Abstract
2,3-Butanediol has been known as a platform green chemical, and the production cost is the key problem for its large-scale production in which the carbon source occupies a major part. Sugarcane molasses is a by-product of sugar industry and considered as a cheap carbon source for biorefinery. In this paper, the fermentation of 2,3-butanediol with sugarcane molasses was studied by reducing the medium ingredients and operation steps. The fermentation medium was optimized by response surface methodology, and 2,3-butanediol production was explored under the deficiency of sterilization, molasses acidification, and organic nitrogen source. Based on these experiments, the fermentation medium with sugarcane molasses as carbon source was simplified to five ingredients, and the steps of molasses acidification and medium sterilization were reduced; thus, the cost was reduced and the production of 2,3-butanediol was enhanced. Under fed-batch fermentation, 99.5 g/L of 2,3-butanediol and acetoin was obtained at 60 h with a yield of 0.39 g/g sugar.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2,3-Butanediol (2,3-BD) has been considered as a platform green chemical due to its potential application in the production of polymers, solvent, food additive, printing inks, perfumes, moistening and softening agents, and plasticizers [1–4], whereas the high cost of 2,3-BD production limits its wide application as chemicals and fuels. New strains [5–9] and fermentation strategies [10–12] were developed to increase the concentration and yield of 2,3-BD which can partially reduce the production cost, but the carbon source still occupies a major part. Therefore, it is essential to utilize cheap carbon sources to produce 2,3-BD. The reported carbon sources include biodiesel-derived raw glycerol [13], lignocellulose hydrolysates [14–17], Jerusalem artichoke tuber and stalk [12, 18, 19], industrial waste gas [20], and molasses [6, 21]. Among them, lignocellulose is the cheapest, but sugar concentration was relatively low and the by-products produced during the acid hydrolysis, such as acetic acid, furfural, and 5-hydroxymethylfurfural, would inhibit the bacterial growth. Although the sugar concentration in hydrolysate can be increased by concentration, the concentrations of inhibitors were also increased which have to be removed by detoxification and sugar loss occurred [17]. Till now, the concentration of 2,3-BD obtained from lignocellulose was still relatively low (<40 g/L) which would greatly increase the separation cost, whereas the concentration of 2,3-BD was relatively high with molasses as carbon source. These studies showed that molasses was a suitable carbon source for 2,3-BD production at present.
Sugarcane molasses is a by-product prepared from the liquid waste of sugar production which contains about 50 % total sugar (sucrose, glucose, and fructose), 10 % inorganic salts, such as K+, Na+, Ca2+, Mg2+, Mn2+, Fe2+, Zn2+, Cl−, SO4 2−, etc., 10 % ash, proteins, vitamins, organic acids, and crude fat [22, 23]. As a relatively cheap and abundant raw material, sugarcane molasses was studied to produce a variety of products, including ethanol [22], sorbitol [24], microalgal [25], hydrogen [26], succinic acid [23, 27], lactic acid [28, 29], citric acid [30], acetoin [31], etc. In the study of 2,3-BD, beet molasses [32], corncob molasses [21], and sugarcane molasses [6] were tried to produce 2,3-BD. Wang et al. used a high 2,3-BD producer Klebsiella pneumoniae SDM to produce 2,3-BD with corncob molasses as carbon source, and 78.9 g/L 2,3-BD was obtained at a yield of 81.4 % after 61-h fed-batch fermentation [9, 21]. Jung et al. deleted a sucrose regulator (ScrR) to enhance the production of 2,3-BD, and a high concentration of 98.69 g/L 2,3-BD was obtained in a fed-batch fermentation with sugarcane molasses as carbon source [6]. These studies successfully increased the concentration of 2,3-BD or produced 2,3-BD with a cheap carbon source, but none of them was considered to further reduce the fermentation cost by reducing medium ingredients and operation steps based on the composition and characteristics of carbon source.
The composition of fermentation medium was very complex, which includes many types of inorganic salts such as phosphate source (KH2PO4, Na2HPO4), metal ions for enzyme activity (Mg2+, Zn2+, Mn2+), KCl, citric acid, etc. except carbon source and nitrogen source [6, 9, 33]. On the other hand, some of these inorganic salts such as K+, Na+, Ca2+, Mg2+, Mn2+, Fe2+, Zn2+, Cl−, SO4 2−, etc. have already existed in sugarcane molasses, so part of the inorganic salts that added into the medium can be replaced by these existed salts. Thus, the ingredients which were added into fermentation medium could be reduced. In addition, sugarcane molasses was generally pretreated [22, 31] and the fermentation medium was generally autoclaved which consumed a large amount of energy. If these operation steps were reduced, a large amount of energy could be saved. Based on this idea, in this work, 2,3-BD fermentation from sugarcane molasses was studied by reducing the ingredients of fermentation medium and operation steps to enhance the production. The results showed that sugarcane molasses-based fermentation medium can be simplified to five ingredients and used without sterilization.
Material and Methods
Microorganism and Medium
The bacterium used to produce 2,3-BD was a mutated strain of Enterobacter cloacae (CGMCC 6053). The wild type was isolated from soil sample and treated by diethyl sulfate and dielectric barrier discharge plasma, and the mutated strain with highest production of 2,3-BD was used in this study. Stock cultures were maintained in Luria–Bertani (LB) medium containing 20 % glycerin at −70 °C.
The medium for seed culture contained glucose 80 g/L, (NH4)2HPO4 6.0 g/L, KCl 1.8 g/L, EDTA 0.51 g/L, MgSO4 0.6 g/L, FeSO4 · 7H2O 0.0225 g/L, ZnSO4 · 7H2O 0.0075 g/L, MnSO4 · 7H2O 0.0038 g/L, citric acid 0.21 g/L, and sodium citrate 0.294 g/L.
Sugarcane molasses was produced in Liuzhou, China, which contained 49.5 % sugar (25.7 % sucrose, 9.8 % glucose, and 14.0 % fructose). In the experiments of medium optimization, the sugarcane molasses was pretreated by acid according to the published method [31], while used directly without pretreatment in the batch and fed-batch fermentation experiments. All the media containing sugarcane molasses were freshly prepared and used for fermentation without sterilization.
Medium Optimization
The fermentation medium was optimized using response surface methodology (RSM) with the total concentration of diol (2,3-BD and acetoin) as experimental response. All the experiments were carried out in triplicate by shaking flask cultivation at 37 °C and 200 rpm with an initial pH of 7.0.
First, Plackett–Burman (PB) design was used to pick the key medium components influencing diol production. A 12-run PB design was used to screen eight factors, and the experimental responses were analyzed by the method of least squares to fit the following first-order model:
where Y was the predicted response, a i was the regression coefficient, and X i was the coded level of the variable.
Then, a series of experiments were carried out in the direction of the steepest ascent based on the first-order model obtained from PB design.
Finally, a three-level three-factor Box–Behnken design (BBD) was performed to optimize the medium composition. In order to predict the optimal point, a second-order polynomial was fitted to correlate the relationship between independent variables and response:
where Y was the predicted response, β 0 was the offset term, β i was the linear coefficient, β ii was the quadratic coefficient, β ij was the cross coefficient, and X i and X j represented independent variables. The fit of the quadratic polynomial model was evaluated by the determination coefficient (R 2) and analysis of variance (ANOVA).
Production of 2,3-BD by Batch and Fed-Batch Fermentation
The batch and fed-batch experiments were carried out in a 7-L stirring bioreactor (LiFlus GX, Korea) with a working volume of 2 L. The bioreactor equipment (such as vessel or tubing parts) was not sterilized prior to inoculation. The seed culture prepared previously was inoculated (10 %, v/v) into the optimized fermentation medium with an initial pH of 7.0. During the fermentation process, the pH value was decreased automatically to 5.8 and then maintained invariably by automatic addition of 5 mol/L NaOH. The cultivation was stirred at 300 rpm and 37 °C with an aeration rate of 0.1 vvm.
Analytical Method
The concentrations of sucrose, glucose, and fructose were analyzed by HPLC (Waters 600) equipped with a Hypersil APS-2 column (5 mm × 200 mm × 5 μm) and a refractive index detector (Waters 2414). The fermentation products of 2,3-BD and acetoin were determined by GC (Agilent Technologies 7890A) equipped with a column of AT OV-1701 (30 m × 0.32 mm × 0.25 μm) and a FID detector. The density of bacteria was measured at 620 nm with the fermentation medium as blank.
Results and Discussion
PB Design for Selection of Key Medium Components
The effects of medium supplements on 2,3-BD production have been well reviewed by Garg and Jain [34]. Mg2+ is essential for the key enzyme α-acetolactate synthase [35] but inhibits the activity of meso-2,3-butanediol dehydrogenase [36], and trace concentration of metal ions Fe2+ and Mn2+ greatly stimulates the production of 2,3-BD [34]. Ammonium sulfate was a general inorganic nitrogen source in fermentation, whereas urea has been proved to be a more effective and cheap nitrogen source in 2,3-BD production [33]. Therefore, these three metal ions and phosphate plus acidified molasses (a-molasses), urea, citrate, and a cheap organic nitrogen source—corn steep liquor powder (CSLP)—were selected as variables (Table 1), and the experimental design and responses are listed in Table 2.
The statistical analysis showed that factors having greatest impacts on the production of 2,3-BD were X 1 (a-molasses; p < 0.0001), X 2 (urea; p = 0.001), and X 4 (K2HPO4; p = 0.021). The positive factors (X 1, X 6, and X 5) were set at their high levels, while the negative factors (X 2, X 4, X 8, X 3, and X 7) were set at their low levels which meant that the ions Fe2+ and Mn2+ were not needed to add into the medium. This result does not mean that the existence of these two salts can reduce the concentration of diol. In fact, Fe2+ and Mn2+ were essential for 2,3-BD production [9, 34], but in this work, the salts in sugarcane molasses were enough for fermentation. Thus, the composition of fermentation medium was reduced to six ingredients.
To approach the neighborhood of the optimum response, the fitted first-order model equation for the production of diol was obtained from the PB design experiments:
The coefficient of each variable in Eq. ( 3 ) demonstrates the strength of the effect of this variable on diol production. The quality of fit of the polynomial model equation was expressed by the coefficient of determination (R 2). The value 0.9982 for R 2 indicates that 99.82 % of the variability in the response could be explained by the model. The high value of the adjusted determination coefficient (Adj R 2 = 0.9935) advocates for a high significance of the model. The closer the R 2 value is to 1.00, the stronger the model is and the better it predicts the response. These results indicated that the response equation provided a suitable model for the PB design experiment.
The Path of Steepest Ascent Experiments
According to Eq. ( 3 ), the steepest ascent direction was proportional to (6.00, −1.84, −0.682), approximately equivalent to (1:−0.3:−0.1), meaning that if the concentration of a-molasses increased one unit (10 g/L), the concentration of urea would decrease 0.3 unit (0.6 g/L) and K2HPO4 0.1 unit (0.2 g/L). The design and results of the steepest ascent experiment are shown in Table 3. The concentration of target products was increased along the path and then decreased sharply after the third step. Highest production (41.7 g/L) was obtained from the combination of a-molasses (115 g/L), urea (8.8 g/L), and K2HPO4 (0.7 g/L). This combination would be used as the center point for BBD experiments.
Medium Optimization with BBD Experiments
Based on the results of PB design and steepest ascent experiments, Box–Behnken design (BBD) with three coded levels was adopted to optimize the fermentation medium and the responses of diol production are shown in Table 4.
Data obtained from BBD experiments was analyzed by linear multiple regression using software Minitab 15. The corresponding second-order response model for Eq. ( 4 ) which was founded after analysis for the regression was as follows:
The regression analysis of the data showed coefficient of determination (R 2) value of 0.9822, and adjusted R 2 value was 0.9502, which showed a high correlation between observed values and predicted values. Among the terms of the model, the linear and quadratic coefficients of molasses (X 1) (p < 0.01) are more significant than the other factors (see supporting materials, Table S1). Therefore, the concentration of molasses has a great influence on the diol production, which means that a little variation of its concentration will alter diol production. The concentrations of KH2PO4 and urea are almost significant in the quadratic level (p < 0.05). The interaction between the variables has no significant influence on diol production.
Based on these experiments, the optimized medium for diol production with sugarcane molasses as carbon source was as follows: a-molasses 118 g/L, urea 8.7 g/L, CSLP 2 g/L, K2HPO4 0.77 g/L, sodium citrate 0.3 g/L, and MgSO4 0.9 g/L.
Effects of Culture Conditions on Diol Production
Generally, the fermentation medium was sterilized before cultivation to avoid contamination of microbes. Sugarcane molasses is stored at a high sugar concentration of 45 ~ 50 % which can inhibit the growth of bacteria. In 2,3-BD fermentation, the target strain grew quickly, so it is possible to perform the fermentation via a nonsterile process with freshly prepared medium of sugarcane molasses. Before the medium preparation, sugarcane molasses was usually pretreated by acidification because the direct use of crude material might reduce the production of target product [6, 31]. Moreover, it was observed that large amount of foam was formed during the fermentation in a stirred bioreactor using the optimized medium but could be reduced without the addition of CSLP. On the other hand, the results of PB design showed that CSLP had minor impact on diol production and the effect was negative (Table S1), which meant that the less amount of CSLP added, the better the diol production. Based on these above analyses, we compared the production of diol under different conditions by shaking flask cultivation. As shown in Table 5, there is little difference on diol production using sterile and nonsterile medium, and the acidification of sugarcane molasses did not show obvious increment on the production of diol. When the strain was cultured in a medium without CSLP under a nonsterile condition, there is no apparent decrease on the diol production. As a result, the fermentation medium was simplified to five ingredients and prepared with molasses without acidification. To control the contamination of the nonsterile process, the fermentation medium must be freshly prepared. Otherwise, the cell growth would be affected and the diol production would reduce.
Production of 2,3-BD via a Nonsterile Process
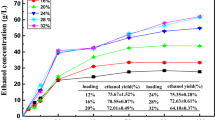
The time courses of batch and fed-batch fermentation are shown in Fig. 1. During batch fermentation (Fig. 1a), biomass increased quickly in the first 10 h and kept constant in the following 24 h. The concentration of 2,3-BD reached 38.4 g/L at 12 h, and the diol concentration was 45.6 g/L. By feeding molasses, the concentration of 2,3-BD increased to 90.8 g/L at 60 h, and the diol concentration was 99.5 g/L with a yield of 0.39 g diol/g sugar and productivity of 1.66 g/(L h). When using glucose-based medium, the 2,3-BD concentration of 110.9 g/L and diol of 119.7 g/L were obtained at 56 h with a yield of 0.42 g diol/g glucose (Fig. 2). Although the concentration and yield of 2,3-BD were a little lower with sugarcane molasses as carbon source, the cost of carbon source was reduced. According to the above results, about 2.38 t glucose was consumed to produce 1 t diol, while 5.2 t sugarcane molasses for 1 t diol was required. At present, the price was about ¥2500/T of glucose and ¥1000/T of sugarcane molasses (www. 1688.com) in China; thus, the cost of ¥800 was saved from carbon source.
The nonsterile fermentation has been reported before by Chatzifragkou et al. in 2011 who tried 1,3-propanediol production with biodiesel-derived crude glycerol as carbon source and confirmed that only one microbial community existed inside the chemostat [37]. The successful work of 1,3-propanediol interested us, and we tried it in 2,3-BD fermentation. The results of batch and fed-batch fermentation showed that the microbe can grow well in the simplified medium without sterilization and a high concentration of diol was obtained, which meant that the nonsterile fermentation was feasible for 2,3-BD production.
The concentration of diol in fermentation broths and simple calculation of carbon source cost above showed that sugarcane molasses is a good alternative for glucose, but the utilization of sugarcane molasses introduced more impurities into the fermentation broth. The most obvious phenomenon is the dark-brown color of fermentation broth which came from sugarcane molasses. Meanwhile, the reduced step of molasses acidification could make the solid–liquid separation more difficult because one of the purposes of molasses acidification was to remove the solid impurities. Finally, viscosity, coloring matters, and impurities in the fermentation broth from sugarcane molasses were greatly increased compared with those in glucose-based fermentation broth. As a result, the downstream processing became more complex. In fact, this problem has been encountered before when we used Jerusalem artichoke stalk and tuber as carbon source to produce 2,3-BD [12, 38]. The viscosity, coloring matters, and impurities of the Jerusalem artichoke-based broth were increased, and the solid–liquid separation was achieved by the combination of centrifugation and salting-out extraction, while only salting-out extraction was enough to realize this purpose for the glucose-based fermentation broth [38, 39]. With the wide utilization of biomass in the production of biochemicals, the viscosity, coloring matters, and impurities become the common problems and more efforts are required to be made on the downstream processing. As for this work, further studies of 2,3-BD separation from molasses-based fermentation broth are exploring now in our lab and the results will be reported later.
Conclusion
The purpose of this work was to enhance 2,3-BD production by reducing the cost of operation steps and raw materials for fermentation, so sugarcane molasses was selected as cheap carbon source.
Based on the optimization of fermentation medium and the study of CSLP addition and molasses acidification on diol production, the fermentation medium was simplified to five ingredients: sugarcane molasses, urea, K2HPO4, sodium citrate, and MgSO4. Using this simplified medium, 90.8 g/L 2,3-BD (99.5 g/L diol) was obtained at 60 h by fed-batch fermentation without sterilization of medium; thus, large energy consumption was saved. The cost of carbon source was reduced by using sugarcane molasses compared with glucose, but more impurities were introduced to the fermentation broth, and further study of downstream processing is needed.
References
Celinska, E., & Grajek, W. (2009). Biotechnology Advances, 27, 715–25.
Ji, X.-J., Huang, H., & Ouyang, P.-K. (2011). Biotechnology Advances, 29, 351–364.
Zeng, A.-P., & Sabra, W. (2011). Current Opinion in Biotechnology, 22, 749–757.
Sabra, W., Quitmann, H., Zeng, A.-P., Dai, J.-Y., & Xiu, Z.-L. (2011). Comprehensive biotechnology. In M.-Y. Murray (Ed.), Biofuels and bioenergy: microbial production of 2,3-butanediol (2nd ed., Vol. 3, pp. 87–97). Amsterdam: Elsevier.
Biswas, R., Yamaoka, M., Nakayama, H., Takashi, K., Yoshida, K., Bisaria, V. S., & Kondo, A. (2012). Applied Microbiology and Biotechnology, 94, 651–658.
Jung, M.-Y., Park, B.-S., Lee, J., & Oh, M.-K. (2013). Bioresource Technology, 139, 21–27.
Gaspar, P., Neves, A. R., Gasson, M. J., Shearman, C. A., & Santos, H. (2011). Applied and Environmental Biotechnology, 77, 6826–6835.
Ji, X. J., Nie, Z. K., Huang, H., Ren, L. J., Peng, C., & Ouyang, P. K. (2011). Applied Microbiology and Biotechnology, 89, 1119–1125.
Ma, C., Wang, A., Qin, J., Li, L., Ai, X., Jiang, T., Tang, H., & Xu, P. (2009). Applied Microbiology and Biotechnology, 82, 49–57.
Nie, Z. K., Ji, X. J., Huang, H., Du, J., Li, Z. Y., Qu, L., Zhang, Q., & Ouyang, P. K. (2011). Applied Biochemistry and Biotechnology, 163, 946–953.
Ji, X. J., Huang, H., Du, J., Zhu, J. G., Ren, L. J., Hu, N., & Li, S. (2009). Bioresource Technology, 100, 3410–4.
Li, D., Dai, J.-Y., & Xiu, Z.-L. (2010). Bioresource Technology, 101, 8342–8347.
Metsoviti, M., Paraskevaidi, K., Koutinas, A., Zeng, A.-P., & Papanikolaou, S. (2012). Process Biochemistry, 47, 1872–1882.
Jiang, L.-Q., Fang, Z., Guo, F., & Yang, L.-B. (2012). Bioresource Technology, 107, 405–410.
Zhao, X., Song, Y., & Liu, D. (2011). Enzyme Microb. Technology, 49, 413–419.
Jiang, L.-Q., Fang, Z., Li, X.-K., & Luo, J. (2013). AMB Express, 3, 48.
Cheng, K.-K., Liu, Q., Zhang, J.-A., Li, J.-P., Xu, J.-M., & Wang, G.-H. (2010). Process Biochemistry, 45, 613–616.
Gao, J., Xu, H., Li, Q. J., Feng, X. H., & Li, S. (2010). Bioresource Technology, 101, 7087–7093.
Sun, L.-H., Wang, X.-D., Dai, J.-Y., & Xiu, Z.-L. (2009). Applied Microbiology and Biotechnology, 82, 847–52.
Köpke, M., Mihalcea, C., Liew, F., Tizard, J. H., Ali, M. S., Conolly, J. J., Al-Sinawi, B., & Simpson, S. D. (2011). Applied and Environmental Microbiology, 77, 5467–5475.
Wang, A. L., Wang, Y., Jiang, T. Y., Li, L. X., Ma, C. Q., & Xu, P. (2010). Applied Microbiology and Biotechnology, 87, 965–970.
Kaseno, & Kokugan, T. (1997). Journal of Fermentation and Bioengineering, 83, 577–582.
Liu, Y.-P., Zheng, P., Sun, Z.-H., Ni, Y., Dong, J.-J., & Zhu, L.-L. (2008). Bioresource Technology, 99, 1736–1742.
Cazetta, M. L., Celligoi, M. A. P. C., Buzato, J. B., Scarmino, I. S., & Silva, R. S. F. (2005). Process Biochemistry, 40, 747–751.
Yan, D., Lu, Y., Chen, Y.-F., & Wu, Q. (2011). Bioresource Technology, 102, 6487–6493.
Han, W., Wang, B., Zhou, Y., Wang, D.-X., Wang, Y., Yue, L.-R., Li, Y.-F., & Ren, N.-Q. (2012). Bioresource Technology, 110, 219–223.
Chan, S., Kanchanatawee, S., & Jantama, K. (2012). Bioresource Technology, 103, 329–336.
Farooq, U., Anjum, F. M., Zahoor, T., Sajjad-Ur, R., Randhawa, M. A., Ahmed, A., & Akram, K. (2012). Pakistan Journal of Botany, 44, 333–338.
Meziane, M., Dilmi Bouras, A., El Hameur, H., Boukrabouza, S., & Bensehaila, S. (2011). African Journal of Biotechnology, 10, 16953–16962.
Ikram-ul, H., Ali, S., Qadeer, M. A., & Iqbal, J. (2004). Bioresource Technology, 93, 125–130.
Xiao, Z. J., Liu, P. H., Qin, J. Y., & Xu, P. (2007). Applied Microbiology and Biotechnology, 74, 61–68.
Canepa, P., Cauglia, F., Gilio, A., & Perego, P. (2000). Chemical and Biochemical Engineering Quarterly, 14, 53–56.
Ji, X. J., Huang, H., Du, J., Zhu, J. G., Ren, L. J., Li, S., & Nie, Z. K. (2009). Bioresource Technology, 100, 5214–5218.
Garg, S. K., & Jain, A. (1995). Bioresource Technology, 51, 103–109.
Poulsen, C., & Stougaard, P. (1989). European Journal of Biochemistry, 185, 433–439.
Takusagawa, Y., Otagiri, M., Ui, S., Ohtsuki, T., Mimura, A., Ohkuma, M., & Kudo, T. (2001). Bioscience, Biotechnology, and Biochemistry, 65, 1876–8.
Chatzifragkou, A., Papanikolaou, S., Dietz, D., Doulgeraki, A. I., Nychas, G.-J. E., & Zeng, A.-P. (2011). Applied Microbiology and Biotechnology, 91, 101–112.
Dai, J.-Y., Zhang, Y.-L., & Xiu, Z.-L. (2011). Chinese J. Chemical Engineering, 19, 682–686.
Dai, J.-Y., Sun, Y.-Q., & Xiu, Z.-L. (2014). Engineering in Life Sciences, 14, 108–117.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (No. DUT12ZD209) and National High Technology Research and Development Program of China (863 Program) (No. 2012AA021202-3).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 66 kb)
Rights and permissions
About this article
Cite this article
Dai, JY., Zhao, P., Cheng, XL. et al. Enhanced Production of 2,3-Butanediol from Sugarcane Molasses. Appl Biochem Biotechnol 175, 3014–3024 (2015). https://doi.org/10.1007/s12010-015-1481-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1481-x