Abstract
Bagasse was subjected to a liquefaction process with polyethylene glycol/glycerol using sulfuric acid as catalyst. The effects of various liquefaction conditions, such as reaction time, liquefaction temperature, catalyst content, and liquid ratio (liquefaction solvents/bagasse), on the liquefied residue (LR) content and hydroxyl and acid numbers of liquefied products were investigated. The preferred liquefaction condition of bagasse was determined through orthogonal experiments. The results showed that the catalyst content and reaction time have a greater influence than liquid ratio and liquefaction temperature on the percentage of LR. The hydroxyl and acid numbers of the liquefied products were influenced by many factors, including liquefaction temperature, reaction time, acid content, and liquid ratio. The hydroxyl number of liquefied products decreased as the liquefaction reaction progressed, but the acid number of liquefied products increased. Based on the obtained data, the kinetics for liquefaction was modeled using the first-order reaction rate law and the apparent activation energy for the liquefaction of bagasse was estimated to be 38.30 kJ mol−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyurethane is one of the most important and versatile polymeric material that has been used in a wide range of areas. Currently, polyurethane is a petroleum-based polymer because its major feedstocks (polyol and isocyanate) are largely petroleum-derived. However, lignocellulosic biomass, such as wood and agricultural residues, has attracted significant interest as raw materials for the preparation of polyurethanes in recent years [1]. This interest is economically driven because lignocellulosic biomass is a relatively cheap and renewable resource [1]. In addition, polyurethane produced from lignocellulosic biomass is an environmentally friendly material due to the biodegradable nature of lignocellulosic biomass [2].
Lignocellulosic biomass is mainly composed of cellulose, hemicellulose, and lignin, which typically contain two or more hydroxyl groups per molecule. In general, the conversion of lignocellulosic biomass to high value-added products can be achieved by a thermochemical process which includes gasification, pyrolysis, and liquefaction [3–5]. The liquefaction process is a convenient and effective technology for converting lignocellulose into liquid products, and polyhydric alcohols with appropriate molecular weight are usually used as liquefaction solvent. It is usually carried out at elevated temperature and in the presence of a catalyst. Through liquefaction, the three main components of lignocellulose may be converted to small molecules with hydroxyl groups (liquefied product) which could be directly used as polyol to prepare polyurethane without any additional reaction or treatment [6, 7]. Liao et al. [1, 2] used the liquefied cornstalk and liquefied wood to prepare the rigid polyurethane foam. Lee et al. [8] used polyethylene glycol (PEG)-liquefied wood to prepare polyurethane adhesives. Izumo et al. investigated the influence of wood species on the chemical structure and mechanical properties of polyurethane which was prepared from liquefied wood [9].
The liquefaction of lignocellulose in polyhydric alcohol is a complex series of reactions, including hydrolysis, degradation, condensation, and repolymerization [10–12], and the mechanisms of liquefaction are not entirely understood at present. It has been shown that cellulose is first converted to glycosides which are then hydrolyzed to levulinates [13]. Lignin produces free radical fragments which are easily condensed after a certain reaction time, resulting in insoluble residues [14]. Generally, hemicellulose is hydrolyzed in polyhydric alcohols much faster than cellulose [10, 15].
Liquefied product of lignocellulose has a high reactivity as it contains a large amount of highly active hydroxyl groups. The hydroxyl number of the liquefied wood is usually higher than 200 mg of KOH/g. Kunaver et al. [16] have carried out liquefaction of different southern European hardwoods and softwoods using glycerol/diethylene glycol. They found that the hydroxyl number of the liquefaction products ranged from 400 mg KOH/g at the beginning of the liquefaction process to 200 mg KOH/g at the end of the reaction. The amount of remaining glycols in the liquefied wood was also measured by gas chromatographic analysis, and the results showed that the remaining glycols have a major influence on the total hydroxyl number. Yao et al. [17] subjected polyhydric alcohols alone to the same liquefaction condition without adding lignocellulose and measured the hydroxyl number. The hydroxyl number of the liquefaction solvent did not change significantly; they concluded that the major decrease in the hydroxyl number of the liquefaction products was due to the dehydration and thermal oxidation of the polyhydric alcohols, as well as due to the reactions between the lignocellulose components and the liquefaction solvent [17].
The hydroxyl number and the structure of the hydroxyl group (i.e., primary or secondary) are key characteristics for using liquefied products as raw materials in the preparation of polyurethane, as well as for calculations of the reactant ratios in other condensation reactions [18]. The hydroxyl number of liquefied products is also one of the important factors in the preparation of polyurethane having the desired mechanical properties [12]. In the previous literature, there is very little information on the relations between liquefaction conditions and the hydroxyl number and acid number of liquefied products. Therefore, the aim of this work was to study the liquefaction of bagasse using PEG 400 (average molecular weight, 400)/glycerol blended solvents and to evaluate the effects of liquefaction conditions, such as reaction time, liquefaction temperature, acid concentration, and liquid ratio, on the hydroxyl and acid numbers of liquefaction products. The liquefaction reaction kinetics of the bagasse liquefaction in polyhydric alcohols was also studied.
Materials and Methods
Materials
Bagasse from Guangdong province (Southern China) was used as a raw material; the bagasse sample was milled in a knife mill to pass through a 165-μm sieve, which was dried in an oven at 105 °C for 24 h and kept in a desiccator at room temperature before use. PEG 400 and glycerol were used as the liquefaction reagents. Sulfuric acid (98 %) was used as the acidic catalyst. All other chemicals in this study were analytical reagent grade, which were obtained from commercial sources, and used without further purification.
Liquefaction Reaction
The mixture of liquefaction solvents (PEG 400/glycerol = 80/20, w/w) was placed in a 250-ml four-neck flask, equipped with a mechanical stirrer, reflux condenser, and thermometer. The reaction system was preheated at a set temperature, and then the bagasse and sulfuric acid were added to the flask under stirring. The amount of sulfuric acid was calculated as weight content (in percent) based on the amount of the liquefaction solvent. Time 0 was considered as the time at which the bagasse and sulfuric acid were added to the flask. Liquefaction was conducted under constant stirring and refluxing for a certain time. A sample was withdrawn from the flask periodically and immersed in cold water to quench the reaction as fast as possible.
Measurement of Percentage Liquefied Residue
About 5 g liquefaction product was diluted with 150 ml mixture of dioxane–water (4:1, v/v), the liquefied residue (LR) was separated by filtration, and then rinsed thoroughly with the mixture of dioxane–water. The LR was dried in oven at 105 °C to constant weight and weighed for the determination of the content of LR, and the percentage of LR (α) was calculated with the following equation:
Measurement of Hydroxyl Number
The hydroxyl number is an important parameter in the production of polyurethane from liquefied lignocellulose. It is usually expressed as hydroxyl number in the equivalents of milligrams KOH per gram of sample. The hydroxyl number of liquefied bagasse was determined according to ISO 14900-2001: 1.0–1.5 g of liquefied bagasse was dissolved in 25 ml of a phthalation reagent (116 g phthalic anhydride, 16 g imidazole, and 700 ml pyridine) and heated at 115 °C for 0.5 h under reflux. After the heating period, the assembly was removed from the bath and allowed to cool to room temperature. The condenser was washed down with 50 ml of pyridine, and the mixture was titrated with 0.5 M sodium hydroxide solution to the equivalence point using a pH meter. The hydroxyl number in milligrams KOH per gram of sample was calculated as follows:
where A is the volume (in milliliters) of the 0.5 M sodium hydroxide solution required for titration of the sample, B is the volume (in milliliters) of the sodium hydroxide solution required for titration of the blank solution, N is the normality of the sodium hydroxide solution, and w is the amount of the sample (in grams) to be analyzed.
Measurement of Acid Number
The acid number was determined as follows [12, 16]: 5 g of sample was dissolved in 100 ml mixture of dioxane–water (4:1, v/v). The mixture was titrated with 1 M sodium hydroxide solution to the equivalence point using a pH meter. The acid number (in milligrams KOH per gram of sample) was calculated as follows:
where C is the titration volume (in milliliters) of the sodium hydroxide solution at the equivalence point, B is the volume of the blank solution (in milliliters), N is the normality of the sodium hydroxide solution, and w is the weight of the sample to be analyzed.
The content of LR and the hydroxyl and acid numbers were the average values from two replicate samples, and the run would be repeated if the deviation of the value obtained from the average was higher than 5 %.
Gel Permeation Chromatography
The molecular weight of the liquefied product was determined by gel permeation chromatography (GPC; Waters, Milford, MA, USA) equipped with a Waters 410 Differential Refractometer and Ultrahydrogel Column. The mobile phase was tetrahydrofuran, which had a flow rate of 1.0 ml/min. The column temperature was 40 °C. The average molecular weight of the sample was calculated by using a calibration curve of monodisperse polystyrene standards.
Results and Discussion
Effects of Liquefaction Conditions on the Content of LR
The influences of various liquefaction conditions, including sulfuric acid percent, liquefaction temperature, liquid ratio (liquefaction solvents/bagasse, w/w), and reaction time, on the content of LR are shown in Table 1. It can be seen from Table 1 that the degree of the effects of the four liquefaction factors on the content of LR is in a descending order as follows: sulfuric acid addition (in percent), reaction time (in minutes), liquefaction temperature (in degrees Celsius), and liquid ratio.
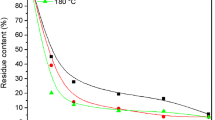
The effect of reaction time on the LR content under different liquid ratios is presented in Fig. 1a (H2SO4, 3 %; liquefaction temperature, 150 °C). The content of LR was rapidly decreased at the beginning of the liquefaction process, and about 75 % of the bagasse was liquefied within 30 min. The whole liquefaction process could be divided into two phases: the fast one corresponded to the depolymerization of the susceptible components of bagasse, such as lignin and the amorphous region of cellulose, and the slow one corresponded to the degradation of the crystalline regions of cellulose [6]. Budija et al. observed the increase of LR after a certain reaction time and explained this phenomenon as a recondensation of decomposed wood components [19]. However, the content of LR decreased slowly in the latter liquefaction stage, and the recondensation reaction of liquefied bagasse components did not appear in the observed time when the liquid ratio was in the range between 3:1 and 5:1 in this study. It is probably due to the existence of glycerol in the liquefaction system, which had a significant effect on the retardation of the recondensation reaction due to their larger molecular numbers compared to PEG 400 [12].
In general, with an increase in acid concentration, the content of LR decreased. It is evident from Fig. 1b (liquefaction temperature, 150 °C; liquid ratio, 3:1; reaction time, 120 min) that the LR decreased drastically when the acid concentration was increased from 1 to 3 %. However, when the acid concentration was increased to 5 %, LR content slightly increased. Similarly, Zhang et al. [6, 7] reported that the content of LR decreased first and then increased with the increase of catalyst concentration and explained this phenomenon as a recondensation or reprecipitation of liquefied products at a higher concentration of acid.
Figure 1c shows that the LR content decreased as the temperature in the system increased (H2SO4, 3 %; liquid ratio, 3:1; reaction time, 120 min), which indicated that increased temperature could accelerate the liquefaction reaction. This is probably because the permeation of acid into the bagasse and diffusion of decomposed components of bagasse into the liquefaction solvents were accelerated at higher temperature [7]. Furthermore, it can also be seen that liquefaction temperature above 150 °C has no influence on LR, and thus, the preferred temperature for the liquefaction of bagasse was about 150 °C.
It is well known that the liquefaction of lignocellulose using organic solvent is a solvolysis process and the solvent is very important to the decomposition of lignocellulose [1]. Figure 1d shows the effect of the liquid ratio on the percentage of LR (H2SO4, 3 %; liquefaction temperature, 150 °C; reaction time, 120 min). The amount of LR decreased with the increase of liquid ratio, and then decreased slowly when liquid ratio was above 3. A similar trend has been also observed in the case of liquefaction of wood, cornstalk, and lignin [1, 6, 20, 21]. These results suggested that liquefaction solvents/bagasse ratios of 3–4 are enough to get a residue percentage of <10 %. Therefore, the preferred liquefaction conditions for bagasse with the PEG 400/glycerol were liquefaction temperature of 150 °C, reaction time of 120 min, liquid ratio of 3:1–4:1, and 3 % of sulfuric acid addition. GPC was used to study the molecular weight distribution of the liquefied products. The average molecular weight of the liquefied products was 2,615 g mol−1 and the polydispersity was 6.74.
Effects of Liquefaction Conditions on the Hydroxyl and Acid Numbers of Liquefied Product
Figure 2 shows the time dependence of the hydroxyl and acid number curves of liquefied products with the liquid ratio ranging from 3:1 to 5:1. The inverse relationship between the hydroxyl number of liquefied products and reaction time for different liquid ratios, except the acid number of liquefied products, was proportional to the reaction time. As shown in Fig. 2, a remarkable increase in the acid number of liquefied products and a subsequent slow increase process were observed, which indicated that the acidic substances content of the liquefaction solvents and/or liquefied products is increased during the liquefaction reaction. For comparison, a control experiment under the same liquefaction condition without adding bagasse was also carried out, and the changes in the hydroxyl and acid numbers of the liquefaction solvents are shown in Fig. 3. The horizontal line of the acid number showed that the acid number of the liquefaction solvents do not obviously change during the reaction. These results suggested that the increases in acid number of the liquefied products of bagasse during reaction are largely due to the depolymerized bagasse components or to the oxidation of the saccharides during the liquefaction [22]. Jasiukaitytė et al. [11] proposed the liquefaction mechanism of cellulose in the presence of polyol under acid catalysis. It was suggested that cellulose liquefaction in acidified polyol seemed to be analogous to hydrolysis. The primary reaction is hydrolysis, followed by glycosidation of the new reducing groups, leading to large quantities of levulinic acid and its derivatives [11, 23]. Zhang et al. [24] reported that the liquefied product of bagasse contained large amounts of levulinic acid, formic acid, and their derivatives, which are mainly produced by the degradation of cellulose and hemicellulose.
Figure 2 also shows that the hydroxyl number of the liquefied products decreased significantly in the initial reaction stage and almost leveled off with the increase in the reaction time, which is consistent with the results from Yao et al. and Lee et al. [17, 22]. Similarly, the hydroxyl number of the liquefaction solvent also has the same change tendency, as shown in Fig. 3, which reveals that the reaction of the hydroxyl groups of the liquefaction solvents occurred during the reaction. In addition, according to the comparison of the hydroxyl numbers of the liquefied product with and without adding bagasse, it can be found that liquefied bagasse with much lower hydroxyl number is obtained at the same liquefaction condition. It is suggested that the decrease in the hydroxyl number of liquefied bagasse with increasing reaction time could also be attributed to a reaction between the liquefaction solvent and the decomposed components of bagasse. Similar results have been also reported in previous investigations: Yao et al. [17] subjected the liquefaction solvents alone to the same liquefaction conditions, and they found that no significant decrease in the hydroxyl number could be observed. Therefore, it was suggested that the decrease in the hydroxyl number of liquefied products is due to the dehydration and thermal oxidation reaction of the liquefaction solvents and/or the reactions between the bagasse components and the liquefaction solvents. Furthermore, the influence of the liquid ratio on the hydroxyl and acid numbers was also investigated (Fig. 2). It can be seen that the hydroxyl and acid numbers showed no obvious differences among the liquid ratios of 4:1–5:1, but changed significantly at a ratio of 3:1. This was probably due to the increase in the concentration of liquefied products at low liquid ratio; thus, the reaction between the liquefaction solvent and the decomposed components of bagasse was accelerated.
The hydroxyl and acid numbers of liquefied products at various acid concentrations are shown in Fig. 4. As shown in Fig. 4, the hydroxyl number of liquefied products drastically decreased from 410 to 356 mg KOH/g with increasing acid concentrations from 0.8 to 3 % and then slightly decreased. On the other hand, acid number increased with increasing acid concentration. This could be considered that recondensation of the decomposed components and dehydration of the hydroxyl group-rich liquefaction solvents were accelerated at high acid concentration [12].
Figure 5 indicates the relationship between liquefaction temperature and the hydroxyl and acid numbers of liquefied products of bagasse. It is also obvious from Fig. 5 that the hydroxyl numbers of liquefied products decrease from 385 to 305 mg KOH/g and the acid numbers of liquefied products increase from 35.4 to 42.2 mg KOH/g, when changing liquefaction temperature from 130 to 170 °C. At higher temperature, the surface tension of the liquefaction solvents was lower, which could promote permeation of sulfuric acid into the bagasse samples and diffusion of decomposed components of bagasse from the solid matrix into the liquefaction solvent [25]. On the other hand, the dehydration and thermal oxidative degradation of the liquefaction solvent, as well as the reactions between the liquefaction solvent and bagasse components, such as cellulose, hemicelluloses, and lignin, were accelerated at higher temperature [4, 16]. Consequently, the higher the temperature of the liquefaction system, the lower the LR content and the hydroxyl number of the liquefied product, but the higher the acid number of the liquefied product.
First-Order Reaction Kinetics of Bagasse Liquefaction
The liquefaction curve of bagasse in polyhydric alcohol is considered to follow the first-order kinetics reaction as in Eq. 4 [26, 27]:
where W is the mass of bagasse, t is the reaction time, and k is a rate constant. Equation 4 could be written as:
Assuming that, initially (at t in = 0), the mass of bagasse was W in, the logarithmic form of the previous kinetics equation was:
where W f is the final oven-dried weights of the LR at the final reaction time (t f).
Figure 6 shows the obtained temperature dependence of the liquefaction rate constant of bagasse. It could be seen that the liquefaction rate constant increases with the increase in liquefaction temperature, which indicated that the increase of liquefaction temperature can accelerate the liquefaction of bagasse. This change agreed well with the tendencies reported in the literatures [12, 28].
Assuming the Arrhenius form of dependence of the rate constant on temperature, the reaction rate constant k is expressed by the Arrhenius equation:
The activation energy (E a) and the preexponential factor (A) are determined from the logarithmic form of the Arrhenius equation:
The apparent activation energy value and preexponential factor of bagasse liquefaction could be obtained from a linear fitting of lnk against 1,000/T, the slope at any point is equal to −E a /R, and the intercept is lnA. The Arrhenius plot for the liquefaction of bagasse in polyhydric alcohol is shown in Fig. 7. The apparent activation energy value for the liquefaction of bagasse was 38.30 kJ mol−1, which was lower than cornstalk (73.6 kJ mol−1) [27], which suggested that cornstalk requires much higher energy for its liquefaction as compared to bagasse.
Conclusions
Bagasse was liquefied in PEG 400/glycerol catalyzed by sulfuric acid under atmospheric pressure. The results showed that the amount of LR decreases as liquefaction temperature, catalyst concentration, reaction time, and liquid ratio increase. Sulfuric acid concentration and reaction time had a greater influence on the content of LR rather than liquefaction temperature and liquid ratio. The hydroxyl and acid numbers of liquefied products depended on the acid concentration, liquefaction temperature, and reaction time. With an increase in acid concentration, liquefaction temperature, and reaction time, the hydroxyl number of liquefied products decreased and the acid number of liquefied products, by contrast, increased. The decrease in the hydroxyl number of liquefied products may be due to the dehydration and thermal oxidation reaction of liquefaction solvents, as well as the reactions between the bagasse components and the liquefaction solvent. The reason for the increased acid number of liquefied products during reaction could be the depolymerized bagasse components or the oxidation of the saccharides. A first-order reaction model was used to explain the kinetics of bagasse liquefaction, and the apparent activation energy for the liquefaction of bagasse was estimated to be 38.30 kJ mol−1.
References
Yan, Y. B., Pang, H., Yang, X. X., Zhang, R. L., & Liao, B. (2008). Journal of Applied Polymer Science, 110, 1099–1111.
Zhang, H. R., Pang, H., Zhang, L., Cheng, X. D., & Liao, B. (2012). Journal of Polymers and the Environment. doi:10.1007/s10924-012-0542-2.
Zhang, B., Huang, H. J., & Ramaswamy, S. (2008). Applied Biochemistry and Biotechnology, 147, 119–131.
Yu, F., Le, Z. P., Chen, P., Liu, Y. H., Lin, X. Y., & Ruan, R. (2008). Applied Biochemistry and Biotechnology, 148, 235–243.
Chen, H. Z., Zhang, Y. Z., & Xie, S. P. (2012). Applied Biochemistry and Biotechnology, 167, 250–258.
Zhang, H. R., Pang, H., Ji, H. G., Fu, T. Z., & Liao, B. (2012). Journal of Applied Polymer Science, 123, 850–856.
Zhang, H. R., Ding, F., Luo, C. R., Xiong, L., & Chen, X. D. (2012). Industrial Crops and Products, 39, 47–51.
Lee, W. J., & Lin, M. S. (2008). Journal of Applied Polymer Science, 109, 23–31.
Izumo, K., & Fukushima, M. (2010). Journal of Applied Polymer Science, 118, 2109–2115.
Shin, H., Kim, C. J., & Kim, S. (2009). Biotechnology and Bioprocess Engineering, 14, 349–353.
Jasiukaitytė, E., Kunaver, M., & Strlič, M. (2009). Cellulose, 16, 393–405.
Kurimoto, Y., Doi, S., & Tamura, Y. (1999). Holzforschung, 53, 617–622.
Yamada, T., Aratani, M., Kubo, S., & Ono, H. (2007). Journal of Wood Science, 53, 487–493.
Kobayashi, M., Asano, T., Kajiyama, M., & Tomita, B. (2004). Journal of Wood Science, 50, 407–414.
Krzan, A., & Zagar, E. (2009). Bioresource Technology, 100, 3143–3146.
Kunaver, M., Medved, S., Cuk, N., Jasiukaityte, E., Poljansek, I., & Strnad, T. (2010). Bioresource Technology, 101, 1361–1368.
Yao, Y. G., Yoshioka, M., & Shiraishi, N. (1996). Journal of Applied Polymer Science, 60, 1939–1949.
Ionescu, M. (2005). Chemistry and technology of polyols for polyurethanes. Shawbury: Rapra Technology Limited.
Budija, F., Tavzes, C., Zupancic-Kralj, L., & Petric, M. (2009). Bioresource Technology, 100, 3316–3323.
Bonini, C., D’Auria, M., Ernanuele, L., Ferri, R., Pucciariello, R., & Sabia, A. R. (2005). Journal of Applied Polymer Science, 98, 1451–1456.
Briones, R., Serrano, L., Llano-Ponte, R., & Labidi, J. (2011). Chemical Engineering Journal, 175, 169–175.
Lee, S. H., Yoshioka, M., & Shiraishi, N. (2000). Journal of Applied Polymer Science, 78, 319–325.
Yamada, T., & Ono, H. (1999). Bioresource Technology, 70, 61–67.
Zhang, T., Zhou, Y. J., Liu, D. H., & Petrus, L. (2007). Bioresource Technology, 98, 1454–1459.
Demirbas, A. (2008). Energy Sources, Part A: Recovery Utilization and Environmental Effects, 30, 1120–1126.
Yu, F., Ruan, R., Lin, X. Y., Liu, Y. H., Fu, R., Li, Y. H., Chen, P. & Gao, Y. Y.. (2006). Applied Biochemistry and Biotechnology, 130, 563–573.
Yan, Y. B., Hu, M. M., & Wang, Z. H. (2010). Industrial Crops and Products, 32, 349–352.
Mishra, G., & Saka, S. (2011). Bioresource Technology, 102, 10946–10950.
Acknowledgments
The authors acknowledge the financial support of the National Science and Technology project (2012BAD32B07), Foundation of the Xuyi Center of Attapulgite Applied Technology Research Development and Industrialization (20121004), Foundation of the Director of Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences (y107re1001) (y107re1001, y107rf1001), and Natural Science Foundation of Guangdong Province (S2012040007546).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Luo, J., Li, Y. et al. Acid-Catalyzed Liquefaction of Bagasse in the Presence of Polyhydric Alcohol. Appl Biochem Biotechnol 170, 1780–1791 (2013). https://doi.org/10.1007/s12010-013-0300-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0300-5