Abstract
Adulteration of meat products and costly animal-derived commodities with their inferior/cheaper counterparts is a grievous global problem. Species authentication is still technical challenging, especially to those deep processed products. The present study described the design of seven sets of species-specific primer based on a high heterozygous region of mitochondrial cytochrome c oxidase subunit I (COI) gene. These primers were proven to have high species specificity and no cross-reactions and unexpected products to different DNA source. Multiplex PCR assay was achieved for rapid and economical identification of four commonly consumed meats (pork, beef, chicken, and mutton). The conventional PCR assay was sensitive down to 0.001 ng of DNA template in the reactant. The developed method was also powerful in detecting as low as 0.1-mg adulterated pork (0.05 % in wt/wt) in an artificial counterfeited mutton. Validation test showed that the assay is specific, reproducible, and robust in commercial deep processed meats, leatherware, and feather commodities. This proposed method will be greatly beneficial to the consumers, food industry, leather, and feather commodity manufacture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to WHO’s report, the annual production of meat and meat products will rise to 376 millions of tonnes in 2030, whereas it was 218 millions of tonnes between 1997 and 1999. The steadily growing numbers of output, unlisted, mislabeled, or fraudulent ingredients in animal products are becoming a grievous problem and impacts humans in a number of ways. Those meat adulterants are not only related with economic fraud but also directly point to a crisis of consumer confidence and food safety. Meat adulteration regained public attention in spring of 2013, soon after the disclosure of “European horsemeat crisis” and “Chinese lamb scandal,” in which unlabelled horse meat was found in beef product, or fox meat even murine meat substituted lamb or beef. Due to the possible diseases, such as avian influenza virus [1], bovine spongiform encephalopathy (BSE) [2], and foot and mouth disease (FMD) [3] in chicken, cattle, and pork, respectively, meat species adulteration are also a threat to public health. Moreover, the adulterated meat with pork was severe against the religious beliefs of Muslims. Apart from meat species falsification, illegal poaching and trading of endangered game species were often found posing threat to wildlife populations [4]. It is also significant to limit the transmission of food-borne allergens. What is more, fraudulent animal also had extensive affection to animal product, such as leather, drug, cashmere, and feedstuff. For example, collacoriiasini, a well-known and expensive traditional Chinese medicine (TCM) that is primarily prepared by donkey-hide gelatin, was found counterfeit prepared by cowhide leftover. Similar adulteration cases are also frequently found in goat cashmere by mixing with sheep wool since 1990s, for the sake of high profit [5]. As a consequence, a reliable, rapid, economic, and highly sensitive analytical tool that facilitates routine control tests of meat species in different foods and animal products is urgently needed.
For the sake of species identification in animal products, there are a multitude of selections available: proteins, lipid, volatile organic compounds, and DNA analyses [6]. Among these, abundant methods have been developed based on protein and DNA analysis. Unfortunately, methods based on the protein traction such as electrophoretic, chromatographic, and immunological techniques are often not suitable for complex animal products, are not sensitive in processed materials to differentiate closely related species, and are time-consuming, inadequate, and/or expensive [7]. It is reported that DNA as the most appropriate biomarker to identify the source of animal-derived materials and DNA-based techniques are reliable, robust, and rapid, especially species-specific PCR which has the potential to reach higher identification simplicity, sensitivity, and specificity. The target genes and DNA fragments used as markers for identifying animal species are mainly coming from the mitochondrial genome, such as cytochrome oxidase subunit I (COI) [8], the mitochondrial D-loop region [9], and cytochrome b [10], 16 s rRNA [11], and 12 s rRNA [12]. Many techniques based on the DNA fractions, such as species-specific PCR [12], direct PCR [13], real-time PCR [14], polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) [15], and sequencing [16]. PCR has a high success rate and a very low false-positive rate, making it an extremely popular and valuable tool for the identification of meats of different animal species in recent years and came into prominence as an alternative method that can replace the existing methods. Species-specific PCR has been shown to be suitable for the detection of meat adulteration for a specific target sequence that can be detected in sequences of different origin without further sequencing or digestion of the PCR products with restriction enzymes. Based on PCR, Hebert et al. (2003) argued that sequence diversity in the cytochrome c oxidase subunit I (COI) gene could be used to create a “DNA barcoding” system that would be capable of offering a means of identifying all animal life and may become a standard tool. Recent evidence from better-studied taxa suggests that upon most occasions, DNA barcoding permits rapid and accurate identification. Although PCR is commonly used in the identification of meat products, the application of the COI gene is still very limited and it has not been deeply investigated to distinguish different raw meats and meat product species from nucleotide variation in almost the same area of the mitochondrial COI gene yet.
Thus, the aim of the study was to establish a reliable and rapid assay for authenticating common meats, such as horse (Equus caballus), beef (Bubalus bubalis), mutton (Capra hircus), pork (Sus scrofa), dog (Canis lupus familiaris), chicken (Gallus gallus domesticus), and mice (Mus musculus) meat or other products according to species-specific PCR of the mitochondrial COI gene.
Materials and Methods
Animal Materials and Reagents
Authentic fresh meats of horse, beef, mutton, pork, dog, chicken, and mice were obtained from morphological-verified specimens. Animal products including leather, heat-treated foods, pickled foods, street foods, spiced foods, and instant frozen foods were directly purchased from local retail shops and supermarkets of Nanchang. The processed meats were rinsed twice with 70 % ethyl alcohol and three times with ddH2O to eliminate oil and spices. All the referenced samples were cut into small pieces, labeled and stored at −20 °C until the isolation of the DNA in order to prevent sample spoilage and the enzymatic degradation of DNA.
PCR mixture 2× and 100-bp DNA ladder were products of Tiangen (China). Proteinase K (sigma) was dissolved in Tris-HCl buffer (pH 7.4). Seventy-five and 99 % ethyl alcohol, isopropyl alcohol, chloroform, and other reagents were analytical grade.
Primer Design
Mitochondrial COI DNA sequences of the tested meat species were retrieved from Genbank (www.ncbi.nlm.nih.gov). Multiple sequence alignment was carried by GenDoc software; a 22nt DNA segment was found heterogeneous among the seven species. The 22nt DNA fragments were then used as antisense primers, and the optimum corresponding sense primers were designed by Primer premier 6.0, avoiding self-dimer, cross-dimer, and hairpin structures. Details of primers applied in this study are shown in Table 1.
DNA Extraction
Two hundred-milligram meat sample was taken and homogenized with 400 μL TE solution [10 mmol/L Tris-HCl (pH8.0), 1 mmol/L EDTA]. Meat slurry was then mixed with 400 μL DNA lysis buffer [5 mol/L guanidinium isothiocyanate, 50 mmol/L Tris-HCl (pH 6.4), 20 mmol/L EDTA, 1.3 % Triton X-100] overnight at room temperature. The lysed meat mixture was heated to 37 °C, then 100 μL (20 mg/ml) proteinase K was added and incubated for 30 min at 56 °C. The aqueous supernatant was collected by centrifugation at 13,000g for 10 min and carefully transferred to a new 1.5-mL tube, followed by the standard phenol-chloroform-isoamyl alcohol method to isolate and purify the genomic DNA. DNA samples, concentrated by ethanol precipitation, were dissolved in 50 μL TE solution and kept at −20 °C until further use. DNA concentration was measured by spectrophotometric analysis. The pretreatment to solid materials like leather and feather was slightly modified; ultrasonication with a programmed interval working and resting at 400 W was adopted to improve tissue lysis.
Polymerase Chain Reaction
For PCR amplification, primer pairs for COI gene (Table 1) were used. Each PCR reaction was set in a volume of 20 μL with 10 μL 2× PCR mixture, 1 μL each of (10 μmol/L) sense and antisense primers, and 2 ng DNA sample. Volume was made up to 20 μL by adding autoclaved Milli-Q water. PCR conditions were programmed as follows: 5 min at 95 °C for initial denaturation, followed by 35 cycles of amplification (30 s each at 95, 58, and 72 °C) and final extension for 5 min at 72 °C. PCR amplicons were analyzed by electrophoresis in 2.0 % agarose gel for 30 min at 120 V in TAE buffer and stained with ethidium bromide.
Simplex PCR Specificity
In an elementary part of this research, simplex PCRs were carried out on DNA isolated from the seven raw meats to verify the specificity of the primers.
Mixed and Multiplex PCR Specificity
Further determination of primers’ specificity was performed by mixing binary DNA templates with a single set of primer and by mixing binary primer pairs with a single template. The most common and highest consumption meats, namely pork, mutton, beef, and chicken were adopted for species-specificity detection. The purpose was to investigate the cross-reactions between different combinations of DNA and primers.
Multiplex PCR was then carried out by mixing genomic DNAs and their primer pairs in arbitrary combination. The experiment aimed to establish a fast, economical, and simultaneous assay for meat authentication.
Sensitivity Test and Mimic Counterfeiting
For the sensitivity test, serial diluted DNA templates of beef and pork were used. Four concentrations (1.0, 0.1, 0.01, and 0.001 ng) were prepared by dilution and amplified by the assay to determine the minimum amount that can be detected.
Owing to the grievous problem of mutton falsification, we prepared a serial mixture of pork and mutton meat with the following ratios: 0.05, 0.1, 1, and 5 % pork in mutton by weight/weight. Each 200-mg mixture was used for DNA isolation and PCR assay followed by the above described method. This experiment is to determine the sensitivity of our method in detecting market meat counterfeiting.
Application to Commercial Animal Products
Besides raw meats, the processed meats and animal products such as instant food, street food, pickled and spiced meat, leatherware, etc. are also subject to severe falsification. Those meats and products are technically challenging. For the real-world validation, five typical processed meats and two animal products were selected and identified by the developed PCR assay. These commercial products include stewed beef, fried beef, preserved pork, pork sausage, chicken feet with pickled pepper, cow leather waist belt, and chicken feather. The processed meats were pretreated to eliminate oil and spices by rinsing with 70 % ethyl alcohol and ddH2O repeatedly. In the pretreatment of leather belt and feather samples, they were subjected to an extra ultrasonication at 400 W for 10 min, with a program of interval working for 3 s and resting for 5 s. Same DNA extraction and PCR amplification were followed; PCR amplicons from those commercial products were tested by polyacrylamide gel electrophoresis (PAGE) and ethidium bromide staining.
Results
Multiple Alignment and Primer Design
Multiple alignment of COI gene of the seven species was shown in Fig. 1. COI genes are evolutionary conservative and contain widely distributed single nucleotide polymorphism (SNP) sites. According to the alignment, SNPs and conserved sites are evenly distributed in the 2009-bp-length COI gene, except a 22nt heterogeneous region from 1681 to 1702 bp (framed in Fig. 1). The 22nt segment happens to be a primer’s length; therefore, the common region was used for anti-sense primers. The species-specific corresponding sense primers were then designed by Primer premier 6.0 software, avoiding self-dimer, cross-dimer, and hairpin structures. Due to the possible DNA degradation in meat processing like cooking, primers were restricted to generate PCR amplicons less than 350 bp. The primers, PCR product size, and annealing temperature of the seven meat species are listed in Table 1.
The framed rectangular segment shows the heterogenous sequences of COI in the seven meat species. Antisense primers were predesigned according to the region, and the corresponding sense primers were matched by primer 6.0 software.
Meat Identification by Species-Specific Primers
Simplex PCR Specificity
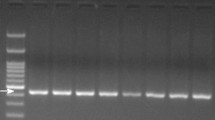
We successfully performed PCR amplification by applying the above primers with all seven meat DNA templates. The amplicons of the seven animal COI genes showed different sizes as expected in Table 1. No extra PCR products were produced in each reaction (Fig. 2), which indicated a high specificity of these primers in the simplex PCR identification. Since most of these PCR products were over 30 bp different in size, their migration on agarose gel was easy to discriminate except pork (268) and chicken (251), mice (128), and horse (124). Better resolution could be achieved by using polyacrylamide gel electrophoresis (PAGE) (see Fig. 6).
Mixed and Multiplex PCR Specificity
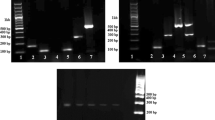
In order to investigate the cross-reactivity among these primers and different genomic DNA, mixed PCR and multiplex PCR were performed. Figure 3a showed the results of reactions of binary templates with a single primer pair; the sole band in the diagram indicated that the primers’ specificity were high enough for common meat authentication. PCR results of a single DNA template with mixed primers were shown in Fig. 3b; no primer dimers were detected. Obviously, these primers have no cross-reaction in any combination. Also in Fig. 3c, the multiplex PCR with mixed DNA templates and primers showed no cross-contamination and unexpected band in each lane, which indicated that the specific and efficient method was adequate enough for complicated species identification.
Specificity test of primers. a Mixed templates with a single primer pair. Lanes 1–3 represent beef primer with binary templates of B+(M/C/P); lanes 4–6, mutton primer with binary DNA of M+(C/P/B); lanes 7–9, chicken primer with DNA of C+(M/P/B); lanes 10–12, pork primer with DNA of P+(M/C/B). b Mixed primers with single templates. Lanes 1–3 represent mutton DNA with binary primers M+(C/P/B); lanes 4–6, chicken DNA with primers of C+(B/P/M); lanes 7–9, pork DNA with primers of P+(M/C/B); lanes 10–12, beef DNA with primers of B+(M/C/P). c Mixed templates and mixed primers. Lanes 1–3 represent binary DNA of B+(P/C/M) with corresponding primers; lane 4, P+C DNA and primers; lane 5, P+M DNA and primers; lane 6, C+M DNA and primers. Abbreviation: B beef, M mutton, P pork, C chicken
Sensitivity Test
To determine the sensitivity, PCR amplification was performed with pork and beef DNA. Both the two animal DNA templates were diluted by ddH2O to reach final amount of 1, 0.1, 0.01, and 0.001 ng in each PCR reaction. The sensitivity, also known as detection limit, was determined to be 0.001 ng DNA for each species (Fig. 4).
Mimic Counterfeiting
Two hundred milligram pork-mutton binary meat mixture was premixed separately according to the ratios of 0.05:99.95, 0.1:99.9, 1:99, and 5:95. Figure 5 showed that pork PCR product (268 bp) was detected in all the mixtures, even the amount of pork was as low as 0.1 mg (0.05 % of 200-mg binary meat mixture). The result implied that at least 0.1-mg fraudulent meat could be detected by the method mentioned in this paper.
Application to Commercial Products
The real-world use of the developed assay with processed meat and animal-derived products was validated. Whether these meats were dried, fried, stewed, or pickled, even more complicated processing like belt manufacture, the animal species were successfully identified with good specificity, reproducibility, sensitivity, and robustness, as shown in Fig. 6.
Discussion
Several researchers have previously reported species-specific PCR-based assays for meat species identification in meat productions, such as real-time PCR assays for the food microbiologist [17], pork detection in poultry meat products [18], and assay for the specific identification of meats from red deer, roe deer, pyrenean ibex, and chamois by PCR-sequencing and capillary electrophoresis techniques [19], but suitable equipments and trained professionals are required to carry out the analysis which limits the use of the technique in many food control laboratories that cannot afford these expensive equipment. This study developed a simple and sensitive conventional PCR method for identification of seven meat species, and it does not require expensive equipment such as real-time PCR instrument and capillary electrophoresis.
Different from most of previous reports, in this research, PCR assays were totally designed with the amplification of mitochondrial COI gene in horse, beef, mutton, pork, dog, chicken, and mice DNA based on a 22nt heterogenous region from 1681-1702 bp. The 5′ region 658 bp of COI has high variants among inter- and intra-species, by which DNA barcode database was established up for species identification (http://www.barcodinglife.org). Thousands of species, such as insects, birds, fishes, spiders, reptiles, and mammals are included in the library. However, species identification based on DNA barcode relies on PCR amplification and sequencing, which is both costly and time-consuming. By using the former 700-bp segment of COI, Haider set up a PCR-RFLP method for identification of cow, chicken, turkey, sheep, pig, buffalo, camel, and donkey, in which seven restriction enzymes were tested [8]. Till now, species identification by the rest region (700–2009 bp) of COI was seldom reported, especially to multiple species authentication. Kitpipit used 552∼804-bp and 482∼792-bp regions of COI to identify horse (Equus cabllus) and cow (Bos Taurus) by direct PCR, respectively. But other species (pork, lamb, and ostrich) in his work were identified by cytochrome b or 12s rRNA [13]. The 1681–1702 bp of COI, as shown in Fig. 1, was in high polymorphism among different families and genera. Furthermore, the region is also highly varied in close species. For example, there are six SNPs (single nucleotide polymorphism) in the 22nt region of milk cow (Bos Taurus) and buffalo (Bubalus bubalis), which is the most intensive variant region (every 22nt) in COI (see supplementary 1). Therefore, the 22nt region is really good for species identification.
According to mixed and multiplex PCR results, the seven sets of primers had no cross-reactions and unexpected products, which indicated the COI gene has adequate polymorphism for species identification. Although PCR techniques are widely reported in meat authentication, identification to seven or more species based on just one gene is seldom found.
Due to possible DNA fragmentation in high-temperature processing, PCR primer is suggested restrictive to produce amplicons over than 350 bp [20]. The primers in this research generated specific fragments of 124-,313-,157-, 268-,188-,251-, and 128-bp length for horse, beef, mutton, pork, dog, chicken, and mice meat, respectively. These close PCR products, for example, pig (268 bp) vs chicken (251 bp) and mice (128 bp) vs horse (124 bp), may be less discriminative on agarose gel. The issue can be well solved by modification on primers or altering to polyacrylamide gel electrophoresis (PAGE). Primer modification is to add an adaptor at the 5′ of primer, which leads to longer PCR product. The adaptor is a 10–30nt random sequence which is rarely complementary to DNA template and primer. For example, when the forward primer of mice is modified with an adapter like CCTTCCTTCCTTCCCCCC as described by Bai et al. [21], the resulted PCR product will be 146 bp, which is more reliable to discriminate from horse amplicon. Alternatively, PCR product can be run on polyacrylamide gel which has smaller mesh and better separation to similar-sized DNA fragments (as shown in Fig. 6). PAGE is powerful enough to distinguish identical length DNA fragments with different conformation caused by SNP [22].
A minimum detection limit of 0.1–0.01 % for food products was found in various literatures [18, 23, 24]. Most of these sensitivity data, however, were obtained from PCR amplification with serial diluted foreign DNA in target template DNA. Thus, these data only stood for PCR sensitivity, not for real-world meat identification. Kesmen et al. (2007) prepared sausage from horse-beef, donkey-beef, and pork-beef binary meat mixtures, and he demonstrated a detection possibility at 0.1 % level (0.1 % horse, donkey, or pork with 99.9 % beef meat) and PCR sensitivity at 0.01-ng template DNA [25]. Safdar et al. (2014) increased the detection threshold to 0.01 % by using DNA extraction kit and qPCR reagent in sausage gradient identification [26]. We achieved a 0.05 % (0.1-mg pork/199.9-mg mutton) detection level by using common DNA extraction method and conventional PCR assay. The PCR sensitivity in our research is 0.001 ng (of template DNA), which is lower than most of the reported data [18, 24]. By using semi-nested PCR, the detection limit to further processed meat was enhanced to 0.001 ng by Zhang’s effort [27]. In a word, our new method is much more attractive as compared to others due to its minimum detection threshold of meat sample and high PCR sensitivity.
Multiplex PCR, in which many primers are used together for amplification of multiple target genes, is preferred over those of simplex as they can detect multiple species at the same time, reducing both the cost and time [6]. Although we only performed duplex PCR of the four kinds of widely consumed meats (pork, beef, mutton, chicken), but all the binary combinations were tested meanwhile (Fig. 3c), so a quadplex PCR to simultaneous detection of the four meats is also practicable. The specificity, reproducibility, and robustness were also validated with commercial further processed meat products, cow leather belt, and chicken feather. All these points reveal confidence in the use of the developed assay not only for various meat falsification but also leatherware, cashmere, feather commodities, and animal traditional Chinese medicine (TCM) counterfeiting.
Conclusion
The obtained results showed that COI is a suitable target gene for species identification. The developed PCR assay had high sensitivity, specificity, and robustness for raw meat, further processed meat products, leatherware, and others animal source commodities. Multiplex PCR is realizable in the four widely consumed meat (pork, beef, mutton, chicken) authentication. The developed assay is cost-saving and practicable to food safety agencies.
References
Swayne, D. E. (2006). Microassay for measuring thermal inactivation of H5N1 high pathogenicity avian influenza virus in naturally infected chicken meat. International Journal of Food Microbiology, 108(2), 268–271.
Yamamoto, T., Tsutsui, T., Nonaka, T., Kobayashi, S., Nishiguchi, A., & Yamane, I. (2006). A quantitative assessment of the risk of exposure to bovine spongiform encephalopathy via meat-and-bone meal in Japan. Preventive Veterinary Medicine, 75(3–4), 221–238.
Roche, S. E., Garner, M. G., Wicks, R. M., East, I. J., & de Witte, K. (2014). How do resources influence control measures during a simulated outbreak of foot and mouth disease in Australia? Preventive Veterinary Medicine, 113(4), 436–446.
Rajput, N., Shrivastav, A. B., Parmar, S. N. S., Ranjan, R., Singh, S., & Joseph, E. (2013). Characterization of 12S rRNA gene for meat identification of common wild and domestic small herbivores as an aid to wildlife forensic. Veterinary World, 6(5), 254.
Ji, W., Bai, L., Ji, M., & Yang, X. (2011). A method for quantifying mixed goat cashmere and sheep wool. Forensic Science International, 208(1–3), 139–142.
Ali, M. E., Kashif, M., Uddin, K., Hashim, U., Mustafa, S., & Che Man, Y. B. (2012). Species authentication methods in foods and feeds: the present, past, and future of Halal forensics. Food Analytical Methods, 5(5), 935–955.
Martin, I., Garcia, T., Fajardo, V., Lopez-Calleja, I., Rojas, M., Pavon, M. A., Hernandez, P. E., Gonzalez, I., & Martin, R. (2007). Technical note: detection of chicken, turkey, duck, and goose tissues in feedstuffs using species-specific polymerase chain reaction. Journal of Animal Science, 85(2), 452–458.
Haider, N., Nabulsi, I., & Al-Safadi, B. (2012). Identification of meat species by PCR-RFLP of the mitochondrial COI gene. Meat Science, 90(2), 490–493.
Karabasanavar, N. S., Singh, S. P., Kumar, D., & Shebannavar, S. N. (2014). Detection of pork adulteration by highly-specific PCR assay of mitochondrial D-loop. Food Chemistry, 145, 530–534.
Aida, A. A., Che Man, Y. B., Wong, C. M., Raha, A. R., & Son, R. (2005). Analysis of raw meats and fats of pigs using polymerase chain reaction for Halal authentication. Meat Science, 69(1), 47–52.
Sarri, C., Stamatis, C., Sarafidou, T., Galara, I., Godosopoulos, V., Kolovos, M., Liakou, C., Tastsoglou, S., & Mamuris, Z. (2014). A new set of 16S rRNA universal primers for identification of animal species. Food Control, 43, 35–41.
Ahmed, M. U., Hasan, Q., Hossain, M. M., Saito, M., & Tamiya, E. (2010). Meat species identification based on the loop mediated isothermal amplification and electrochemical DNA sensor. Food Control, 21(5), 599–605.
Kitpipit, T., Sittichan, K., & Thanakiatkrai, P. (2014). Direct-multiplex PCR assay for meat species identification in food products. Food Chemistry, 163, 77–82.
Kesmen, Z., Gulluce, A., Sahin, F., & Yetim, H. (2009). Identification of meat species by TaqMan-based real-time PCR assay. Meat Science, 82(4), 444–449.
Chen, S. Y., Liu, Y. P., & Yao, Y. G. (2010). Species authentication of commercial beef jerky based on PCR-RFLP analysis of the mitochondrial 12S rRNA gene. Journal of Genetics and Genomics, 37(11), 763–769.
Girish, P. S., Anjaneyulu, A. S., Viswas, K. N., Anand, M., Rajkumar, N., Shivakumar, B. M., & Bhaskar, S. (2004). Sequence analysis of mitochondrial 12S rRNA gene can identify meat species. Meat Science, 66(3), 551–556.
Hanna, S. E., Connor, C. J., & Wang, H. H. (2005). Real-time polymerase chain reaction for the food microbiologist: technologies, applications, and limitations. Journal of Food Science, 70(3), R49–R53.
Soares, S., Amaral, J. S., Oliveira, M. B. P. P., & Mafra, I. (2013). A SYBR Green real-time PCR assay to detect and quantify pork meat in processed poultry meat products. Meat Science, 94(1), 115–120.
La Neve, F., Civera, T., Mucci, N., & Bottero, M. T. (2008). Authentication of meat from game and domestic species by SNaPshot minisequencing analysis. Meat Science, 80(2), 216–224.
Hird, H., Chisholm, J., Sanchez, A., Hernandez, M., Goodier, R., Schneede, K., Boltz, C., & Popping, B. (2006). Effect of heat and pressure processing on DNA fragmentation and implications for the detection of meat using a real-time polymerase chain reaction. Food Additives and Contaminants, 23(7), 645–650.
Bai, W., Xu, W., Huang, K., Yuan, Y., Cao, S., & Luo, Y. (2009). A novel common primer multiplex PCR (CP-M-PCR) method for the simultaneous detection of meat species. Food Control, 20, 366–370.
Zhu, W., Deng, Y., Jie, K., Luo, D., Liu, Z., Yu, L., Zeng, E., & Wan, F. (2013). Detection of single nucleotide polymorphisms by PCR-conformation-difference gel electrophoresis. Biotechnology Letters, 35, 515–522.
Ghovvati, S., Nassiri, M. R., Mirhoseini, S. Z., Moussavi, A. H., & Javadmanesh, A. (2009). Fraud identification in industrial meat products by multiplex PCR assay. Food Control, 20(8), 696–699.
Amaral, J. S., Santos, C. G., Melo, V. S., Oliveira, M. B. P. P., & Mafra, I. (2014). Authentication of a traditional game meat sausage (Alheira) by species-specific PCR assays to detect hare, rabbit, red deer, pork and cow meats. Food Research International, 60, 140–145.
Kesmen, Z., Sahin, F., & Yetim, H. (2007). PCR assay for the identification of animal species in cooked sausages. Meat Science, 77(4), 649–653.
Safdar, M., Junejo, Y., Arman, K., & Abasiyanik, M. F. (2014). A highly sensitive and specific tetraplex PCR assay for soybean, poultry, horse and pork species identification in sausages: development and validation. Meat Science, 98(2), 296–300.
Zhang, C. (2013). Semi-nested multiplex PCR enhanced method sensitivity of species detection in further-processed meats. Food Control, 31(2), 326–330.
Acknowledgment
The authors acknowledge the funding of National Training Programs of innovation and entrepreneurship for undergraduates (201310403029).
Conflicts of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhenyu Dai, Jiao Qiao and Siran Yang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 136 kb)
Rights and permissions
About this article
Cite this article
Dai, Z., Qiao, J., Yang, S. et al. Species Authentication of Common Meat Based on PCR Analysis of the Mitochondrial COI Gene. Appl Biochem Biotechnol 176, 1770–1780 (2015). https://doi.org/10.1007/s12010-015-1715-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1715-y