Abstract
There are several problems limiting an industrial application of fossil fuel biodesulfurization, and one of them is the cost of culture media used to grow the microorganisms involved in the process. In this context, the utilization of alternative carbon sources resulting from agro-industrial by-products could be a strategy to reduce the investment in the operating expenses of a future industrial application. Recently, Gordonia alkanivorans 1B was described as a fructophilic desulfurizing bacterium, and this characteristic opens a new interest in alternative carbon sources rich in fructose. Thus, the goal of this study was to evaluate the utilization of sugar beet molasses (SBM) in the dibenzothiophene (DBT) desulfurization process using strain 1B. SBM firstly treated with 0.25 % BaCl2 (w/v) was used after sucrose acidic hydrolysis or in a simultaneous saccharification and fermentation process with a Zygosaccharomyces bailii Talf1 invertase (1 %), showing promising results. In optimal conditions, strain 1B presented a μ max of 0.0795 h−1, and all DBT was converted to 2-hydroxybiphenyl (250 μM) within 48 h with a maximum production rate of 7.78 μM h−1. Our results showed the high potential of SBM to be used in a future industrial fossil fuel biodesulfurization process using strain 1B.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The technology used to reduce sulfur in fossil fuels consists of a process called hydrodesulfurization (HDS). This process is expensive since it is carried out at high temperature and pressure and does not work well on various polyaromatic sulfur heterocycles always present in crude oil such as dibenzothiophene (DBT) and substituted DBTs (methylated DBTs and benzo-DBTs), which are particularly recalcitrant to HDS [1]. To achieve lower sulfur concentrations, refiners need to increase the capital investment and/or the operating costs [2]. Moreover, increasing the severity of HDS elicits undesirable effects on fuel quality, as other chemical components are reduced [3].
The microbiological process, biodesulfurization (BDS), could be a potential complementary technology to the physicochemical process, removing HDS recalcitrant molecules [4]. Lower capital and operating costs, ability to produce substantially less greenhouse gasses, and high valuable by-products are the potential benefits of BDS. Moreover, BDS takes advantage of the specificity of enzymes, especially for DBT and substituted DBTs. Therefore, BDS offers an alternative way to obtain “zero sulfur” products [2]. Recent discoveries related to biodesulfurization mechanisms may lead to commercial applications of biodesulfurization through engineering recombinant strains for overexpression of biodesulfurization genes, removal of end-product repression, and/or by combining relevant industrial and environmental traits with improvements in bioprocess design [5]. DBT and its derivatives are often used in fossil fuel biodesulfurization as a model compound. Gordonia alkanivorans strain 1B was one of the previously reported good DBT desulfurizing bacteria [6].
However, there are several problems limiting an industrial application of fossil fuel BDS, and one of them is the cost of culture media used to grow the microorganisms involved in the process. In this context, the utilization of alternative carbon sources resulting from agro-industrial by-products could be a strategy to reduce the investment in the operating expenses of a future industrial application. Recently, G. alkanivorans strain 1B was described as a fructophilic bacterium [7], and this characteristic opens a new interest in alternative carbon sources rich in fructose. The utilization of alternative carbon sources that can efficiently be used for the formulation of the culture medium for biodesulfurization studies was already described [4, 8, 9]. Moreover, G. alkanivorans could be a good choice for a future industrial application since it is a better desulfurizing bacterium compared with Rhodococcus erythropolis, as it can desulfurize both BT and DBT and also exhibits higher desulfurization activity [10]. The importance of the emerging genus Gordonia in industrial and environmental biotechnology is evidenced by the recent increase in associated publications, gene sequence depositions, and patent applications [11].
Sugar beet molasses (SBM) is a very abundant industrial by-product rich in sucrose (∼50 %, w/v) which can be hydrolyzed to glucose and fructose with a low price of commercialization ($100–$150 per ton). Several biotechnological applications related with fossil fuel field using SBM were reported such as for biohydrogen and biogas production [12], ethanol production [13], lactic acid production [14], and 2,3-butanediol production [15]. The utilization of this low-cost carbon source can contribute to decrease the fossil fuel BDS cost using G. alkanivorans strain 1B.
The goal of this study was to evaluate the potential of SBM as a cheaper carbon source in the dibenzothiophene desulfurization process using G. alkanivorans strain 1B. As far as we know, this is the first report describing the SBM utilization in fossil fuel BDS studies.
Materials and Methods
Chemicals and SBM
DBT (99 %) was obtained from Acros Organics, 2-hydroxybiphenyl (2-HBP) was from Sigma, and dimethylformamide (DMF) was from Riedel de Haën. BaCl2·2H2O (>99 %) was obtained from Merck. 4-Methyldibenzothiophene (4-mDBT) (96 %) was from Aldrich Chem. Co. All other reagents were of the highest grade commercially available. The industrial sugar beet molasses was kindly supplied by Tate & Lyle Açúcares Portugal S.A, located at Santa Iria de Azoia, Lisboa, and its initial sugar concentration was about 500 g l−1 of sucrose.
Microorganism and Culture Media
The microorganism used in this study was the bacterium G. alkanivorans strain 1B, isolated in our laboratory [6]. The basal salt medium used for the cultivation/maintenance of this microorganism and further on for the desulfurization assays was a sulfur-free mineral culture medium (SFM) containing 1.22 g l−1 NH4Cl, 2.5 g l−1 KH2PO4, 2.5 g l−1 Na2HPO4·2H2O, and 0.17 g l−1 MgCl2·6H2O. This medium was supplemented with 0.5 ml l−1 of a sulfur-free trace element solution [7], and its final pH was adjusted to 7.5, before autoclaved at 121 °C, 1 atm for 15 min. Filter sterile samples of SBM (untreated and treated with different conditions as described below) were added and diluted to the culture medium as the only carbon source, to an initial concentration of 10–18 g l−1 total sugars. Bacterial cultures were performed in shake flasks with 150 ml of culture medium at 30 ºC and 150 rpm of agitation. For all desulfurization tests performed in triplicates, 250 μM of DBT, dissolved in DMF, was added into the sterilized medium.
SBM Sulfate Precipitation
The sulfate precipitation was performed with the commonly used method of barium chloride (BaCl2). Five samples of SBM were treated with a range of BaCl2 concentrations (0, 0.25, 0.5, 1.0, and 1.5 %, w/v) for sulfate precipitation during overnight at 30 ºC, and then they were centrifuged to remove the pellet that contains the precipitated sulfates.
SBM Acidic Hydrolysis
SBM treated with BaCl2 was diluted (dilution 1/20 (v/v) in water) and submitted to an acidic hydrolysis to convert sucrose into fructose and glucose. The molasses’ pH was lowered to 2 using HCl 4.0 M, and it was left for 48 h at 55 °C to allow complete hydrolysis. After, this hydrolysed SBM is adjusted to pH 7.5 and filter sterilized (0.22-μm cellulose acetate membranes) to further BDS assays (described above).
SSF Assay
Simultaneous saccharification and fermentation (SSF) assay was performed using a crude enzymatic extract containing inulinase/invertase activity produced by the yeast Zygosaccharomyces bailii strain Talf1 as previously described [9]. This enzymatic extract was previously dialyzed using a dialysis membrane with a cutoff of 10 kDa overnight to remove sulfur sources that can inhibit the desulfurization process and further added, 1 % (v/v), to BDS assays (above described). The initial invertase activity in the dialyzed enzyme extract used was about 90 U ml−1. One unit of invertase activity (U) was defined as the amount of enzyme responsible for the production of 1 μmol of reducing sugar per minute.
Analytical Methods
The cell growth was monitored by measuring the optical density (OD) of the culture broth samples at 600 nm (Thermo Electron Corporation Spectrophotometer, model Genesys 6, Madison, USA). Dry cell weight (DCW) was determined using 0.22-μm cellulose acetate membranes after 18 h at 100 ºC. Sugar concentrations (glucose, fructose, and sucrose) were measured by high-performance liquid chromatography as described by Alves et al. [4]. The DBT desulfurization was evaluated measuring the 2-HBP production (the final product of DBT desulfurization). The 2-HBP was extracted from the culture broth, previously acidified with HCl, with ethyl acetate during a 5-min vortex. After the phase separation occurs, the organic phase was analyzed by gas chromatography (GC) in a gas chromatograph (Model CP9001, Chrompack, Middelburg, The Netherlands) equipped with a flame ionization detector. An Alltech 10 % SE-30 on 80/100 Chromosorb W-HP column was used with nitrogen as the carrier gas. The chromatograph oven start temperature was of 190 ºC for 2 min and the end temperature 225 ºC maintained for 1 min (heating rate of 6 ºC min−1). The injector and detector temperatures were set for 250 and 290 ºC, respectively. In all GC measurements, 4-mDBT was used as an internal standard to minimize variations.
Results and Discussion
SBM as Carbon Source
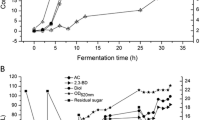
Sugar beet molasses was used as alternative carbon source to grow G. alkanivorans strain 1B in the DBT desulfurization process. In a first set of assays, untreated SBM was added to the bacterial culture medium to an initial concentration of 18 g l−1 of total sugars to ensure total DBT desulfurization. The time-course profiles for growth and desulfurization characteristics obtained with G. alkanivorans strain 1B for this untreated SBM are presented in Fig. 1. The growth occurs during 3 days with a μ max of 0.067 h−1 and with a biomass yield of 0.471 g g−1 sugar. Although the high bacterial growth, there is no DBT desulfurization, suggesting that the untreated SBM used have enough quantity of sulfur sources to allow complete bacterial growth and to inhibit desulfurization. In addition, the high μ max obtained can also be explained by the presence of more easily assimilated sulfur sources, which promotes faster bacterial growth than DBT. A previous study with strain 1B growing in commercial sucrose and DBT as the only sulfur source showed a μ max of 0.038 h−1 [7].
Time-course profile displaying the growth, sucrose consumption, and the desulfurization characteristics of G. alkanivorans strain 1B in batch cultures with 250 μM DBT and diluted SBM (18 g l−1 total sugars). All results were obtained in triplicate, and standard deviation is indicated by the error bars
Effect of BaCl2 Concentration on Sulfate Removal from SBM
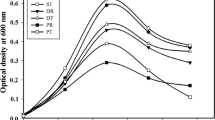
The presence of small amounts of sulfates inhibits the DBT desulfurization by the desulfurizing microorganisms [8, 16, 17]. The strategy is to remove these sulfates from SBM before its utilization in the desulfurization process as have already been performed with success for other alternative carbon source [8]. So, to obtain a low sulfate concentration, SBM was treated with four barium chloride concentrations ranging from 0.25 to 1.5 % (w/v). The four treated SBM samples and the untreated SBM were diluted into the culture medium (10 g l−1) and used as the only carbon source to promote the bacterial growth and DBT desulfurization by strain 1B, during 5 days (Fig. 2). The maximum desulfurization was obtained in the SBM samples treated both with 0.25 and 0.5 % BaCl2, where the 2-HBP produced attained about 200 μM in comparison with the insignificant level of 2-HBP produced using the untreated SBM (18 μM). In these set of assays, the prior dilution of the untreated SBM to an initial concentration of 10 g l−1 of total sugars have also diluted the amount of the existent sulfates to a final concentration that did not inhibit completely the DBT desulfurization and thus a low desulfurization was observed (9 % of that obtained in the best conditions). For the SBM treated with 1 and 1.5 % of BaCl2, a slight decrease in the desulfurization ability by strain 1B was observed, reducing the production of 2-HBP from 200 to 187 and 186 μM, respectively, probably due to BaCl2 toxicity. In a previous work, it was shown that 0.5 % BaCl2 was the best concentration to remove sulfates from carob pulp liquor [8]. But in the case of SBM, these results highlight that the treatment with 0.25 % BaCl2 overnight (16–18 h) was the adequate for sulfate removal from molasses, viewing the maximal 2-HBP production within further BDS assays.
Biodesulfurization Using SBM Treated with BaCl2
Further BDS assays were carried out using SBM treated with 0.25 % BaCl2 (SBMt). The treatment used does not change significantly the total amount of sucrose present in SBM, so the SBMt was added to the bacterial culture medium in a dilution 1:50 to an initial concentration of about 12 ± 2 g l−1 sucrose. Three different set of assays were carried out using this SBMt as carbon source: (i) DBT desulfurization using directly SBMt, (ii) DBT desulfurization using SBMt after acidic hydrolysis (SBMt–AH), and (iii) DBT desulfurization using SBMt in a SSF approach with Z. bailii strain Talf1 crude enzymatic extract exhibiting invertase activity.
Figure 3 shows the time-course profile displaying the bacterial growth and desulfurization characteristics of G. alkanivorans strain 1B in batch cultures using SBMt as carbon source and 250 μM of DBT as sulfur source. These results show that strain 1B started the 2-HBP production after 24 h of growth with the maximum 2-HBP production at 96 h (122 μM). The efficiency of BaCl2 treatment was once again confirmed since the significant production of 2-HBP only was possible because the initial sulfate concentration in SBMt was very low. In these conditions, the bacterial culture presented a μ max of 0.04 h−1 with a biomass yield of 0.377 g g−1 sugar. The decrease of μ max and biomass yield of strain 1B with the utilization of SBMt in comparison with those untreated SBM (Fig. 1) can be explained by the different sulfur source used, respectively, DBT and sulfates. Sulfates are a more easily assimilated sulfur source in comparison with DBT, which support a faster growth of strain 1B. In this process, strain 1B presented a maximum 2-HBP production rate of 2.56 μM h−1 and a maximum 2-HBP specific production rate (q 2-HBP) of 2.20 μmol g−1 DCW h−1. In a previous study with the same bacterial strain growing on commercial sucrose, the values of maximum 2-HBP production rate and q 2-HBP reported were 1.91 μM h−1 and 0.718 μmol g−1 DCW h−1, respectively [7]. The higher desulfurization ability of strain 1B when growing in SBMt was probably due to the presence of some nutrients in this complex alternative carbon source which can promote a faster bacterial metabolism.
Time-course profile displaying the growth, sucrose consumption, and the desulfurization characteristics of G. alkanivorans strain 1B in batch cultures with 250 μM DBT and diluted SBM treated with 0.25 % w/v BaCl2 (10 g l−1 total sugars). All results were obtained in triplicate, and standard deviation is indicated by the error bars
Knowing from previous works that G. alkanivorans strain 1B is a desulfurizing bacterium able to use several carbon sources, such as glucose and sucrose [6], but that prefers fructose due to its fructophilic behavior [7], with which achieves an enhanced DBT desulfurization (9.29 μM h−1); further BDS assays were carried out based on hydrolyzed SBMt. A set of assays were carried out using SBMt which was acidified to hydrolyze sucrose into glucose and fructose (SBMt−AH) and then used as carbon source for DBT desulfurization by strain 1B. Figure 4 shows the time course of bacterial growth, 2-HBP production, and sugar consumption during the 3 days of culture with SBMt−AH. The strain 1B presented a μ max of 0.0575 h−1, almost 44 % higher than in the growth with SBMt not hydrolyzed. The 2-HBP was detected after 19 h of culture with the maximum production of 168 μM after 66 h. The maximum 2-HBP production rate and q 2-HBP obtained in these conditions were 5.76 μM h−1 and 2.61 μmol g−1 DCW h−1, respectively. As expected, these 2-HBP production rates increased significantly in comparison with the rates obtained for SBMt directly. Alves and Paixão [7] have showed that G. alkanivorans strain 1B growing in a mixture of glucose-fructose as carbon sources presented a q 2-HBP of 1.9 μmol g−1 DCW h−1 which is 27 % lower than the q 2-HBP obtained for SBMt−AH. This enhancement of DBT desulfurization using the SBMt−AH can indicate, once again, the presence of inducers in this complex carbon source. Moreover, an increase of biomass yield was also observed from 0.377 to 0.488 g g−1 sugar, a value very similar to that of the untreated SBM. Figure 4 shows strain 1B utilized glucose and fructose simultaneously but with a faster consumption of fructose, as expected due to its fructophilic behavior [7]. The maximum fructose consumption rate was 0.167 g l−1 h−1, and the maximum glucose consumption rate changed from 0.078 g l−1 h−1 (during the high availability of fructose) to 0.134 g l−1 h−1 (after the low availability of fructose).
Time-course profile displaying the growth, sugars consumption, and the desulfurization characteristics of G. alkanivorans strain 1B in batch cultures with 250 μM DBT and diluted SBM treated with 0.25 % w/v BaCl2 and hydrolyzed (12 g l−1 total sugars). All results were obtained in triplicate, and standard deviation is indicated by the error bars
Thus, the acidic hydrolysis of SBMt allowed the achievement of an enhanced DBT desulfurization by strain 1B using an alternative carbon source cheaper than commercial sucrose. However, in order to turn the process more eco-friendly and also decrease the costs associated with the sucrose hydrolysis of SBMt, the utilization of invertases in a simultaneous saccharification and fermentation approach was also studied. The application of invertases to DBT desulfurization using SBMt was based on the promising results recently obtained by Paixão et al. [9], when they applied inulinases to a BDS using inulin/Jerusalem artichoke juice as carbon source. So, another set of DBT desulfurization assays were performed using SBMt and a crude enzymatic extract containing inulinase/invertase activity produced by the yeast Z. bailii strain Talf1 (1 % v/v) in SSF approach. The results obtained are presented in Fig. 5, where the time course of bacterial growth, 2-HBP production, sucrose hydrolysis, and glucose and fructose consumption during 55 h of strain 1B culture with SBMt can be observed. The SSF process allowed a faster growth of strain 1B with a μ max of 0.08 h−1 and a higher biomass yield of 0.72 g g−1 sugar. The amount of invertase crude extract (1 % v/v) was adequate since all the sucrose present in culture medium was hydrolyzed after 30 h. In addition, after 7 h, about 85 % of sucrose was converted to glucose and fructose, allowing that no fructose/glucose limitation occurred in the beginning of the strain 1B growth. Once again, glucose and fructose are assimilated simultaneously but the fructose was consumed faster than the glucose. The presence of some nutrients/inducers in the enzymatic extract can also explain the increase of desulfurization ability of G. alkanivorans strain 1B.
Time-course profile displaying the growth, sugars production/consumption, and the desulfurization characteristics during a SSF approach with G. alkanivorans strain 1B and 1 % invertase crude extract (v/v) in batch cultures with 250 μM DBT and diluted SBM treated with 0.25 % w/v BaCl2 (13 g l−1 total sugars). All results were obtained in triplicate, and standard deviation is indicated by the error bars
The 2-HBP was detected after 7 h of culture, being the maximal quantity produced of 249.5 μM at 47.5 h of culture, which corresponds to the total desulfurization of DBT present in the culture medium (250 μM). The maximum 2-HBP production rate achieved was 7.78 μM h−1, and the q 2-HBP was 3.12 μmol g−1 DCW h−1. Comparing this q 2-HBP value to others obtained with the same strain grown in pure commercial glucose [6] and fructose [7], an enhancement of about 284 and 47 % can be stated, respectively. In literature, other Gordonia sp. strains have been described for BDS, showing a lower or similar 2-HBP production rate [18–20] but also exhibiting higher 2-HBP production rates, especially after process optimization using resting cells [21–23].
Moreover, previous works using G. alkanivorans strain 1B grown in cheaper alternative carbon sources derived from agro-industrial materials reported lower values of desulfurization, namely: the achievement of a q 2-HBP of 1.1 μmol g−1 DCW h−1 in a DBT desulfurization process using recycled paper sludge hydrolyzate as carbon source [4], which is about 3fold lower than that observed with SBMt within the SSF, and the achievement of a maximum 2-HBP production rate of 4.8 μM h−1 in a BDS using Jerusalem artichoke juice as carbon source [9], which is about 38 % lower than that obtained with SBMt within the SSF. These results highlight the potential of SBMt as a cheaper carbon source to grow G. alkanivorans strain 1B in a future process optimization with resting cells viewing its upscale application.
Conclusions
This work showed that sugar beet molasses, a renewable substrate since it is an agro-industrial by-product, is a good alternative carbon source that can contribute to the decrease of culture media costs necessary to obtain biocatalysts to be applied in a biodesulfurization process. In comparison with the pure sucrose refined from sugar cane price (about $500–$600 per ton), the use of SBM as carbon source implies a cost reduction of 3fold. Moreover, SBM utilization in an SSF process, with invertase crude extract, contributes to an improved DBT desulfurization due to possible nutrients/inducers from the molasses or from the enzymatic extract. Therefore, our results showed the high potential of SBM to be used in a future industrial fossil fuel BDS process using G. alkanivorans strain 1B.
References
Borgne, L. S., & Quintero, R. (2003). Fuel Processing Technology, 81, 155–169.
Srivastava, V. C. (2012). Royal Society of Chemistry Advances, 2, 759–783.
Folsom, B. R., Schieche, D. R., DiGrazia, P. M., Werner, J., & Palmer, S. (1999). Applied and Environmental Microbiology, 65, 4967–4972.
Alves, L., Marques, S., Matos, J., Tenreiro, R., & Gírio, F. M. (2008). Chemosphere, 70, 967–973.
Mužic, M., & Sertić-Bionda, K. (2013). Chemical and Biochemical Engineering Quarterly, 11, 101–108.
Alves, L., Salgueiro, R., Rodrigues, C., Mesquita, E., Matos, J., & Gírio, F. M. (2005). Applied Biochemistry and Biotechnology, 120, 199–208.
Alves, L., & Paixão, S. M. (2014). New Biotechnology, 31, 73–79.
Silva, T. P., Paixão, S. M., Teixeira, A. V., Roseiro, J. C., & Alves, L. (2013). Journal of Chemical Technology and Biotechnology, 88, 919–923.
Paixão, S. M., Teixeira, P. D., Silva, T. P., Teixeira, A. V., & Alves, L. (2013). New Biotechnology, 30, 598–606.
Aggarwal, S., Karimi, I. A., & Ivan, G. R. (2013). Molecular BioSystems, 9, 2530–2540.
Drzyzga, O. (2012). Critical Reviews in Microbiology, 38, 300–316.
Wu, X., Lin, H., & Zhu, J. (2013). Bioresource Technology, 136, 351–359.
Kasavi, C., Finore, I., Lama, L., Nicolaus, B., Oliver, S. G., Oner, E. T., et al. (2012). Biomass and Bioenergy, 45, 230–238.
Taskin, M., Esim, N., & Ortucu, S. (2012). Food and Bioproducts Processing, 90, 773–779.
Yang, T., Rao, Z., Zhang, X., Xu, M., Xu, Z., & Yang, S. (2013). Applied Microbiology and Biotechnology, 97, 7651–7658.
Mohebali, G., Ball, A. S., Kaytash, A., & Rasek, B. (2008). Microbiology, 154, 878–885.
Kim, Y. J., Chang, J. H., Cho, K.-S., Ryu, H. W., & Chang, Y. K. (2004). Korean Journal of Chemical Engineering, 21, 436–441.
Aminsefat, A., Rasekh, B., & Ardakani, M. R. (2012). Microbiology, 81, 154–159.
Chang, J. H., Chang, Y. K., Cho, K.-S., & Chang, H. N. (2000). Biotechnology Letters, 22, 193–196.
Rhee, S., Chang, J. H., Chang, Y. K., & Chang, H. N. (1998). Applied and Environmental Microbiology, 64, 2327–2331.
Peng, Y., & Wen, J. (2010). Chemical and Biochemical Engineering Quarterly, 24, 85–94.
Mohebali, G., Ball, A. S., Rasekh, B., & Kaytash, A. (2007). Enzyme and Microbial Technology, 40, 578–584.
Jia, X., Wen, J. P., Sun, Z. P., Caiyin, Q. G., & Xie, S. P. (2006). Chemical Engineering Science, 61, 1987–2000.
Acknowledgments
The present work was financed by FEDER funds through POFC-COMPETE and by national funds through FCT (Fundação para a Ciência e a Tecnologia) in the scope of project Carbon4Desulf—FCOMP-01-0124-FEDER-013932 (Ex—PTDC/AAC-AMB/112841/2009).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Alves, L., Paixão, S.M. Enhancement of Dibenzothiophene Desulfurization by Gordonia alkanivorans Strain 1B Using Sugar Beet Molasses as Alternative Carbon Source. Appl Biochem Biotechnol 172, 3297–3305 (2014). https://doi.org/10.1007/s12010-014-0763-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0763-z