Abstract
In general, gamma-aminobutyric acid (GABA) pathway involves the decarboxylation of glutamate, which is produced from sugar by Corynebacterium fermentation. GABA can be used for the production of pharmaceuticals and functional foods. Due to the increasing demand of GABA, it is essential to create an effective alternative pathway for the GABA production. In this study, Escherichia coli were engineered to produce GABA from glucose via GABA shunt, which consists of succinate dehydrogenase, succinate-semialdehyde dehydrogenase, and GABA aminotransferase. The three enzymes were physically attached to each other through a synthetic scaffold, and the Krebs cycle flux was redirected to the GABA pathway. By introduction of synthetic scaffold, 0.75 g/l of GABA was produced from 10 g/l of glucose at 30 °C and pH 6.5. The inactivation of competing metabolic pathways provided 15.4 % increase in the final GABA concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic engineering emerged in the recent past in which optimizing and engineering the metabolic pathway within the cells to achieve the desired product yield. This strategy can be employed to create a pathway which is not found naturally in the host organism. The synergy between metabolic engineering and synthetic biology can play a vital role in the production of next-generation biofuels and other products for bio-based industries.
Synthetic scaffold is a novel strategy and has been used for the optimization of intracellular metabolic fluxes. If the low carbon flux is directed to the desired pathway, the efficient production of metabolites could not be achieved. It was reported that the synthetic scaffold can co-localize the enzymes and enable to increase the local carbon flux to the targeted pathway [2, 7]. The enzymes are closely located within the cells to the maximum possibility of reaction which takes place in targeted pathway. Many metabolites production systems were improved by the introduction of various synthetic scaffolds. Glucaric acid production from Escherichia coli was improved to fivefold by the introduction of synthetic scaffold [13]. The three mevalonate enzymes were closely attached by a synthetic scaffold, which leads to 77-fold increase in the concentration of the final product [3]. Similarly, the gamma-aminobutyric acid (GABA) production was increased by employing synthetic scaffold between glutamate decarboxylase and GABA transporter in E. coli [10, 17].
Considering increasing demand of GABA, GABA is considered as one of the important metabolites in biotechnology industries. GABA is a non-protein amino acid used for the production of biopolymer, nylon 4 [16]. It acts as a core neurotransmitter in mammalian brains [15]. The germinated brown rice was reported to contain rich amount of GABA which is used to blocks the proliferation of various cancer cells [14].
Generally, GABA can be produced from decarboxylation of glutamate by glutamate decarboxylase. Glutamate decarboxylase from various strains has been overexpressed to increase the production of GABA. By overexpression of Lactobacillus plantarum glutamate decarboxylase in Lactobacillus sakei B2-16, the GABA production was elevated to 1.4-fold [8]. The introduction of heterologous enzyme from Pyrococcus horikoshii glutamate decarboxylase in E. coli, the maximum concentration of 5.69 g/l of GABA was obtained from 10 g/l of monosodium glutamate [19]. It was also reported that 5.62 g/l of GABA was obtained by overexpression of Neurospora crassa OR74A glutamate decarboxylase [9].
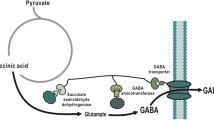
In this study, GABA shunt was tested as novel GABA production pathway. To direct Krebs cycle flux to GABA, succinate dehydrogenase (SdhA), succinate-semialdehyde dehydrogenase (GabD), and GABA aminotransferase (GabT) were co-localized via synthetic scaffold (Fig. 1). The effects of various conditions such as pH, temperature, and glucose concentration were investigated to optimize GABA production condition. The effect of competing metabolic pathway was also investigated.
Materials and Methods
Bacterial Strains and Culture Conditions
E. coli XL1-Blue and E. coli mutant strains (XBM7 and XBM8) were used in this study (Table 1). The strains were cultivated in Luria-Bertani medium (10 g/l of bacto-tryptone, 5 g/l of bacto-yeast extract, and 5 g/l of sodium chloride) supplemented with 50 μg/ml of ampicillin and/or 30 μg/ml of chloramphenicol [18]. Media were solidified by the addition of 2 % agar. The strains were inoculated into 20-ml tubes containing 10 ml of LB and incubated at 200 rpm at 37 °C overnight. Next, 100 ml of LB media supplemented 10 g/l glucose was inoculated with the overnight culture and cultured further at 37 °C and 250 rpm. When the optical density at 600 nm (OD600) increased to 1.2, gene expression was induced with addition of 1 % l-arabinose [5] and 108 nM anhydrotetracycline (ATc) [11, 13]. During the fermentation, samples were withdrawn and stored at −20 °C for subsequent analysis.
Construction of Plasmids for GABA Production
Table 2 shows the list of oligonucleotides used in this study. The sdhA, gabD, and gabT genes were amplified from E. coli XB chromosomal DNA using the expand high-fidelity polymerase chain reaction (PCR) system (Roche Molecular Biochemicals, Mannheim, Germany). The GBD ligand was then fused to the sdhA genes by overlap PCR, while the SH3 and PZD ligand sequences were incorporated into reverse primers for gabD and gabT, respectively. The sdhA-GBD fusion gene was then cloned into plasmid pBAD30C using the restriction enzymes SacI and KpnI, after which the gabD-SH3 gene was cloned downstream of sdhA-GBD using KpnI and XmaI. XmaI and XbaI were employed to clone the gabT-PDZ genes for construction of the expression plasmids pBSDT (Fig. 2a). Nine scaffold architecture plasmids consisting of GBD, SH3, and PDZ protein interaction domains were kindly provided by Professor Dueber (Table 1).
Genome Engineering
The genes ackA, ldhA pflB, adhE, poxB, and frdB were deleted in the chromosomal DNA of E. coli XL1-Blue strain by using the one-step inactivation method as previously reported [1].
Optimization of GABA Production
Various conditions such as temperatures, pH, and glucose concentrations were optimized and tested for the effective GABA production. The strains were incubated at 25, 30, and 37 °C at pH 6.5 to test the effect of temperature. To examine the effect of pH, the strains were cultured at pH 4.5, 5.5, 6.5, and 7.0 at 30 °C. The effect of glucose concentration was tested by varying the initial glucose concentration from 10 to 30 g/l. The OD600 of the cultures were measured at the time of sample withdrawal.
GABA Analysis
GABA bioconversion was quantitatively analyzed by HPLC using an OptimaPak C18 column (4.6 × 150 mm, RS tech Corporation, Daejeon, Korea). Samples were centrifuged at 12,000 rpm for 5 min. One hundred microliters of supernatant from the sample was added to a new eppendorf tube. Next, 200 μl of 1 M sodium bicarbonate buffer pH 9.8 and 100 μl of 80 g/L dansylchloride in acetonitrile was added. Finally, 600 μl of double-distilled water was added to the sample to make a 1-ml reaction mixture. The mixture was then incubated at 80 °C for 40 min, after which 100 μl of 20 μl/ml acetic acid was added to stop the reaction. The reaction mixture was then centrifuged at 12,000 rpm for 5 min. The sample was filtered through a 0.2-μm Millipore filter and analyzed by HPLC in Agilent system using UV detection. The column temperature was set to 30 °C, and the samples were separated using a binary nonlinear gradient with eluant A [tetrahydrofuran/methanol/50 mM sodium acetate pH 6.2 (5:75:420, by vol.)] and eluant B (methanol). The elution conditions were as follows: equilibration (6 min, 20 % B), gradient (20 min, 20–80 % B), and cleaning (3 min, 100 % B). The mobile phase flow rate was set to 1 ml/min, and detection of the samples was carried out at UV 286 nm. The standard curve of GABA ranges from 0.1, 0.2, 0.3, 1, 2, 3, 5, to 10 g/l GABA (Sigma, Missouri, USA) was determined using the same procedure.
Analysis of Metabolites
The metabolites such as glucose and acetate were analyzed by HPLC using the Aminex HPX-87H column (300 × 7.8 mm, Bio-Rad). One milliliter of samples was collected and centrifuged at 12,000 rpm for 5 min. After centrifugation, the supernatant was collected and filtered using a 0.2-μm Millipore filter. It was analyzed on an Agilent system using RI detection. The derivative samples were separated by using 0.008 N H2SO4 as the mobile phase. The column temperature was set to 50 °C, and the flow rate of the mobile phase was 0.6 ml/min. Similarly, the standard curves for glucose and acetate were determined for seven standard solutions of 0.1, 0.5, 1, 2, 3, 5, and 10 g/l (Sigma, Missouri, USA).
Results and Discussion
Construction of the Novel GABA Pathway
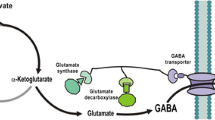
In general, GABA is produced from the Krebs cycle intermediate from alpha-ketoglutarate via glutamate. The alpha-ketoglutarate is converted to glutamate by glutamate synthase, which is then further converted to GABA by glutamate decarboxylase. In this study, a novel GABA pathway was proposed, which directs the conversion of succinate to GABA. To achieve this, SdhA, GabD, and GabT were physically connected via a synthetic scaffold (Fig. 2a). The protein-protein interaction ligands such as GBD, SH3, and PDZ were attached with the C-terminus of SdhA, GabD, and GabT, respectively. The enzyme-ligand complexes were then co-expressed with the protein-protein interaction domain of GBD, SH3, and PDZ scaffold architecture. The overexpressed proteins of each enzyme-ligand protein complex are targeted to the scaffold architecture containing their respective protein-protein interaction domains. Binding of the enzyme-ligand with the domain in the scaffold architecture should allow close localization of the three enzymes together in the cytosol of E. coli. Succinate obtained from the conversion of fumarate by SdhA, would then have a higher chance of reacting with the closely located GabD than with other competing enzymes. Similarly, succinate semialdehydes produced by the enzyme GabD have strong flux towards the GabT for the conversion of GABA rather than other metabolites, due to the co-localization (Fig. 1). The expression of the three enzymes was analyzed by SDS-PAGE after 8 h of cultivation (Fig. 2b).
GABA was not produced when the enzymes are not expressed. When enzymes SdhA, GabD, and GabT were directly overexpressed without the scaffold architecture, 0.27 g/l of GABA was obtained from 10 g/l of glucose. By the introduction of scaffold plasmid pJD757 encoding GBD, SH3, and PDZ domain at 1:1:1 ratio, higher GABA concentration of 0.65 g/l was obtained. This data demonstrate the co-localization of enzyme can increase the metabolic flux from Krebs cycle into the GABA production pathway. This result shows that physical co-localization of enzymes can direct more carbon flux into the targeted pathway [4, 13].
Optimization of GABA Production Conditions
The effect of temperature, pH, scaffold architecture, and glucose concentration was evaluated. Initially, the effect of temperature was evaluated by culturing the recombinant strains at different temperatures at 25, 30, and 37 °C (Fig. 3a). Optimum temperature is required for effective biochemical activity in the cells, and it is one of the major factors affecting GABA yield. Recombinant E. coli at 30 °C produced maximum GABA concentration of 0.63 g/l. Lowest GABA concentration was obtained at 25 °C. Data shows that 30 °C was the optimum temperature for the efficient GABA production.
A previous study shows that the pH of the medium affects the activity of pathway enzymes and leads to the decrease in GABA production. The recombinant cell was cultured in media at various pH (4.5, 5.5, 6.5, and 7.0) at 30 °C (Fig. 3b). Result shows at pH 6.5 recombinant E. coli produced maximum of 0.65 g/l of from 10 g/l of glucose. Lower GABA concentrations were obtained when the pH falls below or above than pH 6.5. Hence, pH 6.5 was considered as the optimum for GABA production.
It was reported that the catalytic activity of SdhA was optimum at pH less than 7.6 and GabD has optimum pH at 8.2 [6, 12]. But GadC reported that acidic pH can favor the mechanism of GABA transport [20]. Previous study reported that effective conversion of GABA occurred at acid environment of media. The employment of different enzymes for the novel GABA pathway leads to differ in their each optimal pH conditions. Hence, the data shows that pH 6.5 is considered as the suitable environment condition for three different enzymes which drives the optimal production of GABA.
Scaffold architecture is used to direct the metabolic flux into the targeted metabolic pathway. Metabolite production can be optimized by enzyme titer ratio. The ratio can be controlled by changing the number of scaffold domains in the scaffold architecture. The plasmids (pJD757 to pJD765) containing the various ratio of domains were transformed into the E. coli which harboring pBSDT plasmid. Totally, nine recombinant strains were constructed and tested for the GABA production. Even though some recombinant strains produce more GABA than other strains, there were no signification changes observed in GABA production (Fig. 3c).
The initial concentration of substrate plays an essential factor in the production of GABA. Different concentrations of glucose were tested in the culture media for GABA production. When 20 g/l of initial glucose was used, GABA production was increased and the maximum of 1.2 g/L of GABA was produced from the recombinant E. coli XB (Fig. 3d). The concentration of GABA was decreased when initial glucose concentration was increased to 30 g/l glucose.
Metabolic Pathway Engineering
To study the impact of synthetic scaffold introduction on recombinant E. coli cells, time profiles of glucose and metabolite concentrations were monitored by HPLC. When the recombinant cells were cultured for the GABA conversion, the glucose concentration was decreased from 10 to 3.41 g/l and acetate concentration was increased to 1.25 g/l (Fig. 4a). Hence, data suggest that carbon flux was also directed to the competing pathway for the production of acetate.
To estimate the effects of competing pathways on GABA production, the pathways which directs the higher carbon flux to other competing pathways are inactivated by knockout their specific genes that lead to direct strong carbon flux towards to desired GABA pathway. In XBM7 strain, the genes for acetate kinase and lactate dehydrogenase were inactivated. While the genes for alcohol dehyrogenase, fumarate reductase, lactate dehydrogenase, pyruvate formate-lysase, and pyruvate oxidase were inactivated in XBM8. The pBSDT plasmid was transformed into E. coli XB, XBM7, and XBM8 along with scaffold architecture plasmid pJD757. The recombinant E. coli strains were expressed, and the GABA concentration was monitored. XBM7 mutant strain produced 0.75 g/L of GABA, which was also about 15.4 % higher than that obtained from E. coli XB (Fig. 4b). The data suggest that the inactivation of competing metabolic pathways in E. coli can increase the quantitative production of GABA.
Conclusion
The demand of GABA is constantly increasing in pharmaceuticals and polymer industries. Traditionally, GABA is produced from the decarboxylation of glutamate by glutamate decarboxylase. Several research papers used glutamate as their substrate for the production of GABA. Considering economically, GABA is produced by a one-step process via glucose which is cheaper and more abundant than glutamate. Current method of construction of a novel GABA production pathway which leads to direct production of GABA from glucose and its feasibility was examined. So, glucose is a more suitable substrate for the direct production of GABA. The scaffold architecture constructed in the pathway helps to increase the carbon flux and further redirection of Krebs cycle flux to the GABA pathway. The inactivation of the competing metabolic pathway leads to enhance the GABA concentration in mutant strains. This novel pathway can be further improved to increase the GABA production efficiency to be used in industrial GABA process.
Abbreviations
- GABA:
-
gamma-aminobutyric acid
References
Datsenko, K. A., & Wanner, B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proceedings of the National Academy of Science of the United States of America, 97, 6640–6645.
Delebecque, C. J., Lindner, A. B., Silver, P. A., & Aldaye, F. A. (2011). Organization of intracellular reactions with rationally designed RNA assemblies. Science, 333, 470–474.
Dueber, J. E., Wu, G. C., Malmirchegini, G. R., Moon, T. S., Petzold, C. J., Ullal, A. V., Prather, K. L. J., & Keasling, J. D. (2009). Synthetic protein scaffolds provide modular control over metabolic flux. Nature Biotechnology, 27, 753–759.
Good, M. C., Zalatan, J. G. and Lim, W. A. (2011). Scaffold proteins: hubs for controlling the flow of cellular information. Science (New York, N.Y.) 332, 680–686.
Guzman, L. M., Belin, D., Carson, M. J., & Beckwith, J. (1995). Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. Journal of Bacteriology, 177, 4121–4130.
Hirst, J., Sucheta, A., Ackrell, B. A. C., & Armstrong, F. A. (1996). Electrocatalytic voltammetry of succinate dehydrogenase: direct quantification of the catalytic properties of a complex electron-transport enzyme. Journal of the American Chemical Society, 118, 5031–5038.
Ignacio Tinoco Jr. KS, J. C. W., Joseph D. Puglisi, Gerard Harbison, David Rovnyak. (2002). Physical chemistry: principle and applications in biological sciences, 4th. Prentice Hall, Upper Saddle River, NJ.
Kook, M.-C., Seo, M.-J., Cheigh, C.-I., Lee, S.-J., Pyun, Y.-R., & Park, H. (2010). Enhancement of γ-amminobutyric acid production by Lactobacillus sakei B2–16 expressing glutamate decarboxylase from Lactobacillus plantarum ATCC 14917. Journal of the Korean Society Applied Biological Chemistry, 53, 816–820.
Le Vo, T., Ko, J.-s., Lee, S., Park, S. and Hong, S. (2013). Overexpression of Neurospora crassa OR74A glutamate decarboxylase in Escherichia coli for efficient GABA production. Biotechnology and Bioprocess Engineering, 18, 1062–1066.
Le Vo, T., Ko, J.-s., Park, S., Lee, S. and Hong, S. (2013). Efficient gamma-aminobutyric acid bioconversion by employing synthetic complex between glutamate decarboxylase and glutamate/GABA antiporter in engineered Escherichia coli. Journal of Industrial Microbiology and Biotechnology, 40, 927–933.
Lederer, T., Kintrup, M., Takahashi, M., Sum, P.-E., Ellestad, G. A., & Hillen, W. (1996). Tetracycline analogs affecting binding to Tn10-encoded Tet repressor trigger the same mechanism of induction. Biochemistry, 35, 7439–7446.
Maklashina, E., Berthold, D. A., & Cecchini, G. (1998). Anaerobic expression of Escherichia coli succinate dehydrogenase: functional replacement of fumarate reductase in the respiratory chain during anaerobic growth. Journal of Bacteriology, 180, 5989–5996.
Moon, T. S., Dueber, J. E., Shiue, E., & Prather, K. L. J. (2010). Use of modular, synthetic scaffolds for improved production of glucaric acid in engineered E. coli. Metabolic Engineering, 12, 298–305.
Oh CH, O. S. (2004). Effect of germinated brown rice extracts with enhanced levels of GABA on cancer cell proliferation and apoptosis. Journal of Medicinal Food, 7, 19.
Park, K.-B., & Oh, S.-H. (2006). Enhancement of γ-aminobutyric acid production in Chungkukjang by applying a Bacillus subtilis strain expressing glutamate decarboxylase from Lactobacillus brevis. Biotechnology Letters, 28, 1459–1463.
Park, S., Kim, E., Noh, W., Oh, Y., Kim, H., Song, B., Cho, K., Hong, S., Lee, S., & Jegal, J. (2013). Synthesis of nylon 4 from gamma-aminobutyrate (GABA) produced by recombinant Escherichia coli. Bioprocess and Biosystems Engineering, 36, 885–892.
Pham, V. D., Lee, S. H., Park, S. J., & Hong, S. H. (2015). Production of gamma-aminobutyric acid from glucose by introduction of synthetic scaffolds between isocitrate dehydrogenase, glutamate synthase and glutamate decarboxylase in recombinant Escherichia coli. Journal of Biotechnology, 207, 52–57.
Sambrook J, R. D. (2001) Molecular cloning: a laboratory manual.
Vo, T., Pham, V., Ko, J.-s., Lee, S., Park, S. and Hong, S. (2014). Improvement of gamma-amino butyric acid production by an overexpression of glutamate decarboxylase from Pyrococcus horikoshii in Escherichia coli. Biotechnology and Bioprocess Engineering, 19, 327–331.
Vo, T. D., & Hong, S. H. (2012). Optimization of gamma-Aminobutyric acid (GABA) bioconversion by recombinant Escherichia coli. Korean Society for Biotechnology and Bioengineering Journal, 27, 127–130.
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (SSAC, grant number: PJ01111601), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pham, V.D., Somasundaram, S., Lee, S.H. et al. Redirection of Metabolic Flux into Novel Gamma-Aminobutyric Acid Production Pathway by Introduction of Synthetic Scaffolds Strategy in Escherichia Coli . Appl Biochem Biotechnol 178, 1315–1324 (2016). https://doi.org/10.1007/s12010-015-1948-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1948-9