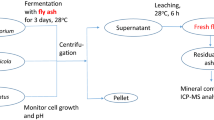
Abstract
Fly ash collected from an Indian thermal power plant was characterised by scanning electron microscope (SEM)-energy dispersive spectrometer, X-ray diffraction and energy dispersive X-ray fluorescence analysis. The effect of fly ash on the growth and morphology of a metal-tolerant tropical marine yeast, Yarrowia lipolytica NCIM 3589, was studied. The growth of the yeast was unaffected by the presence 0.1, 0.2 or 0.3 % fly ash although the surface-to-volume ratio decreased. The yeast formed biofilms on immobilized fly ash as evidenced by SEM observations. The organism produced citric acid and additional extracellular proteins in the presence of fly ash. Leaching of metals from fly ash by Y. lipolytica was compared with chemical leaching by citric acid. Yeast cells were most effective in leaching Cu (59.41 %) although other metals (Zn, Ni, Cu and Cr) were also extracted. Transmission electron microscope images showed the deposition of metals at the cell wall, cell membrane and in the cytoplasm. This paper thus reports a potential application of Y. lipolytica for removal of different metals from solid waste material (fly ash).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermal power plants generate large quantities of fly ash as a by-product. The major portion of fly ash is disposed in ash ponds and landfills which in turn cause environmental pollution [1–3]. The presence of Si, Al, Fe, Cd, Cr, Mn, B, As, Cu, Zn and Mo in this by-product is hazardous for living forms [4–6]. There is thus a need to treat fly ash prior to its disposal into the environment. Since conventional methods for treatment are cost intensive and have a negative impact on the environment, bioleaching has emerged as an effective alternative [7, 8]. Bioleaching is based on the potential of microorganisms (bacteria or fungi) to solubilise and extract elements. Microorganisms can mobilize and leach metal ions from solid wastes due to (1) production of organic or inorganic acids, (2) inherent oxidation and reduction mechanisms and (3) excretion of complexing agents [9, 10]. Microbial leaching methods are important in the recovery of copper, gold, uranium and zinc from low-grade ores [11]. For example, Thiobacillus ferrooxidans and Thiobacillus thiooxidans are effective metal solubilizers that have been applied at an industrial scale [12, 13]. Similarly, fungi such as Aspergillus and Penicillium species are also important in solubilising metal ions [7, 10].
Yarrowia lipolytica is a hemiascomycetous dimorphic fungus that has several biotechnological applications [14–16]. This organism is widely used in the remediation of a variety of polluted environments [17–19]. Y. lipolytica has the ability to accumulate metals such as copper, cobalt, cadmium, nickel, zinc and gold [20–23]. In addition, the organism has also been applied in the biosorption of Cr (VI) ions from aqueous solutions [24]. The fungus inherently produces citric acid, a potential lixiviant [25] that could be used to leach heavy metals from solid materials. Although Y. lipolytica is known to accumulate and adsorb heavy metals, to the best of our knowledge, there are no reports on the bioleaching of metals from fly ash by this fungus. In the current investigation, the ability of a tropical marine strain of Y. lipolytica to (1) grow in the presence of fly ash, (2) solubilise fly ash-associated metals and (3) accumulate metals from this solid waste are reported. This work thus highlights the significance of Y. lipolytica in the treatment of this important solid waste.
Materials and Methods
Fungal Strain and Maintenance
A strain of Y. lipolytica, NCIM 3589, isolated from oil-polluted seawater in Mumbai, India, was used [18]. Stock cultures of this organism were maintained on MGYP slants (malt extract, 3.0; glucose, 10.0; yeast extract, 3.0; peptone, 5.0; agar, 25.0 g L−1 of distilled water) and sub-cultured at monthly intervals. The growth temperature was 30 °C.
Fly Ash Sample Collection
Fly ash used in this work was collected from a dumpsite of a thermal power plant in Maharashtra, India. The sample was collected in clean plastic bags, dried to constant weight and stored at 30 °C.
Characterisation of Fly Ash
Elemental composition of fly ash was determined by using energy dispersive X-ray fluorescence (ED-XRF) (AMETEK Materials Analysis Division). Fly ash (4.0 g dry weight) was placed in sample cups. These cups were arranged in a sample tray (four different variables), exposed to X-rays, and the elemental composition was determined as described earlier [26]. The scanning electron microscope-energy dispersive spectrometer (SEM-EDS) analysis was performed by using an analytical SEM (JEOL JSM 6360 A). X-ray diffraction (XRD) measurements were carried out in the transmission mode on a D8 Advanced Brucker instrument with CuKα radiation (λ = 1.54 °A) over the two theta values from 20° to 80°.
Effect of Fly Ash on Yeast Growth and Morphology
Y. lipolytica NCIM 3589 was grown in a yeast extract peptone dextrose (YPD) liquid medium (yeast extract, 5.0; peptone, 5.0; glucose, 10.0 g L−1 of distilled water) for 36 h. The cells were harvested by centrifugation, washed twice and resuspended in sterile deionised water. Cell suspensions containing 2 × 109 cells mL−1 were used to inoculate 100 mL of YPD medium containing 0.1, 0.2 or 0.3 % (w/v) fly ash. At these concentrations of the fly ash, the YPD liquid medium was not turbid. The flasks were incubated at 30 °C on a rotary shaker at 200 rpm. The growth of the yeast was monitored by measuring the absorbance at 600 nm. In control experiments, fly ash was not included. The microscopic observations were made in the presence of 1.0, 2.0 and 3.0 % of fly ash. Dimensions of cells in control and test (incubated with fly ash) samples were measured by using an Axio Scope-A1 microscope with a photographic attachment (ProgRes® Capture Pro 2.7) and the software AxioVison Rel. 4.8. The average cell volumes in cubic micrometres (V) and surface areas in square micrometres (A) were calculated by using the following equations:
where r was the average radius and h the average length of 100 cells in micrometres [27]. In order to understand the interactions between the yeast cells and fly ash under shake flask conditions, SEM images were obtained as described earlier [24].
Growth Characteristics of Y. lipolytica on Immobilized Fly Ash Surface
Fly ash was immobilized on glass pieces (2 × 2 cm) by heat fixing with an adhesive at 60 °C for 24 h. These glass pieces were placed in Petri plates containing 20 mL of sterile YPD liquid medium; 2 × 109 cells mL−1 of Y. lipolytica cells were added, and the assemblies were incubated at 30 °C for 48 h. The growth of Y. lipolytica on immobilized fly ash was monitored by SEM analysis.
Bioleaching of Fly Ash by Y. lipolytica
In the bioleaching experiments, 2 × 109 cells mL−1 were inoculated into 100 mL of YPD liquid medium containing 8 % (w/v) fly ash. The control flasks were without yeast cells. All flasks were incubated on a rotary shaker (200 rpm, 30 °C, 15 days). After incubation, the reaction mixtures were centrifuged at 7,000×g for 10 min. The citric acid concentration in cell-free supernatant was determined by high performance liquid chromatography (HPLC) on a Waters Associate system (using a μBondpack C18 250 × 4 mm column) equipped with an ultraviolet detector (at 210 nm). The determinations were made under isocratic conditions (10 °C) using 50 mM phosphate buffer (pH 7.0) as the mobile phase. The chemical leaching of fly ash [8 % (w/v)] was also conducted with citric acid (200 mM), a well-known metal ion leaching agent [28]. The content of metal ions in the cell-free supernatants was determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES). All experiments were carried out in triplicates with two biological replicates and mean values indicating standard deviation are presented here.
Extraction of Yeast Biomass-Accumulated and Associated Metals
The pellets containing yeast biomass and residual fly ash particles were dried at 80 °C for 48 h. They were treated with 10 mL of 5 % HNO3 with shaking for 2 h and subsequently heated at 80 °C for 30 min. The resultant extracts were filtered through Whatman papers, and the final volumes were adjusted to 50 mL with deionised water. The metal ions present in the extract were analysed by ICP-AES. Control samples lacking the yeast biomass were also similarly treated to determine dissolution of metals by 5 % HNO3 as reported earlier [28]. The percentage of metal ions leached was calculated from these observations. The extracellular protein profiles in presence and absence of fly ash [8 % (w/v)] were studied by SDS-PAGE. Accumulation of metals by the yeast cells was also demonstrated by transmission electron microscope (TEM) images. The samples were prepared by following a previously reported protocol [29]. All experiments were performed in triplicates with two biological replicates, and representative data or images are presented here.
Statistical Analysis
Experimental parameters were checked for significance of difference by using one-way ANOVA. A probability level of P (0.05) was used. For statistical analysis, the GraphPad InStat [DATASET1.ISD] software was used.
Results and Discussion
Characterisation of Fly Ash
The fly ash used in this study was characterised by EDS, XRD and XRF analyses. Figure 1a is a representative EDS profile of the fly ash sample. This profile showed the presence of heavy metals such as Cr, Co, Ni, Cu, Zn, As, Cd and Pb. The representative XRD profile is depicted in Fig. 1b. The major peaks in the XRD profiles could be indexed to the crystalline oxides of silica, aluminium and iron (SiO2, Al2O3 and Fe3O4, respectively). On the basis of the ED-XRF analysis, the composition of the fly ash was determined and is depicted in Table 1. SiO2 and Al2O3 were the major components of fly ash accounting for 63.85 and 28.98 % (w/w), respectively. As also observed in the EDS profiles, ED-XRF spectra displayed peaks for Cr, Co, Ni, Cu, Zn, As, Cd and Pb (Fig. 1a and Table 1). These results on fly ash composition are in agreement with earlier reports [30].
Growth Pattern and Morphometric Analysis of Yeast
Figure 2 shows the growth pattern of Y. lipolytica NCIM 3589 in absence and presence of different concentrations of fly ash. From the figure, it is evident that the growth in all the cases was comparable. Table 2 shows the morphometric analysis of control experiments and cells grown in the presence of increasing concentrations of fly ash. The average cell length, radius, cell volume and surface area increased, and the surface/volume ratio decreased in a significant manner (P < 0.05). A decreased surface/volume ratio is a well-known response of cells to toxic environmental stress factors [27].
SEM images showed that control cells of Y. lipolytica had an average length of 6 μm. Typical budding cells were observed (Fig. 3a). In the subsequent images, the white arrows point towards yeast cells and the black arrows towards the fly ash particles. SEM images of the test samples revealed the presence of small-sized particles of fly ash adhering to yeast cells (Fig. 3b and c). A representative image of the untreated fly ash displayed spherical, oval, elongated and irregular particles (Fig. 3d). The yeast cells were also seen attached to larger particles of fly ash (Fig. 3e and f). Fly ash is known to be hydrophobic in nature [31]. In the presence of glucose-containing media, cells of Y. lipolytica NCIM 3589 are hydrophobic [32]. The observed attachment of Y. lipolytica to the fly ash particles may thus be mediated via hydrophobic interactions. Other microorganisms such as Rhodococcus strain GIN1(NCIMB 40340) and T. thiooxidans are reported to interact with fly ash particles in a similar manner [33].
Representative scanning electron micrographs of Y. lipolytica NCIM 3589. a Control cells without fly ash magnified ×10,000; bar equivalence, 1 μm. Grown in the presence of fly ash; b magnified ×6,000; bar equivalence, 2 μm; c magnified ×15,000; bar equivalence, 1 μm. Fly ash control; d magnified ×2,000; bar equivalence, 10 μm. Large fly ash particles showing the attachment of yeast cells on the surface after 48 h; e magnified ×1,600 and (f) ×2,000; bar equivalence, 10 μm
Y. lipolytica cells are known to form biofilms on the different substrates [34]. Such biofilms were also observed on immobilized fly ash surfaces. Representative SEM images of Y. lipolytica biofilms on the immobilized fly ash are shown in Fig. 4. In these figures as well, the white arrows point towards yeast cells and the black arrows towards the fly ash particles. Low-magnification images (Fig. 4a and b) show that the fly ash particles were completely covered with Y. lipolytica biofilms. At higher magnifications, the biofilm growth was more distinctly observed (Fig. 4c and d). It is known that the cells in biofilm mode of growth are protected against toxic compounds, desiccation and shock loads. On other solid waste material such as a phosphate-bonded ceramic, cement/manganese tailings or ceramic-coated metal strips, cells of T. thiooxidans are reported to exist as biofilms [35]. Formation of biofilms on the surface of fly ash particles may have importance in developing biofilm-based bioleaching technologies.
Bioleaching of Fly Ash by Y. lipolytica NCIM 3589
The bioleaching of fly ash [8 % (w/v)] by Y. lipolytica was studied in the YPD medium. The pH of the medium decreased from 6.0 to 2.7 after 5 days of incubation. A possible reason for this drop in pH could be the secretion of organic acids. Such organic acids play an important role in the extraction of metal ions from fly ash [36]. Y. lipolytica is known to produce citric acid [25]. Citric acid is reported to display metal leaching properties [7]. Subpanels a and b of Fig. 5 are representative HPLC profiles showing peaks for citric acid in the test (in the presence of fly ash) and control samples, respectively. The concentrations of citric acid in the test and control samples were estimated to be 11 and 15 mM, respectively. These results indicated that citric acid was produced by Y. lipolytica cells even in the presence of fly ash. This is unlike previous reports wherein the presence of metal ions (zinc, manganese, iron and copper) in the fly ash inhibited the production of this acid [37, 38]. Thus, citric acid may be one of the metal leaching agents secreted by Y. lipolytica.
A study on the chemical (using 200 mM citric acid) and biological (using Y. lipolytica) leaching of metals was carried out. Figure 6a is a flask showing growth of Y. lipolytica in the absence of fly ash, and Fig. 6b depicts growth in its presence. Figure 6c is a flask showing leaching of fly ash with 200 mM citric acid, and Fig. 6d is the fly ash in uninoculated YPD medium. Extensive foaming was observed in the presence of fly ash (Fig. 6b, black arrow) which was not so significant in its absence (Fig. 6a, black arrow). Foam formation was neither observed with citric acid nor with the uninoculated YPD medium (Fig. 6c and d, white arrows). This indicated that in the presence of fly ash, some proteins participating in the bioleaching process may be secreted.
Leaching of fly ash under shake flask conditions. A Y. lipolytica in YPD media without fly ash, B Y. lipolytica in YPD medium containing 8 % fly ash, C 200 mM citric acid and 8 % fly ash, D uninoculated YPD medium containing 8 % fly ash. All flasks were incubated at 30 °C for 5 days. E Graphical representation of metal leaching by a Y. lipolytica cells, b cell-free supernatant, c 200 mM of citric acid and d YPD medium after incubation at 30 °C for 5 days at 200 rpm
After treatment of the fly ash with Y. lipolytica, the cell-free supernatants showed the presence of 17.37 % Cr, 13.30 % Zn, 14.19 % Ni, 52.95 % Cu, 1.47 % Si and 0.32 % Al. In addition, yeast-associated and yeast-accumulated metal ions were also estimated to be 5.47 % of Cr, 13.06 % of Zn, 9.59 % of Ni, 8.46 % of Cu, 0.48 % of Si and 0.68 % of Al. When citric acid was used as a leaching agent, 29.29 % of Cr, 33.78 % of Zn, 38.7 % of Ni, 44.61 % of Cu, 3.07 % of Si and 9.14 % of Al were extracted. In uninoculated YPD medium, 6.77 % of Cr, 7.85 % of Zn, 11.69 % of Ni, 8.46 % of Cu, 0.19 % of Si and 0.72 % of Al were extracted (Fig. 6e). From these data, it is evident that citric acid was an efficient leaching agent. Similar to other fungi, the total metal leaching efficiency of Y. lipolytica was due to the bioaccumulation of metal ions and leaching by metabolites such as citric acid in the medium [7, 8]. The combined effect of the metal leaching by metabolites and accumulation was comparable to the results obtained by the chemical method. Amongst all the metals, copper was bio-leached to a maximum greater extent (P < 0.05). The yeast cells were more effective in leaching Cu (II) ions than the chemical method.
In a preliminary experiment on the fly ash-induced production of specific proteins, the cell-free supernatants were subjected to SDS-PAGE. Figure 7 shows a representative profile that was obtained. Lane A depicts the molecular weight markers, lane B is the profile obtained in the absence of fly ash and lane C is that in the presence of fly ash. It was observed that two extracellular proteins with molecular mass of 35.75 and 25.98 kDa were induced in the presence of fly ash (Fig. 7, white arrows). These proteins may be playing a role in the metal leaching process. In response to the presence of metals, cells are often induced to produce different proteins that may be important in the detoxification and sequestration of metals [39].
In this study, it was observed that the bioaccumulation of metal ions by yeast cells also played a role in the bioleaching process. The metal accumulation efficiency of Y. lipolytica was: Zn > Ni > Cu > Cr > Al > Si. The accumulation of metal ions was confirmed by obtaining TEM images. A representative control cell of Y. lipolytica (grown in the absence of fly ash) is shown in Fig. 8a. The image did not show the presence of metal deposits (Fig. 8a, black arrow). In test cells that were exposed to metals, dense metal deposits were observed at the cell wall, cell membrane and in the cytoplasm (Fig. 8b, c and d; white arrows). Cell walls and cell membranes are known to be major sites for metal deposition in Y. lipolytica [21]. These observations confirmed that cells of Y. lipolytica accumulated metals.
Y. lipolytica thus displayed three possible mechanisms by which leaching of metals from fly ash was possible: (1) Secretion of lixiviant metabolites such as citric acid, (2) production of extracellular proteins in response to the presence of fly ash and (3) bioaccumulation of heavy metals at the cell wall, cell membrane and in the cytoplasm. As a result of the combined effect of these three processes, the metals in the fly ash could be leached out in an effective manner.
Conclusions
This study thus highlights the role of the hitherto unexplored cells of Y. lipolytica in the leaching of metal ions from fly ash. The fly ash sample used in this study mainly contained Cr, Co, Ni, Cu, Zn, As, Cd and Pb. Cells of Y. lipolytica were able to grow in the presence of fly ash in the free form. They also grew in the biofilm form on immobilized fly ash when assessed by SEM. Although the growth of the culture in liquid media was unaffected by the presence of fly ash, morphometric analysis showed that the surface-to-volume ratios of the cells decreased in its presence. Y. lipolytica cells secreted citric acid and two extracellular proteins that may be involved in the bioleaching process. Moreover, TEM images revealed the bioaccumulation of heavy metals by cells. It was thus evident that Y. lipolytica could be a potential candidate for the leaching of a variety of metal ions from fly ash. The role of the biofilm form of growth in management of solid wastes is being investigated, and work on the scale-up of this process to develop the technology for the extraction of metal ions is ongoing.
References
Sarkar, A., Rano, R., Mishra, K. K., & Sinha, I. N. (2005). Fuel Processing Technology, 86(11), 1221–1238.
Bhattacharjee, U., & Kandpal, T. C. (2002). Energy, 27, 151–166.
Mathur, R., Chand, S., & Tezuka, T. (2003). Energy Policy, 31, 319–331.
Adriano, D. C., Page, A. L., Elseewi, A. A., Chang, A. C., & Straughan, I. (1980). Journal of Environmental Quality, 9, 333–344.
Wong, M. H., & Wong, J. W. C. (1990). Agriculture, Ecosystems and Environment, 30, 251–259.
Rai, U. N., Pandey, K., Sinha, S., Singh, A., Saxena, R., & Gupta, D. K. (2004). Environment International, 30, 293–300.
Bosshard, P. P., Bachofen, R., & Brandl, H. (1996). Environmental Science and Technology, 30, 3066–3070.
Wu, H. Y. (2002). Master's thesis, National University of Singapore (NUS), Singapore.
Krebs, W., Brombacher, C., Bosshard, P. P., Bachofen, R., & Brandl, H. (1997). FEMS Microbiology Reviews, 20, 605–617.
Brandl, H., Bosshard, R., & Wegmann, M. (2001). Hydrometallurgy, 59, 319–326.
Brombacher, C., Bachofen, R., & Brandl, H. (1997). Applied Microbiology and Biotechnology, 48, 577–587.
Briand, L., Thomas, H., de la Vega Alonso, A., & Donati, E. (1999). Proceedings of the International Biohydrometallurgy Symposium (IBS), part A (pp. 263–271). Amsterdam: Elsevier.
Blaustein, B., Hauck, J. T., Olson, G. J., & Baltrus, J. P. (1993). Fuel, 72, 1613–1618.
Barth, G., & Gaillardin, C. (1997). FEMS Microbiology Reviews, 19, 219–237.
Fickers, P., Benetti, P. H., Waché, Y., Marty, A., Mauersberger, S., Smit, M. S., et al. (2005). FEMS Yeast Research, 5, 527–543.
Bankar, A. V., Kumar, A. R., & Zinjarde, S. S. (2009). Applied Microbiology and Biotechnology, 84, 847–865.
Margesin, R., & Schinner, F. (1997). FEMS Microbiology Ecology, 24, 243–249.
Zinjarde, S. S., & Pant, A. (2002). Marine Pollution Bulletin, 44, 118–121.
Jain, M. R., Zinjarde, S. S., Deobagkar, D. D., & Deobagkar, D. N. (2004). Marine Pollution Bulletin, 49, 783–788.
García, S., Prado, M., Dégano, R., & Domínguez, A. (2002). Journal of Biological Chemistry, 277, 37359–37368.
Strouhal, M., Kizek, R., Vacek, J., Trnkova, L., & Nemec, M. (2003). Bioelectronics, 60, 29–36.
Ito, H., Inouche, M., Tohoyama, H., & Joho, M. (2007). Biometals, 20, 773–780.
Agnihotri, M., Joshi, S., Kumar, A. R., Zinjarde, S., & Kulkarni, S. (2009). Materials Letters, 63, 1231–1234.
Bankar, A. V., Kumar, A. R., & Zinjarde, S. S. (2009). Journal of Hazardous Materials, 170, 487–494.
Papanikolaou, S., Galiotou-Panayotou, M., Fakas, S., Komaitis, M., & Aggelis, G. (2008). Bioresource Technology, 99, 2419–2428.
Nagpal, U. M. K., Bankar, A. V., Pawar, N. J., Kapadnis, B. P., & Zinjarde, S. S. (2011). Water, Air, and Soil Pollution, 215, 177–188.
Neumann, G., Veeranagouda, Y., Karegoudar, T. B., Sahin, Ö., Mäusezahl, I., Kabelitz, N., et al. (2005). Extremophiles, 9, 163–168.
Santhiya, D., & Ting, Y. (2006). Journal of Biotechnology, 121, 62–74.
Byers, B., & Goetsch, L. (1991). Methods in Enzymology, 194, 602–608.
Park, J. S., Taniguchi, S., & Park, Y. J. (2009). Chemosphere, 74, 320–324.
Alam, J., & Akhtar, M. N. (2011). International Journal of Emerging Trends in Engineering and Development, 1, 2249–6149.
Vatsal, A., Zinjarde, S. S., & Kumar, A. R. (2011). Yeast, 28, 721–732.
Seidel, A., Zimmels, Y., & Armon, R. (2001). Chemical Engineering Journal, 83, 123–130.
Dusane, D. H., Nancharaiah, Y. V., Venugopalan, V. P., Kumar, A. R., & Zinjarde, S. S. (2008). Water Science and Technology, 58, 1221–1229.
Idachaba, M. A., Nyavor, K., Egiebor, N. O., & Rogers, R. D. (2000). Journal of Hazardous Materials, B77, 133–147.
Xu, T., & Ting, Y. (2009). Enzyme and Microbial Technology, 44, 323–328.
Burgstaller, W., & Schinner, F. J. (1993). Biotechnology, 27, 91–116.
Grewal, H. S., & Kalra, K. L. (1995). Biotechnology Advances, 13, 209–234.
Hu, Y., Wang, G., Chen, G. Y. J., Fu, X., & Yao, S. Q. (2003). Electrophoresis, 24, 1458–1470.
Acknowledgments
AB wishes to thank the Council of Scientific and Industrial Research, India, for Senior Research Fellowship (CSIR-SRF). The authors thank University Grants Commission, India, for financial support to the Institute of Bioinformatics and Biotechnology, Department of Microbiology and Department of Physics. All the authors wish to thank to DMCB, University of Colorado, Colorado, USA, for providing the TEM facility and Dr. Thomas Giddings Jr. as well as Christina Clarissa, MCDB, University of Colorado, Boulder, Colorado, USA, for providing TEM services.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bankar, A., Winey, M., Prakash, D. et al. Bioleaching of Fly Ash by the Tropical Marine Yeast, Yarrowia lipolytica NCIM 3589. Appl Biochem Biotechnol 168, 2205–2217 (2012). https://doi.org/10.1007/s12010-012-9930-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9930-2