Abstract
We constructed and applied a recombinant, permeabilized Escherichia coli strain for the multistep synthesis of UDP-glucose. Sucrose phosphorylase (E.C. 2.4.1.7) of Leuconostoc mesenteroides was over expressed and the pgm gene encoding for phosphoglucomutase (E.C. 5.4.2.2) was deleted in E. coli to yield the E. coli JW 0675-1 SP strain. The cells were permeabilized with the detergent Triton X-100 at 0.05 % v/v. The synthesis of UDP-glucose with permeabilized cells was then optimized with regard to pH, cell density during the synthesis and growth phase during cell harvest, metal cofactor, other media components, and temperature. In one configuration sucrose, phosphate, UMP, and ATP were used as substrates. At pH 7.8, 27 mg/ml cell dry weight, cell harvest during the early stationary phase of growth and Mn2+ as cofactor a yield of 37 % with respect to UMP was achieved at 33 °C. In a second step, ATP was regenerated by feeding glucose and using only catalytic amounts of ATP and NAD+. A UDP-glucose yield of 60 % with respect to UMP was obtained using this setup. With the same setup but without addition of external ATP, the yield was 54 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The synthesis of metabolic intermediates is of broad industrial interest [1, 2]. Multi-enzyme biocatalysis is a promising alternative to multistep organic synthesis or production by living cells [3, 4]. UDP-glucose is a major reactive intermediate in carbohydrate metabolism, particularly for the biosynthesis of oligomers and polymers of glucose and complex oligomeric carbohydrates. As a key intermediate for glycosylation reactions, it acts as a glucose donor and is an intermediate in galactose metabolism [5]. UDP-glucose was produced using freeze-dried cells of Candida saitoana [6] as catalyst.
Cell permeabilization has been used as a method for the study of metabolic processes for 50 years [7–10]. When cells are treated with mild permeabilizing agents (i.e., detergents), their microscopic structure remains intact but the cell membrane becomes permeable to small molecules. After washing away these molecules, only macromolecules like enzymes stay within the cell and become accessible for any low molecular weight compound that is added to a cell suspension. These cells can then be used as multi-enzyme catalyst [9, 11].
We used permeabilized Escherichia coli for the multi-enzyme synthesis of UDP-glucose under defined conditions. We overexpressed sucrose phosphorylase (SPase) from Leuconostoc mesenteroides [12] in a strain lacking phosphoglucomutase. Using this strain, we could directly produce UDP-glucose using sucrose, UMP, and ATP. ATP could also be regenerated via glycolysis after feeding glucose and adding catalytic amounts of NAD+. Addition of small amounts of ATP further stimulated UDP-glucose production.
Results and Discussion
Cell Permeabilization
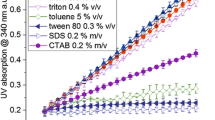
The activity of malate dehydrogenase (MDH) [13] was used to monitor the progress of permeabilization and optimize cell density, Triton X-100 concentration, temperature, and incubation time. At a dry cell density of 13.5 g/l, MDH activity increased until a Triton X-100 concentration of 0.05 % v/v and remained constant at higher detergent concentrations (Fig. 1). The activity of fully permeabilized cells was identical (±2 %) to the activity of cell extract without any detergent.
MDH activity increased linearly with cell density in the range from 1.69 to 13.5 g/l CDW (Fig. 2). At higher densities, it increased less, potentially caused by insufficient availability of Triton X-100. It is likely that a combination of higher cell density and higher Triton concentration would be effective as well. However higher amounts of Triton would require additional washing steps for complete removal of detergents.
The effects of permeabilization temperature (0, 10, 20, 30, 37, and 45 °C) and incubation time with Triton X-100 (1, 2, 5, 10, 20, 30 min) were also examined. Temperature effects on permeabilization were studied applying an incubation time of 10 min. The effect of different incubation times was investigated at 20 °C. In all cases except 45 °C, the MDH activity was identically high (data not shown). A comparison of the protein contents of cell lysate and of samples taken after 2 h of reaction revealed that less than 2 % of cellular protein was released into the supernatant. Overall, the permeabilization process is very robust and efficient.
Synthesis of UDP-Glucose
Initial studies were carried out with permeabilized E. coli K-12 cells. With UMP (10 mM), ATP (20 mM), and D-glucose-1-phosphate (G1P) (10 mM) as substrates, the yield did not exceed 15 % (1.5 mM) with respect to UMP. In WT, E. coli G1P can be isomerized to D-glucose-6-phosphate (G6P) by phosphoglucomutase (Pgm). To prevent related loss of G1P via G6P and glycolytic reactions, E. coli JW 0675-1 where the pgm gene coding for phosphoglucomutase (E.C. 5.4.2.2) was deleted was used [14]. With this strain, a yield of 30 % with respect to applied UMP was reached under optimized conditions after adding stoichiometric amounts of G1P and ATP.
Strain E. coli JW 0675-1 was transformed with the pQE 30-LmSPase plasmid carrying the SPase gene from L. mesenteroides to yield the strain E. coli JW 0675-1 SP. Using this strain G1P was supplied via phosphorolysis of less expensive sucrose by sucrose phosphorylase (SPase) [12]. The resulting reaction-network is shown in Scheme 1. With this strain, the synthesis worked best at 33 °C, pH 7.8, a cell density of 27 g/l, 5 mM manganese as metal cofactor, a phosphate concentration of 5 mM, and a sucrose concentration of 50 mM. Under these conditions, the maximum yield was 37 %.
Since no NADP+ was supplied, reactions of the oxidative branch of the PP-pathway are not possible limiting the formation of byproducts.
Further optimization of the synthesis was achieved by replacing the previously used stoichiometric amounts of ATP with catalytic concentrations combined with a fermentation-based regeneration driven by feeding glucose for glycolytic formation of ATP. If ATP concentrations lower than 2 mM were used, product formation was slower (Fig. 3, full squares). This might be caused by high K m values of UMPK and/or NDPK enzymes under the given conditions. UMPK has a K m around 0.15 mM under similar conditions whereas no kinetic data for NDPK could be found [15, 16].
Time course of UDP-glucose formation under optimized conditions. Reaction mixture: Open triangles: MnSO4 7 mM, NaH2PO4 5 mM, sucrose 50 mM, UMP 10 mM, and ATP 20 mM. Full circles: MgSO4 7 mM; NaH2PO4 5 mM + 4 × 2 mM added after 0, 27, 92, and 162 min; sucrose 50 mM; UMP 10 mM; ATP 2 mM; glucose 100 mM; and NAD+ 2 mM. Full triangles: MnSO4 7 mM, NaH2PO4 5 mM + 4 × 2 mM added after 0, 30, 90, and 150 min; sucrose 50 mM; UMP 10 mM; ATP 2 mM glucose 100 mM; and NAD+ 2 mM. Full squares: MnSO4 7 mM, NaH2PO4 5 mM + 4 × 2 mM added after 0, 30, 90, and 150 min; sucrose 50 mM; UMP 10 mM, glucose 100 mM; NAD+ 2 mM; and no addition of ATP. Open squares: MnSO4 7 mM; NaH2PO4 5 mM + 4 × 2 mM added after 0, 30, 90, and 150 min; sucrose 50 mM; UMP 10 mM; glucose 100 mM; ATP 2 mM; and no addition of NAD+
Remarkably, manganese was superior to magnesium as cofactor. As reported, magnesium is the preferred cofactor for UMPK and SPase, whereas no detailed information was found on the other enzymes [17–19]. Manganese activates the enzyme UDP-sugar diphosphatase (EC 3.6.1.45) which hydrolyses UDP-sugars [20]. However, it seems that the UDP-sugar diphosphatase is not highly expressed and/or manganese does not strongly activate it under the given conditions. Another reason for the superior effect of manganese seems to be that magnesium activates 5′-nucleotidase (EC 3.1.3.5) hydrolyzing UMP much more than manganese [21].
A yield of 37 % with respect to UMP was achieved when ATP was used in a stoichiometric amount (Fig. 3, open triangles). UDP-glucose was formed with a constant rate (0.052 mM/min) during the first 72 min. At this point, UMP was exhausted (as measured by MALDI-TOF-MS) and UDP-glucose started decreasing.
Reactions potentially consuming/degrading UDP-glucose under the given conditions are hydrolysis by the UDP-sugar diphosphatase (EC 3.6.1.45), lipopolysaccharide glucosyltransferase II (EC 2.4.1.73), and glycogenin glucosyltransferase (EC 2.4.1.186) [22]. Part of UMP might have been hydrolyzed by 5′-nucleotidase (EC 3.1.3.5) [21]. UTP can be consumed in the uridylylation of, e.g., protein PII [23].
When ATP was used in catalytic concentrations, the initial rate of product formation remained unchanged but product degradation was drastically reduced. Under these conditions a yield of 60 % with respect to applied UMP was reached within 2.5 h. (Fig. 3, full triangles). When glucose fermentation was applied for ATP regeneration, lactate formation was observed. If magnesium was added instead of manganese in this reaction setup, no product degradation was observed but yields did not increase beyond 39 % after 20 h. When no ATP was added to the reaction mixture, the rate of product formation was reduced but a yield of 54 % was still obtained after 4 h (Fig. 3, full squares). It can be concluded that trace amounts of ADP and/or ATP remain bound to the cells during the washing steps. Due to effective ATP regeneration, these small initial amounts were sufficient to support product formation. When NAD+ was omitted in the reaction mixture, product formation stopped after the initially added ATP was consumed (Fig. 3, open squares).
To classify production with permeabilized cells (in situ) in comparison with alternative approaches, further experiments were carried out with strain E. coli JW 0675-1 SP. Cell lysate (in vitro conditions) showed comparable behavior but inferior performance (not shown). The maximum yield was 53 % after 3.5 h. During cultivation, UDP-glucose could not be detected in the culture medium even after 24 h. Therefore, in vivo production is not a valuable option under these cultivation conditions.
Concluding Remarks
The permeabilization of E. coli has proven to be a simple and effective method to provide a multi-enzyme biocatalyst for the one-pot synthesis of UDP-glucose. Overexpression of heterologous SPase and deletion of pgm gene encoding for phosphoglucomutase permitted a direct synthesis of UDP-glucose starting from UMP with simultaneous regeneration of ATP and G1P. In this way, a yield of 60 % with respect to applied UMP was reached. The resulting procedure is straight forward, requiring only cell cultivation and harvest, permeabilization, washing, and incubation for synthesis of UDP-glucose. The biocatalyst can be separated from the reaction mixture by centrifugation or filtration. As compared to the fermentative method, downstream processing is facilitated due to the much smaller number of metabolites present in the reaction mixture. The system described also lends itself to combination with further enzymatic reactions. Another big advantage of the permeabilization approach is that it allows studying reaction pathways under defined conditions without elaborate enzyme purification and pathway reconstitution.
Experimental Section
E. coli K-12 was obtained from DSMZ (Braunschweig, Germany). E. coli JW 0675-1 was obtained from the Coli Genetic Stock Center (CGSC, Yale) [14]. The expression plasmid pQE 30-LmSPase harboring the SPase gene was generously provided by the group of Bernd Nidetzky from TU Graz [12]. E. coli JW 0675-1 was transformed with pQE 30-LmSPase to yield the E. coli JW 0675-1 SP strain using heat shock transformation [24]. Transformants were selected using LB5G agar plates supplied with 100 μg/ml ampicillin. All cultivations were carried out in LB5G medium (5 g/l glucose, 10 g/l tryptone, 5 g/l NaCl, 5 g/l yeast extract) at 37 °C and 230 rpm in baffled shake flasks. The growth medium for E. coli JW 0675-1 SP contained the same concentration of ampicillin. Cells were harvested by centrifugation (7000 g, 10 min, 4 °C) (Biofuge Stratos, Heraeus, Hanau, Germany). The pelletized cells were then re-suspended in Tris buffer (100 mM Tris, 200 mM KCl, pH 7.8 with HCl) and incubated with Triton X-100 for permeabilization. After that, permeabilized cells were washed three times with NH4HCO3 buffer (100 mM NH4HCO3, pH 7.8 with acetic acid).
A malate dehydrogenase (MDH—E.C. 1.1.1.40) assay was used to determine the degree of permeabilization. To 50 μl of cell suspension/cell free extract, a mixture containing 890 μl Tris-HCl buffer (100 mM Tris-HCl, 200 mM KCl, pH 7.8), 10 μl MgCl2 solution (200 mM), 10 μl NADP+ solution (100 mM), and 40 μl malate solution (1000 mM) was added to start the reaction (total volume 1 ml). UV absorption at 340 nm was measured over a period of 11 min to follow the formation of NADPH (Spectronic Unicam, Helios α-Spectronic Analytical Instruments, West Yorkshire, UK). The reaction temperature was kept at 30 °C using a water bath.
Glass-bead treatment was used to generate cell free extract. Five hundred microliters of cell suspension (13.5 g/l CDW) were mixed with 500 μg of glass beads (<0.25 mM in diameter) in a 2-ml Eppendorf tube and shaken for 15 min at 30 Hz at 0–4 °C using a swing mill of type mM301 (Retsch GmbH, Haan, Germany). The mixture was then centrifuged at 16,000 × g and 4 °C for 15 min using Biofuge Fresco (Heraeus, Germany) to separate cell debris and supernatant.
The synthesis of UDP-glucose was carried out in 100 mM NH4HCO3 buffer. The reaction volume was 1 ml. All reactions were carried out in 1.5-ml Eppendorf tubes.
For sampling 20 μl of reaction mixture were pipetted into 180 μl of cold (0 °C) NH4HCO3 buffer (pH 7.8 adjusted with acetic acid). This mixture was centrifuged (16,000 × g, 4 °C) for 2 min. One hundred eighty microliters of supernatant were then stored at −28 °C until analysis.
For MALDI analysis, 4 μl of sample were mixed with 8 μl of 9-aminoacridine (9 g/l in methanol). Samples were then analyzed with matrix-assisted laser desorption ionization mass spectrometry (MALDI 4800 TOF/TOF Analyzer, Applied Biosystems, Darmstadt, Germany) in negative reflector mode. A pulsed 200 Hz Nd:YAG Laser with a wavelength of 355 nm was used for ionization. Laser energy was set to 3600 units.
Quantifications were carried out using UHPLC (1290 Infinity, Agilent Technologies, CA, USA). The LC separation was carried out isocratically at a flow rate of 0.8 ml/min on a Supelcosil LC-18-T, 250 × 4.6 mM column at 40 °C in 20 min. The Eluent was 100 mM KH2PO4 buffer, pH 5.6 with KOH. Compounds were detected at 254 nm.
The protein content of cell lysate and of samples taken after 2 h of reaction was determined using the Bio-Rad protein assay [25]. Cell lysate was diluted 25-fold, and samples were diluted fivefold prior to analysis. In both cases, this corresponds to a cell density of 0.54 g/l CDW.
All chemicals were purchased from Fluka (Buchs, Switzerland), Sigma-Aldrich (Steinheim, Germany), or Merck (Darmstadt, Germany), and were of analytical grade.
References
Breuer, M., Ditrich, K., Habicher, T., Hauer, B., Kesseler, M., Sturmer, R., & Zelinski, T. (2004). Industrial methods for the production of optically active intermediates. Angewandte Chemie-International Edition, 43, 788–824.
Kuriata-Adamusiak, R., Strub, D., & Lochynski, S. (2012). Application of microorganisms towards synthesis of chiral terpenoid derivatives. Applied Microbiology and Biotechnology, 95, 1427–1436.
Eikmanns, B. J., Eggeling, L., & Sahm, H. (1993). Molecular aspects of lysine, threonine, and isoleucine biosynthesis in Corynebacterium glutamicum. Antonie van Leeuwenhoek International Journal of General and Molecular Microbiology, 64, 145–163.
Chae, H. S., Kim, K. H., Kim, S. C., & Lee, P. C. (2010). Strain-dependent carotenoid productions in metabolically engineered Escherichia coli. Applied Biochemistry and Biotechnology, 162, 2333–2344.
Elling, L., & Bülter, T. (1999). Enzymatic synthesis of nucleotide sugars. Glycoconjugate Journal, 16, 147–159.
Ko, J., Shin, H.-S., Kim, Y., Lee, D.-S., & Kim, C.-H. (1996). Biotransformation of uridine monophosphate (ump) and glucose to uridine diphosphate-glucose (udpg) by candida saitoana kctc7249 cells. Applied Biochemistry and Biotechnology, 60, 41–48.
Felix, H. (1982). Permeabilized cells. Analytical Biochemistry, 120, 211–234.
Niklas, J., Melnyk, A., Yuan, Y. B., & Heinzle, E. (2011). Selective permeabilization for the high-throughput measurement of compartmented enzyme activities in mammalian cells. Analytical Biochemistry, 416, 218–227.
Heinzle, E., Weyler, C., Krauser, S., & Blaß, L. K. (2013). Directed multistep biocatalysis using tailored permeabilized cells. In A.-P. Zeng (Ed.), Fundamentals and application of new bioproduction systems (pp. 185–234). Berlin Heidelberg: Springer.
Kondo, A., Liu, Y., Furuta, M., Fujita, Y., Matsumoto, T., & Fukuda, H. (2000). Preparation of high activity whole cell biocatalyst by permeabilization of recombinant flocculent yeast with alcohol. Enzyme and Microbial Technology, 27, 806–811.
Krauser, S., Kiefer, P., and Heinzle, E. (2012) Multienzyme whole-cell in situ biocatalysis for the production of flaviolin in permeabilized cells of Escherichia coli, ChemCatChem, 4, 786–788.
Goedl, C., Schwarz, A., Minani, A., & Nidetzky, B. (2007). Recombinant sucrose phosphorylase from leuconostoc mesenteroides: Characterization, kinetic studies of transglucosylation, and application of immobilised enzyme for production of [alpha]-d-glucose 1-phosphate. Journal of Biotechnology, 129, 77–86.
Yuan, Y., & Heinzle, E. (2009). Permeabilization of Corynebacterium glutamicum for NAD(P)H-dependent intracellular enzyme activity measurement. Comptes Rendus Chimie, 12, 1154–1162.
Baba, T., Ara, T., Hasegawa, M., Takai, Y., Okumura, Y., Baba, M., Datsenko, K. A., Tomita, M., Wanner, B. L., & Mori, H. (2006). Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Molecular Systems Biology, 2.
Serina, L., Bucurenci, N., Gilles, A. M., Surewicz, W. K., Fabian, H., Mantsch, H. H., Takahashi, M., Petrescu, I., Batelier, G., & Barzu, O. (1996). Structural properties of ump-kinase from escherichia coli: modulation of protein solubility by pH and UTP. Biochemistry, 35, 7003–7011.
Slavova-Azmanova, C. E. M. S. N. ( 2007) Regulatory mechanisms differ in UMP kinases from gram-negative and gram-positive bacteria, The Journal of Biological Chemistry 282.
van den Broek, L. A. M., van Boxtel, E. L., Kievit, R. P., Verhoef, R., Beldman, G., & Voragen, A. G. J. (2004). Physico-chemical and transglucosylation properties of recombinant sucrose phosphorylase from bifidobacterium adolescentis DSM20083. Applied Microbiology and Biotechnology, 65, 219–227.
Vainonen, J. P., Vorobyeva, N. N., Rodina, E. V., Nazarova, T. I., Kurilova, S. A., Skoblov, J. S., & Avaeva, S. M. (2005). Metal-free PPi activates hydrolysis of MgPPi by an Escherichia coli inorganic pyrophosphatase. Biochemistry-Moscow, 70, 69–78.
Meyer, P., Evrin, C., Briozzo, P., Joly, N., Barzu, O., & Gilles, A. M. (2008). Structural and functional characterization of escherichia coli UMP kinase in complex with its allosteric regulator GTP. Journal of Biological Chemistry, 283, 36011–36018.
Glaser, L., Melo, A., & Paul, R. (1967). Uridine diphosphate sugar hydrolase. Purification of enzyme and protein inhibitor. Journal of Biological Chemistry, 242, 1944.
Proudfoot, M., Kuznetsova, E., Brown, G., Rao, N. N., Kitagawa, M., Mori, H., Savchenko, A., & Yakunin, A. F. (2004). General enzymatic screens identify three new nucleotidases in Escherichia coli. Journal of Biological Chemistry, 279, 54687–54694.
Goldraij, A., & Curtino, J. A. (1996). M-glycogenin, the protein moiety of Neurospora crassa proteoglycogen, is an auto- and transglucosylating enzyme. Biochemical and Biophysical Research Communications, 227, 909–914.
Atkinson, M. R., Kamberov, E. S., Weiss, R. L., & Ninfa, A. J. (1994). Reversible uridylylation of the Escherichia coli PII signal-transduction protein regulates its ability to stimulate the dephosphorylation of the transcription factor nitrogen regulator I (NRI or NTrC). Journal of Biological Chemistry, 269, 28288–28293.
Inoue, H., Nojima, H., & Okayama, H. (1990). High efficiency transformation of Escherichia coli with plasmids. Gene, 96, 23–28.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Acknowledgments
We gratefully thank Christiane Goedl and Bernd Nidetzky for supplying pQE 30-LmSPase plasmid used for the creation of the E. coli JW 0675-1 SP strain. We acknowledge the support by BMBF (Federal Ministry of Education and Research, Project MECAT, FKZ 031P7238 within the initiative “Biotechnologie 2020+: Basistechnologien für eine nächste Generation biotechnologischer Verfahren.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weyler, C., Heinzle, E. Multistep Synthesis of UDP-Glucose Using Tailored, Permeabilized Cells of E. coli . Appl Biochem Biotechnol 175, 3729–3736 (2015). https://doi.org/10.1007/s12010-015-1540-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1540-3