. This increase in methane production is consistent with the findings of other studies and indicates that hydrolysis and acidogenesis increase methane production [31, 57].
Discussion
pH has important influences on biological systems. The POMs in complex substrates are hydrolyzed by extracellular enzymes, the activity of which is directly affected by neighboring pH [62]. The pH of the medium also influences the homeostasis of bacteria; for example, cells under non-optimal pH conditions may experience oxidative stress and metabolic suppression [13]. The results of this study showed that pH had a significant effect on most response surfaces; optimal pH was 6 in all cases. Likewise, pH has been considered as an important parameter for acidogenesis of food waste, and maximal acidification has been consistently reported at mildly acidic (6–7) pH [21, 55]. At pH < 5, in contrast, poor acidification has been observed due to the inhibitory effect of undissociated VFAs; more toxic undissociated acids form significantly at pH lower than their pKa values [21, 56]. The growth of lipid-degrading bacteria is also depressed at pH < 5 [9, 10, 27]. pH has been claimed to have greater influence on gelatin degradation efficiency than T OP, with an estimated optimum at pH = 6 [58]. Jiang et al. [21] and Wang et al. [55] also reported that the degree of protein degradation was highest at pH > 6, but low at pH < 5. Therefore, to improve the overall process efficiency of a two-stage anaerobic digester, the first-stage acidogenic reactor should be operated at a mildly acidic pH (near 6). However, due to the low (near 4) pH value of FRW [45], alkalinity must be added to increase the pH of the hydrolytic/acidogenic phase; this task can be accomplished inexpensively by recirculating methanogenic effluent (typical 7 ≤ pH ≤ 8.5) to the acidogenic reactor [62].
The biodegradability of protein is closely related to its solubility, which is in turn affected by pH and T OP [47]. In aqueous environments, protein is minimally soluble at pH near its isoelectric point (pI) [47]. At such pH, protein molecules do not repel each other electrostatically, and the hydration layer alone cannot prevent aggregation, so their biodegradability decreases. The pI of protein depends on the ratio of the free ionized carboxyl groups to the free ionized amino groups and therefore differs widely among proteins [49]. The protein in Korean food waste mainly originates from meat and fish, which constitute 14 % of the wet weight of food waste [36]. The pI of meat and fish proteins has been reported as around 5 and can be reduced by the presence of NaCl [8, 40]. The FRW contains a high (4.1 g/L) concentration of NaCl [29]. Therefore, the estimate that protein degradation efficiency was smaller at pH 4.7 (Fig. 4a) in this study may be a result of the low solubility of fish and meat protein at this pH.
The optimal T OP for hydrolysis and lipid degradation was estimated as 45 °C (Figs. 1 and 5), whereas the optimal T OP for acidification and carbohydrate and protein degradation was 34–40 °C (Figs. 2, 3, and 4). The influence of T OP on hydrolysis and acidogenesis may be explained by its effect on enzyme kinetics, microbial growth, and substrate solubility [1]. The hydrolysis efficiency of biowaste has been claimed to increase with T OP at 20 ≤ T OP ≤ 40 °C [51] and 35 ≤ T OP ≤ 50 °C [24]. Likewise, an increase in T OP accelerates mass transfer of lipid in an aqueous reactor, thereby increasing accessibility to lipolytic microorganisms and enzymes [7]. Following hydrolysis of POMs by extracellular enzymes, acidogenesis from hydrolysates (i.e., monomers such as monosaccharides and amino acids) is mediated by acidogenic bacteria.
Microbes grow strictly within a favorable temperature range. The source of microorganisms used in this study was mesophilic (35 °C) sludge. Many mesophilic bacteria grow optimally at T OP < 40 °C [48]; this is probably the reason that maximal TVFA + EtOH production occurred at T OP = 36.6 °C (Fig. 2), unlike the maximal hydrolysis of POMs at 45 °C (Fig. 1). Similarly, the discrepancy of optimal T OP for hydrolyzing enzymes and for fermenting bacteria has been well documented in bioethanol production systems [39, 44]. The solubility of protein increases with rising temperature between 0 and 40–50 °C. However, protein denaturation, which causes irreversible reduction of solubility, may also occur at >40 °C [38, 61], so maximal protein degradation was probably observed near 40 °C (Fig. 4b).
HRT regulates the contact time between microorganisms and substrates and thereby affects the degree of biochemical reactions. HRT also equals the solid retention time in a CSTR and functions as a selective pressure against slower-growing organisms [43]. Therefore, a longer HRT in a microbial system is generally considered to increase the process efficiency by extending the reaction time and increasing microbial diversity. Accordingly, three out of five dependent variables (i.e., VSS degradation, TVFA + EtOH production, and carbohydrate removal) showed their highest responses at HRT = 3 days (Figs. 1, 2, and 3), which was the upper boundary in this study. Likewise, Lim et al. [30] have reported an increasing acidification yield from food waste as HRT increased (4–12 days), from 0.26–0.32 g VFA/VS at HRT = 4 days to 0.36–0.39 g VFA/VS at HRT = 12 days, with pH = 5.5 and T OP = 35 °C. In contrast, Guerrero et al. [18] observed the highest acidification efficiency at 1 day HRT when five HRT conditions (0.25, 0.5, 1, 1.5, and 2 days) were investigated. In this study, the protein and lipid degradation efficiencies at their estimated optimal conditions were 17.6 % (at HRT = 2.4 days) and 74.2 % (HRT = 1 days), respectively; the model values with the adjusted HRT input of 3 days were 15.4 and 69.9 % (Figs. 4 and 5). To conclude, the selection of HRT for a commercial-scale hydrolysis/acidogenesis tank must be based on a compromise between the different performance parameters as well as on consideration of the capital cost, which is greatly influenced by HRT [18].
DGGE analysis highlighted the major bacterial species that may participate in the hydrolysis/acidogenesis of FRW. Korean food waste often contains Lactobacillus spp. [22, 23], likely due to the dietary culture of eating fermented foods such as kimchi. In this study, three bands (B10, B11, and B12) detected from DGGE analysis were closely associated with Lactobacillus spp. Considering the high fraction (48 %) of carbohydrate to VS in the FRW (Table 1), they may have contributed greatly to carbohydrate degradation in the acidogenesis of FRW.
S. acetigenes is capable of the Stickland reaction, which is the simplest way to degrade amino acid [19, 41]. In addition, S. acetigenes can grow optimally at 40 °C, which is consistent with the T OP (39.4 °C) estimated using RSA for maximum protein removal efficiency. Eubacterium pyruvativorans can also utilize amino acids; it produces mainly caproate as the fermentation product [54]. Although B13 was related to E. pyruvativorans with sequence similarity of 92.8 %, taking into account the high concentration of caproate (13.3 g/L, data not shown) produced in trial 8, E. pyruvativorans-related species that corresponds to B13 may have contributed to the protein removal in cooperation with a S. acetigenes-like microorganism that corresponds to B9 (Table 3). However, among the three organic components, protein showed the lowest degradation efficiency in all trials (Table 2). This low value may be partly attributable to the presence of bacteria, which consist of 40–50 % (dry weight basis) protein, in the FRW [3, 32]. The population of bacteria in the FRW was 3.4 × 108 copies/mL [26], and so the removal efficiency might have been underestimated due to the existence and growth of hydrolytic/acidogenic bacteria [45]. In addition, a high concentration of carbohydrates could suppress the synthesis of exopeptidases, an enzyme complex that enhances protein hydrolysis [33, 58]. Therefore, the relatively low efficiency of protein degradation in this study can also be attributable to the residual carbohydrate content, which ranged from 6.7 to 27.3 g/L. DGGE has inherent limitations caused by biases related to PCR amplification, so multiple bands (i.e., B10 and B11) derived from a single microorganism were detected. In addition, due to the low sensitivity of DGGE analysis in detecting rare populations (<1 % of the total target population), microbial diversity might have been underestimated in trial 8, in which VSS removal efficiency was maximal [17, 37]. For example, B9–B13, the major bands from trial 8, were detected neither from the seed nor the FRW (Fig. 6), potentially due to their low abundance in the two samples. However, DGGE is useful to identify major populations in mixed cultures, so DGGE is still widely applied [17]. In particular, characterization of the main contributors in a biological system can provide evidence to understand the performance of the process. In this study, we revealed that S. acetigenes, Lactobacillus spp., and E. pyruvivorans-like species were likely the main contributors to the hydrolysis and acidogenesis of FRW using DGGE.
In many anaerobic systems that treat wastewater with high levels of suspended organic materials, the specific effects of individual components in the mixture on the overall waste stabilization are usually not determined. Instead, mixtures are studied under controlled conditions to determine their collective responses under the conditions imposed [25, 43, 60]. Therefore, the detailed analyses of the responses of carbohydrate, protein, and lipid given here provide deeper insight into the anaerobic digestion process than have previous studies. These results can be used to guide design and operation of full-scale anaerobic plants.
This study has revealed that combinations of HRT, pH, and T OP have differing effects on the microbial digestion of carbohydrate, protein, and lipids. The equations that describe the changes in the concentrations of substrates and products with respect to the operational parameters can be used to guide choice of operating conditions. For example, regions of the steepest ascent and saddle points in TVFA formation could indicate conditions at which the process would experience rapid and/or minimal changes in pH coupled with acid production. Thus, understanding of the differing influences of the operating conditions on carbohydrate, protein, and lipid may help operators understand how fluctuations in feedstock characteristics affect the overall responses of anaerobic digestion and may improve operators’ ability to maintain stable operation of full-scale digesters.
Conclusions
RSA was used to investigate the combined effect of HRT, pH, and T OP on the mesophilic hydrolysis and acidogenesis of FRW. The proportion of POMs in this FRW was 64.1 %, and its major organic components of the VS were carbohydrate (48.0 %), protein (31.5 %), and lipid (25.6 %). The maximum hydrolysis efficiency was estimated as 47.2 % at HRT = 3 days, pH = 6, and T OP = 45 °C. The methane yield from the effluent at these conditions was 40.4 % greater than that from raw FRW. The estimated maximum TVFA + EtOH production was 0.4 g TVFA + EtOH/g VSadded at HRT = 3 days, pH = 6, and T OP = 34.6 °C. The operating conditions for the maximal degradation of carbohydrate (92.0 %), protein (17.7 %), and lipid (73.7 %) were different. The responses were strongly influenced by pH in most cases, whereas the effects of HRT and T OP, and their interaction, were significant in some cases. Bacterial DGGE revealed that microorganisms similar to S. acetigenes, Lactobacillus sp., and E. pyruvivorans were likely the main contributors to the hydrolysis/acidogenesis of FRW.
References
Angelidaki, I., & Sanders, W. (2004). Assessment of the anaerobic biodegradability of macropollutants. Reviews in Environmental Science and Biotechnology, 3, 117–129.
APHA–AWWA–WEF. (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington, DC: American Public Health Association.
Atkinson, B., & Mavituna, F. (1991). Biochemical engineering and biotechnology handbook (2nd ed.). New York: Nature Press.
Batstone, D. J., Keller, J., Angelidaki, I., Kalyuzhnyi, S. V., Pavlostathis, S. G., Rozzi, A., Sanders, W. T., Siegrist, H., & Vavilin, V. A. (2002). The IWA Anaerobic Digestion Model No 1 (ADM1). Water Science and Technology: A Journal of the International Association on Water Pollution Research, 45, 65–73.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.
Bodkhe, S. (2008). Development of an improved anaerobic filter for municipal wastewater treatment. Bioresource Technology, 99, 222–226.
Cammarota, M. C., & Freire, D. M. G. (2006). A review on hydrolytic enzymes in the treatment of wastewater with high oil and grease content. Bioresource Technology, 97, 2195–2210.
Choi, Y. J., Hur, S., Choi, B.-D., Konno, K., & Park, J. W. (2009). Enzymatic hydrolysis of recovered protein from frozen small croaker and functional properties of its hydrolysates. Journal of Food Science, 74, C17–C24.
Cirne, D. G., Delgado, O. D., Marichamy, S., & Mattiasson, B. (2006). Clostridium lundense sp. nov., a novel anaerobic lipolytic bacterium isolated from bovine rumen. International Journal of Systematic and Evolutionary Microbiology, 56, 625–628.
Dighe, A. S., Shouche, Y. S., & Ranade, D. R. (1998). Selenomonas lipolytica sp. nov., an obligately anaerobic bacterium possessing lipolytic activity. International Journal of Systematic Bacteriology, 48, 783–791.
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356.
Elefsiniotis, P., & Oldham, W. (1994). Substrate degradation patterns in acid-phase anaerobic digestion of municipal primary sludge. Environmental Technology, 15, 741–751.
Follmann, M., Ochrombel, I., Kramer, R., Trotschel, C., Poetsch, A., Ruckert, C., Huser, A., Persicke, M., Seiferling, D., Kalinowski, J., & Marin, K. (2009). Functional genomics of pH homeostasis in Corynebacterium glutamicum revealed novel links between pH response, oxidative stress, iron homeostasis and methionine synthesis. BMC Genomics, 10, 621.
Foresti, E., Zaiat, M., & Vallero, M. (2006). Anaerobic processes as the core technology for sustainable domestic wastewater treatment: consolidated applications, new trends, perspectives, and challenges. Reviews in Environmental Science and Bio/Technology, 5, 3–19.
Ganesh, R., Torrijos, M., Sousbie, P., Lugardon, A., Steyer, J. P. and Delgenes, J. P. (2014). Single-phase and two-phase anaerobic digestion of fruit and vegetable waste: comparison of start-up, reactor stability and process performance. Waste Management, 34, 875–885.
Garrity, G., Brenner, D. J., Krieg, N. R. and Staley, J. R. (2005). Bergey’s manual® of systematic bacteriology, 2nd ed. New York: Springer.
Green, S. J., Leigh, M. B. and Neufeld, J. D. (2010). Denaturing gradient gel electrophoresis (DGGE) for microbial community analysis. In K. N. Timmis (Ed.), Handbook of hydrocarbon and lipid microbiology (pp. 4137–4158). Heidelberg: Springer.
Guerrero, L., Omil, F., Méndez, R., & Lema, J. M. (1999). Anaerobic hydrolysis and acidogenesis of wastewaters from food industries with high content of organic solids and protein. Water Research, 33, 3281–3290.
Hernandez-Eugenio, G., Fardeau, M.-L., Cayol, J.-L., Patel, B. K. C., Thomas, P., Macarie, H., Garcia, J.-L., & Ollivier, B. (2002). Sporanaerobacter acetigenes gen. nov., sp. nov., a novel acetogenic, facultatively sulfur-reducing bacterium. International Journal of Systematic and Evolutionary Microbiology, 52, 1217–1223.
Hung, C.-H., Chang, Y.-T., & Chang, Y.-J. (2011). Roles of microorganisms other than Clostridium and Enterobacter in anaerobic fermentative biohydrogen production systems—a review. Bioresource Technology, 102, 8437–8444.
Jiang, J., Zhang, Y., Li, K., Wang, Q., Gong, C., & Li, M. (2013). Volatile fatty acids production from food waste: effects of pH, temperature, and organic loading rate. Bioresource Technology, 143, 525–530.
Jo, J. H., Jeon, C. O., Lee, D. S., & Park, J. M. (2007). Process stability and microbial community structure in anaerobic hydrogen-producing microflora from food waste containing kimchi. Journal of Biotechnology, 131, 300–308.
Kim, M., & Chun, J. (2005). Bacterial community structure in kimchi, a Korean fermented vegetable food, as revealed by 16S rRNA gene analysis. International Journal of Food Microbiology, 103, 91–96.
Kim, M., Gomec, C. Y., Ahn, Y., & Speece, R. E. (2003). Hydrolysis and acidogenesis of particulate organic material in mesophilic and thermophilic anaerobic digestion. Environmental Technology, 24, 1183–1190.
Kim, M., Song, M., Jo, M., Shin, S., Khim, J., & Hwang, S. (2010). Growth condition and bacterial community for maximum hydrolysis of suspended organic materials in anaerobic digestion of food waste-recycling wastewater. Applied Microbiology and Biotechnology, 85, 1611–1618.
Kim, W., Ryu, B.-G., Kim, S., Heo, S.-W., Kim, D., Kim, J., Jo, H., Kwon, J.-H., & Yang, J.-W. (2014). Quantitative analysis of microbial community structure in two-phase anaerobic digesters treating food wastewater. Korean Journal of Chemical Engineering, 31, 381–385.
Kim, Y.-O., Kim, K.-K., Park, S., Kang, S.-J., Lee, J.-H., Lee, S.-J., Oh, T.-K., & Yoon, J.-H. (2010). Photobacterium gaetbulicola sp. nov., a lipolytic bacterium isolated from a tidal flat sediment. International Journal of Systematic and Evolutionary Microbiology, 60, 2587–2591.
Lee, J., Hwang, B., Koo, T., Shin, S. G., Kim, W., & Hwang, S. (2014). Temporal variation in methanogen communities of four different full-scale anaerobic digesters treating food waste-recycling wastewater. Bioresource Technology, 168, 59–63.
Lim, B. S., Kim, B., & Chung, I. (2012). Anaerobic treatment of food waste leachate for biogas production using a novel digestion system. Environmental Engineering Research, 17, 41–46.
Lim, S. J., Kim, B. J., Jeong, C. M., Choi, J. D. R., Ahn, Y. H., & Chang, H. N. (2008). Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Bioresource Technology, 99, 7866–7874.
Lu, J., Gavala, H. N., Skiadas, I. V., Mladenovska, Z., & Ahring, B. K. (2008). Improving anaerobic sewage sludge digestion by implementation of a hyper-thermophilic prehydrolysis step. Journal of Environmental Management, 88, 881–889.
Mahmoud, N., Zeeman, G., Gijzen, H., & Lettinga, G. (2004). Anaerobic stabilisation and conversion of biopolymers in primary sludge—effect of temperature and sludge retention time. Water Research, 38, 983–991.
McInerney, M. J. (1988). Anaerobic hydrolysis and fermentation of fats and proteins. In A. J. B. Zehnder (Ed.), Biology of anaerobic microorganisms (pp. 373–416) New York: Wiley.
Min, K. S., Khan, A. R., Kwon, M. K., Jung, Y. J., Yun, Z., & Kiso, Y. (2005). Acidogenic fermentation of blended food-waste in combination with primary sludge for the production of volatile fatty acids. Journal of Chemical Technology & Biotechnology, 80, 909–915.
Min, K. S., Park, K. S., Jung, Y. J., Khan, A. R., & Kim, Y. J. (2002). Acidogenic fermentation: utilization of wasted sludge as a carbon source in the denitrification process. Environmental Technology, 23, 293–302.
MOE. (2013). National waste statistical survey, 제4차 전국폐기물통계조사. Republic of Korea: Ministry of Environment.
Muyzer, G., De Waal, E., & Uitterlinden, A. (1993). Profiling of complex microbial population by DGGE analysis of polymerase chain reaction amplified genes coding for 16S rRNA. Applied and Environmental Microbiology, 59, 695–700.
Myerson, A. (Ed.) (2002). Handbook of industrial crystallization. Newton, MA: Butterworth-Heinemann.
Ofori-Boateng, C., & Lee, K. T. (2014). Ultrasonic-assisted simultaneous saccharification and fermentation of pretreated oil palm fronds for sustainable bioethanol production. Fuel, 119, 285–291.
Puolanne, E., & Halonen, M. (2010). Theoretical aspects of water-holding in meat. Meat Science, 86, 151–165.
Ramsay, I. R., & Pullammanappallil, P. C. (2001). Protein degradation during anaerobic wastewater treatment: derivation of stoichiometry. Biodegradation, 12, 247–256.
Romanenko, L., Lysenko, A., Rohde, M., Mikhailov, V., & Stackebrandt, E. (2004). Psychrobacter maritimus sp. nov. and Psychrobacter arenosus sp. nov., isolated from coastal sea ice and sediments of the Sea of Japan. International Journal of Systematic and Evolutionary Microbiology, 54, 1741.
Romero Aguilar, M. A., Fdez-Güelfo, L. A., Álvarez-Gallego, C. J., & Romero García, L. I. (2013). Effect of HRT on hydrogen production and organic matter solubilization in acidogenic anaerobic digestion of OFMSW. Chemical Engineering Journal, 219, 443–449.
Ruiz, E., Cara, C., Ballesteros, M., Manzanares, P., Ballesteros, I., & Castro, E. (2006) Ethanol production from pretreated olive tree wood and sunflower stalks by an SSF process. Applied Biochemistry and Biotechnology, 130, 631–643.
Shin, S. G., Han, G., Lee, J., Cho, K., Jeon, E.-J., Lee, C., & Hwang, S. (2015). Characterization of food waste-recycling wastewater as biogas feedstock. Bioresource Technology, 196, 200–208.
Shin, S. G., Han, G., Lim, J., Lee, C., & Hwang, S. (2010). A comprehensive microbial insight into two-stage anaerobic digestion of food waste-recycling wastewater. Water Research, 44, 4838–4849.
Sikorski, Z. E. (Ed.) (2006) Chemical and functional properties of food components, third edition. Boca Raton, FL: CRC.
Speece, R. E. (1996). Anaerobic biotechnology for industrial wastewaters. Nashville, TN: Archae Press.
Vaclavik, V. and Christian, E. W. (2013). Essentials of food science. New York: Springer.
Vavilin, V. A., Fernandez, B., Palatsi, J., & Flotats, X. (2008). Hydrolysis kinetics in anaerobic degradation of particulate organic material: an overview. Waste Management, 28, 939–951.
Veeken, A., & Hamelers, B. (1999). Effect of temperature on hydrolysis rates of selected biowaste components. Bioresource Technology, 69, 249–254.
Vos, P. D. , Garrity, G. M., Jones, D., Krieg, N. R., Ludwig, W., Rainey, F. A., Schleifer, K. H. and Whitman, W. B. (Ed.) (2009). Bergey’s manual of systematic bacteriology: volume 3: The Firmicutes. New York: Springer.
Wallace, R. J., Chaudhary, L. C., Miyagawa, E., McKain, N., & Walker, N. D. (2004). Metabolic properties of Eubacterium pyruvativorans, a ruminal ‘hyper-ammonia-producing’ anaerobe with metabolic properties analogous to those of Clostridium kluyveri. Microbiology, 150, 2921–2930.
Wallace, R. J., McKain, N., McEwan, N. R., Miyagawa, E., Chaudhary, L. C., King, T. P., Walker, N. D., Apajalahti, J. H. A., & Newbold, C. J. (2003). Eubacterium pyruvativorans sp. nov., a novel non-saccharolytic anaerobe from the rumen that ferments pyruvate and amino acids, forms caproate and utilizes acetate and propionate. International Journal of Systematic and Evolutionary Microbiology, 53, 965–970.
Wang, K., Yin, J., Shen, D., & Li, N. (2014). Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: effect of pH. Bioresource Technology, 161, 395–401.
Warnecke, T., & Gill, R. (2005). Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microbial Cell Factories, 4, 1–8.
Yang, K., Yu, Y., & Hwang, S. (2003). Selective optimization in thermophilic acidogenesis of cheese–whey wastewater to acetic and butyric acids: partial acidification and methanation. Water Research, 37, 2467–2477.
Yu, H. Q., & Fang, H. H. P. (2003). Acidogenesis of gelatin-rich wastewater in an upflow anaerobic reactor: influence of pH and temperature. Water Research, 37, 55–66.
Yu, Y., Lee, C., Kim, J., & Hwang, S. (2005). Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnology and Bioengineering, 89, 670–679.
Yuan, Q., Sparling, R., & Oleszkiewicz, J. A. (2011). VFA generation from waste activated sludge: effect of temperature and mixing. Chemosphere, 82, 603–607.
Zayas, J. F. (Ed.) (1996). Functionality of proteins in food. Heidelberg: Springer.
Zhang, B., He, P.-J., Fan, L., Shao, L.-M., & Wang, P. (2007). Extracellular enzyme activities during regulated hydrolysis of high-solid organic wastes. Water Research, 41, 4468–4478.
Zhang, B., Zhang, L.-L., Zhang, S.-C., Shi, H.-Z., & Cai, W.-M. (2005). The influence of pH on hydrolysis and acidogenesis of kitchen wastes in two-phase anaerobic digestion. Environmental Technology, 26, 329–339.
Acknowledgments
This work was financially supported by Korea Ministry of Environment (MOE) as “Knowledge-based environmental service (Waste to energy recycling) Human resource development Project” and also supported by “Human Resources Program in Energy Technology” of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea (no. 20144030200460).
Author information
Authors and Affiliations
Corresponding author
Additional information
Gyuseong Han and Seung Gu Shin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Han, G., Shin, S.G., Lee, J. et al. Mesophilic Acidogenesis of Food Waste-Recycling Wastewater: Effects of Hydraulic Retention Time, pH, and Temperature. Appl Biochem Biotechnol 180, 980–999 (2016). https://doi.org/10.1007/s12010-016-2147-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2147-z