Abstract
Aspergillus niger culture supernatant is used for bioleaching process. Before starting bioleaching process, fly ash was washed with distilled water. This removed 100 % sodium, 47 % (±0.45) boron, 38.07 % (±0.12) calcium, 29.89 % (±0.78) magnesium, and 11.8 % (±0.05) potassium. The pH was reduced from 10.5 to 8.5 after water washing. During bioleaching process, around 100 % metal removal was achieved in 4 h for all metals except chromium 93 % (±1.18), nickel 83 % (±0.32), arsenic 78 % (±0.52), and lead 70 % (±0.20). The process parameters including temperature, shaking speed, and solid/liquid ratio were optimized for bioleaching process. Experiments were conducted to evaluate effect of fly ash on growth of mung bean (Vigna radiata). At 20 g/100 ml fly ash concentration no germination of V. radiata seeds was observed. With an increasing concentration of untreated fly ash, a gradual decrease in root/shoot length was observed. After bioleaching process 78 % (±0.19) germination of V. radiata was observed with 20 g/100 ml fly ash. This study will help to develop an efficient process to remove the toxic metals from fly ash.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal is one of the major fossil fuels for electricity generation. These coal-fired power plants generate enormous amounts of fly ash [1]. Incineration produces fly ash just as is the case when coal is combusted. The difference lies in the composition of the ash produced. The physical and chemical properties of coal ash are influenced by coal source, moisture, particle size, and type of coal-burning process [2, 3]. Fly ash is normally classified into two main categories based on the percentage of CaO and on the type of coal used for burning as class F and class C fly ash [4]. The chemical and physical characterization of municipal solid waste (MSW) fly ash will depend on the compositions of the raw MSW, the operational conditions, and the type of incinerator. The composition of MSW fly ash varies over time and from country to country, due to the differences in lifestyle and waste recycling processes of a country; the ash content will vary too [5]. Petroleum refineries generate large quantities of sludge containing metals, oil, and suspended solids. Incineration of this sludge produces ash containing toxic metals and sulfur [6]. The thermal power plant fly ash is generated by burning coal. Therefore its composition will be different from other fly ash source. As per available estimate for the year 2005, India generated highest quantities (112 million tons/year) of coal ash, while Denmark, Italy, and Netherlands generated lower quantities (two million tons/year) of coal ash [7]. Even though these power plants take precautionary measures, considerable amounts of fine fly ash particles are emitted to atmosphere due to the large amount of production of fly ash [3]. Also, the major portions of fly ash produced go for disposal in ash ponds and landfills which require big open spaces [8]. Such disposal may be a potential hazard to environment, since the fly ash contain several toxic elements, such as lead (Pb), zinc (Zn), cadmium (Cd), nickel (Ni), and cobalt (Co) [1]. Therefore, the treatment of fly ash for removal of toxic metals becomes a necessity. Also, fly ash can become a source for metal extraction within appropriate economic constraints [9]. Conventionally, thermal treatment, chloride evaporation [10], and chemical leaching [10, 11] are used in the detoxification or decontamination of incineration fly ash. Unfortunately, these techniques suffer from the main disadvantage of high-energy requirement, as well as the liability of hazardous chemical usage during the treatment. This leads to a negative impact on the environment [12]. Direct acid leaching process is used for recovering aluminum from coal fly ash. This process, which involves leaching in relatively intense conditions of reflux and high acid concentration, has not found commercial application yet. Sulfuric acid is often used as the leachant, in leaching processes, for recovery of metals. However, it is less amenable to subsequent process recovery steps and has inferior properties as a dissolving agent. Sulfuric acid forms insoluble sulfates such as CaSO4. This can hinder the leaching process by coating the CFA particles with precipitates of low permeability [9, 13]. Application of biohydrometallurgy for recovering valuable metals from fly ash can overcome these constraints [14]. The detoxified fly ash may be then safely deposited or utilized for construction purposes. The relatively low cost and mild conditions of the process make bioleaching more attractive compared with conventional technologies [9]. Lots of work has been done on use of microorganisms for bioleaching of metals from solid waste [14, 15]. Only few reports are available on the bioremediation of metals from fly ash. Seidel and Zimmels [9] showed mechanism involved in bioleaching of coal fly ash by Thiobacillus thiooxidans. Singh et al. [16] showed the feasibility of removal of environmentally sensitive trace elements, from the coal samples, using bacterial consortium. Roy et al. [17] used in situ bioprecipitation and sorption processes for immobilization of heavy metals. Bioleaching by spent microbial culture have been reported by several researchers [18, 19]. For industrial applications, bioleaching by spent culture is believed to be desirable to increase leaching efficiency [20]. Several researchers studied bioleaching of metals from MSW fly ash using Aspergillus niger [18, 21–23]. However, no report is available on use of fungal culture supernatant for remediation of metals from thermal power plant fly ash. Therefore, this study was planned to explore the potential of A. niger culture supernatant for solublization of metals from thermal power plant fly ash.
Apparent high economic cost of disposal and subsequent environmental management necessitate the safe utilization and disposal of fly ash. In view of this, utilization of fly ash to improve agriculture productivity would not only be a solution to the problem but might also decrease use of inorganic fertilizers [24]. Lots of work has been done on use of fly ash as a soil amendment to boost up crop production [7, 25, 26]. Increase in crop production will depend on proper amendments of fly ash, the method of application, soil types, and plant species coupled with regional practices. This has great limitations because of the high content of toxic concentrations of certain elements and heavy metals in fly ash [7]. Although it is a common practice to mix fly ash with soil for plant growth, very less data is available on direct effect of fly ash on plant growth. The present investigation was carried out to study phytotoxicity effect of fly ash on the growth of Vigna radiata. Also, efficiency of bioleaching process to remove phytotoxicity of fly ash was studied.
Materials and Methods
Growth of A. niger 34770 and Collection of Cell-Free Culture Supernatant
A. niger (BCRC 34770) was obtained from the Food Industry Research and Development Institute (FIRDI), Taiwan. Sucrose medium was used for the growth of fungi. The medium contained the following substances per liter of glass-distilled water: 100 g sucrose, 1.5 g NaNO3, 0.5 g KH2PO4, 0.025 g MgSO4·7H2O, 0.025 g KCl, and 1.6 g yeast extract [22, 27]. Cell-free culture supernatant was collected after 10 days as per the method of Jadhav and Hocheng [19].
Collection of Fly Ash
The fly ash samples were supplied by thermal power plant in Taiwan. The pH, moisture content, and specific gravity readings were provided by thermal power plant.
Determination of Metal Content of Fly Ash
To determine the total metal content of the fly ash, samples were digested with aqua regia as per the method of Ure [28]. This method was slightly modified to help better mixing and to avoid use of high temperature while using aqua regia. Ten-milliliter aqua regia was added to 1 g fly ash, in 100-ml beaker and agitated overnight at 150 rpm. Then, the contents were heated at 50 °C for 1 h. After acid leaching, the small amount of residue was removed by filtration. Sample was analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES).
Water Washing of Fly Ash
A portion of the fly ash was prewashed with distilled water. A 1 g fly ash sample was mixed with distilled water (100 ml) in a conical flask and stirred at 30 °C for 24 h. Aliquots of the water sample were taken and sent to metal analysis. The fly ash was allowed to settle, and the water was decanted to collect washed fly ash. The prewashed sample was dried at 60 °C overnight and subjected to bioleaching tests.
Bioleaching of Fly Ash by A. niger 34770 Culture Supernatant
Typically, 1 g of the prewashed fly ash sample was mixed with A. niger 34770 culture supernatant (100 ml) and incubated in a shaker incubator at 50 rpm and 30 °C for 6 h. The pH of supernatant was 1.1 (±0.02). Aliquots of the samples were withdrawn at 2, 4, and 6 h and filtered. The clear samples were sent for heavy metal content analysis by means of ICP.
Effect of Physicochemical Parameters on Bioleaching of Metals
A 1 g of the prewashed fly ash sample was mixed with culture supernatant (100 ml) in 250-ml flasks separately. These flasks were incubated at variable shaking speeds (0–200 rpm) at 30 °C for 4 h. Effect of varying temperature on bioleaching efficiency was studied. The prewashed fly ash samples were covered with 100 ml of culture supernatant in 250-ml flasks separately. For this study, the flask were incubated at various temperatures (30–50 °C) in a shaker incubator at 50 rpm shaking speed for 4 h. Variable concentrations (2–8 g) of fly ash were employed for this study. The prewashed fly ash sample was mixed with culture supernatant (100 ml) in 250-ml flasks separately. These flasks were incubated at 50 rpm shaking speed at 30 °C for 4 h. The samples were sent for heavy metal content analysis by means of ICP.
Plant Material and Phytotoxicity Study
The seeds of mung bean (V. radiata) were collected from local market. The germination index which combined germination and root growth has proven to be very sensitive parameter. This will help to assess low toxicity (which affect root growth) and increased toxicity (which affect germination) [29]. Therefore, in the present study, the effect of increasing fly ash concentration on germination index has been studied. The phytotoxicity bioassay was evaluated using the seed germination technique [30]. The seeds were germinated in sterile 10-cm Petri dishes, layered with filter paper. Seeds were sterilized according to Jadhav et al. [31] before transferring to the surface of the paper in the Petri dishes (ten seeds per plate). The germination index was determined using the values of relative seed germination and relative root elongation [30].
Analytical Methods
After exposure to the leaching solution, the suspension was filtered. It was acidified using nitric acid. Then, the clear solution was used for metal analysis. The concentration of metals in the leach liquors was analyzed by Kontron S-35, ICP-OES.
Results and Discussion
Properties of Fly Ash and Water Washing Treatment
The thermal power plant fly ash used in this study was gray to black in color. The moisture content of fly ash was 0.17 %, while the specific gravity was 2.46. It has alkaline pH (10.5). Metal content of fly ash is shown in Table 1. Total 19 metals were found in fly ash. Among these 19 metals, manganese (Mn) (66 ± 1.00 μg/g) and magnesium (Mg) (50.34 ± 0.95 μg/g) were found in higher amounts while amounts of titanium (Ti) (0.80 ± 0.01 μg/g) and Cd (0.35 ± 0.015 μg/g) were the lowest. Before using this alkaline fly ash for bioleaching experiment, water washing treatment was carried out. This reduced pH of fly ash to 8.5 from 10.5. Out of 19 metals, some metals were also dissolved during water washing process. Sodium (Na) (100 %), boron (B) (47 ± 0.45 %), calcium (Ca) (38.07 ± 0.12 %), Mg (29.89 ± 0.78 %), and potassium (K) (11.8 ± 0.05 %) were dissolved in water (data not shown). These results are comparable with Wang et al. [32] which showed more than 50 % of potassium and sodium extraction after water washing of fly ash.
Bioleaching of Fly Ash by A. niger 34770 Culture Supernatant
Various methods have been employed for leaching of metals from fly ash. Less attention has been paid to the possible effectiveness of bioleaching process for recovery of metals from thermal power plant fly ash [1]. Effective leaching of metals from fly ash using T. thiooxidans [13] and tropical marine yeast, Yarrowia lipolytica NCIM 3589 [12] has been reported previously. These studies were carried out by incubating microorganisms with fly ash for metal leaching. However, this kind of process has a drawback. Alkaline nature of fly ash and the high metal concentrations in it can inhibit microbial growth and thereby decrease the metal extraction efficiency [13, 33, 34]. Therefore, in the present study, the microbial cells were separated from metal leaching process. The bioleaching system studied in the present work use A. niger 34770. Organic acid production by this fungus was studied previously. It produced only citric acid using sucrose as a carbon source. A. niger 34770 produced 20 g/l citric acid at 4.0 pH, 25 °C temperature, and 120-rpm shaking speed, in 10 days. The pH of the medium decreased from 4.0 to 1.1 (±0.02). The citric acid containing culture supernatant of this fungus was used for biomachining of various metals [27]. This previous study showed application of A. niger 34770 culture supernatant in the two-step biomachining of metals. Hence, in the present study, the fungus was first cultured in sucrose medium for 10 days and then the culture supernatant was used for bioleaching of metals from the fly ash. Before bioleaching process, water washing was carried out. Na was completely removed from fly ash during water washing process. Therefore, in bioleached solution, no Na was observed. The effect of time on bioleaching efficiency was studied using 1 g fly ash and 100 ml culture supernatant. Around 60 % (±0.38) metal extraction was achieved for Cd in the first 2 h. For metals Co, Se, Ca, Cu, Mn, Fe, Al, Zn, Ti, V, and B, the metal extraction varied in between 58 and 51 %. Lowest metal extraction was achieved for Pb (around 32 ± 0.77 %) in the first 2 h. For bioleaching process, 4-h incubation time was found optimum. During bioleaching process, around 100 % dissolution of metals from Cd to Mg was observed, while for chromium (Cr), nickel (Ni), arsenic (As), and lead (Pb) around 93 (±1.18), 83 (±0.32), 78 (±0.52), and 70 % (±0.20) dissolution of metals was observed in 4 h, respectively (Fig. 1). Incubation time was further increased to 6 h to find out whether metal extraction increased or not for Cr, Ni, As, and Pb. An increase in incubation time did not increase the metal extraction. Therefore, 4-h incubation time was selected for further study. In the present study, the time required (4 h) for bioleaching of metals was less as compared to Seidel et al. [13] (required 3 weeks for extraction of aluminium and iron) and Bankar et al. [12] (required 15 days for extraction of various metals). One possible reason behind this might be the presence of fly ash in culture broth. This reduced microbial metabolite production which thereby affected metal dissolution [35]. Hence, two-stage application of A. niger 34770 culture supernatant for metal bioleaching is advantageous as compared to direct application of microbial cells.
Effect of Shaking Speed on Metal Bioleaching
Effect of shaking speed on metal bioleaching from fly ash was studied. It was found that 50 rpm was an optimum shaking speed for dissolution of metals. Decrease in metal dissolution was observed below and above 50-rpm shaking speed (Fig. 2). These results are in contrast to Bankar et al. [12]. They obtained metal dissolution at 200 rpm. In their study, they incubated microbial cells with fly ash. This might have affected metal dissolution process in their study. According to Bosshard et al. [15], incubation conditions of the leaching step can be different from one in the growth phase.
Effect of Temperature on Metal Bioleaching
Three temperatures, viz., 30, 40, and 50 °C were used to study the effect of temperature on bioleaching efficiency. The results of the present study show that varying temperatures have no drastic effect on bioleaching efficiency (data not shown). The bioleaching of metals from fly ash by A. niger 34770 culture supernatant is independent of temperature. In a previous study, authors obtained similar results. In that study A. niger 34770 culture supernatant was used for biomachining of tin metal. Complete metal removal was observed from workpieces at all the temperatures used [27]. Saito et al. [36] also reported similar result. It is always easy to control the processes at low temperatures. Therefore, in further experiments, 30 °C temperature is used.
Effect of Increasing Concentration of Fly Ash on Metal Bioleaching
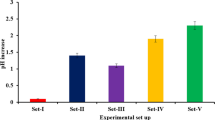
Dissolution of metals from fly ash at different solid/liquid (w/v) ratios is shown in Fig. 3. Different effects of the solid/liquid ratio were observed for the percentage removal of different metals from the fly ash. For example, at solid/liquid ratios 1:100 (g/ml) and 2:100 (g/ml), metal dissolution of around 100 (±0.50) and 75 % (±0.32) for Cd, 100 (±0.16) and 91 % (±0.79) for Co, 100 (±0.66) and 80 % (±0.63) for Se were observed, respectively (Figs. 1 and 3). Surprisingly, dissolution of Ni (89 ± 0.91 %), As (86 ± 0.31 %), and Pb (99 ± 0.75 %) increased at 2:100 (g/ml) fly ash concentration. A further increase in the value of the solid/liquid ratio led to a decrease in the metal dissolution (Fig. 3). Decrease in metal dissolution due to an increase in fly ash (from municipal waste incineration) concentration has been reported by several researchers [15, 18, 37]. Wu and Ting [18] showed that inhibition of the fungal growth occurred in one-step bioleaching at higher pulp densities. This occurred due to the higher concentration of toxic metals. An increase in the pulp density will increase the pH of the fly ash suspension. Therefore, more acid is required to solubilize increased concentration of metals.
Removal of Phytotoxicity of Fly Ash by Bioleaching
In the present study, the phytotoxicity of fly ash toward V. radiata has been studied. It was observed that with an increase in fly ash concentration, there was a gradual decrease in germination index. At 20 g/100 ml fly ash concentration, 0 % germination index was observed, while 100 % germination index was observed in control having only distilled water (Fig. 4). Fly ash was collected after bioleaching process and applied at 20 g/100 ml concentration to study the toxicity effect on V. radiata seeds. In this case, around 78 % (±0.19) germination index was observed as shown in Fig. 4. Similar results were reported by Vollmer et al. [38]. In another study, Teaca and Bodirlau [39] showed that the germination rate of wheat seeds was decreased with increasing application of fly ash as compared to control. In similar way, Kumari [40] found that the high amount of deposition of fly ash during sowing season reduced seed germination as well as seedling growth and development. This is considered due to the higher concentration of trace elements such as Cu, Co, Ni, Se, Al, and Cr. At higher application rates, these metals delayed or inhibited the germination process [41].
Effect of increasing concentration of fly ash on root and shoot length of V. radiata was also studied. Effect of untreated and treated 10 g/100 ml fly ash concentration on root and shoot length is shown in Fig. 5. It clearly shows that this concentration affected the V. radiata growth. In control, with only distilled water, average root and shoot length was 8.51 (±0.31) and 19.6 mm (±0.29), respectively. At 10 g/100 ml fly ash concentration (untreated), average root and shoot length observed was 0.35 (±0.38) and 3.3 mm (±0.11), respectively, while bioleached 10 g/100 ml fly ash showed 6.68 and 18.59 mm, average root and shoot length, respectively (Fig. 6). Further increase in fly ash (untreated) concentration up to 20 g/100 ml affected V. radiata growth drastically and no root and shoot observed. The bioleached 20 g/100 ml fly ash showed 5.86 (±0.24) and 17.89 mm (±0.16), average root and shoot length, respectively (Fig. 6). Thus, these results clearly indicate that untreated fly ash is toxic at higher concentrations and the method described in the present study help to remove it. These results are in agreement with Kumari [40]. Kumari [40] showed that excess levels of fly ash induced hazardous effects in plant roots and the rhizosphere. Also, Kumari [40] reported that the toxic effects of specific fly ash constituents such as B, As, Se, Mo, Al, and Cd caused stunted growth, weak feeling of seeds, and early etiolation of leaves. In contrast to the results of the present study, some researchers showed that amendment of fly ash to soil was beneficiary for plant growth up to certain concentrations [24–26]. In the present study, much smaller concentrations of fly ash were found toxic for V. radiata growth. This is because in the present study, fly ash is applied to V. radiata seeds by mixing in distilled water. An amendment of fly ash with soil for the previous studies might have helped to reduce toxicity of higher concentrations of fly ash. Even though untreated fly ash is found toxic for V. radiata growth, after bioleaching, fly ash did not show such effect. This indicates effectiveness of present bioleaching method for removal of phytotoxicity of fly ash.
Conclusion
A two-step bioleaching process for metal extraction from thermal power plant fly ash was investigated in the present work. Organic acid (citric acid) present in A. niger 34770 culture supernatant promoted effective removal of metals from fly ash due to its strong chelating ability.
Optimum metal removal was achieved under the following conditions: 4-h incubation time, 50-rpm shaking speed, 30 °C temperature, and 1 g/100 ml solid/liquid ratio. The present method is found to be advantageous as compared to reported bioleaching processes, since it needs less time for metal extraction. The presence of heavy metals in fly ash poses an environmental threat. The results of the present study showed that in the seed germination, the root and shoot growth of V. radiata was drastically affected by the presence of fly ash. The bioleaching process presented here was found to be effective in reducing toxicity of fly ash. This approach will help to utilize fly ash in agriculture and thereby to overcome pollution created by fly ash.
References
Meawad, A., Bojinova, D., & Pelovski, Y. (2010). An overview of metals recovery from thermal power plant solid wastes. Waste Management, 30, 2548–2559.
Soco, E., & Kalembkiewicz, J. (2009). Investigations on Cr mobility from coal fly ash. Fuel, 88, 1513–1519.
Bhangare, R., Ajmal, P., Sahu, S., Pandit, G., & Puranik, V. (2011). Distribution of trace elements in coal and combustion residues from five thermal power plants in India. International Journal of Coal Geology, 86, 349–356.
Adriano, D., Weber, J., Bolan, N., Paramasivam, S., Koo, B., & Sajwan, K. (2002). Effects of high rates of coal fly ash on soil, turfgrass, and groundwater quality.Water, Air, and Soil Pollution, 139, 365–385.
He, P., Zhang, H., Zhang, C., & Lee, D. (2004). Characteristics of air pollution control residues of MSW incineration plant in Shanghai. Journal of Hazardous Materials, 116, 229–237.
Moura, M., Ribeiro, B., Sousa, J., & Costa-Ferreira, M. (2008). Leaching of petroleum refinery ash by acidophilic sulfur-oxidizing microbial cultures. Bioresource Technology, 99, 8840–8843.
Pandey, V., & Singh, N. (2010). Application of fly ash on the growth performance and translocation of toxic heavy metals within Cajanus cajan L.: Implication for safe utilization of fly ash for agricultural production. Agriculture Ecosystems & Environment, 136, 16–27.
Bhattacharjee, U., & Kandpal, P. (2002). Potential of fly ash utilization in India. Energy, 27, 151–156.
Seidel, A., & Zimmels, Y. (1998). Mechanism and kinetics of aluminum and iron leaching from coal fly ash by sulfuric acid. Chemical Engineering Science, 53, 3835–3852.
Tateda, M., Ike, M., & Fujita, M. (1998). Comparative evaluation of processes for heavy metal removal from municipal solid waste incineration fly ash. Journal of Environmental Sciences, 10, 458–465.
Hong, K., Tokunaga, S., Ishigami, Y., & Kajuichi, T. (2000). Extraction of heavy metals from MSW incinerator fly ash using saponins. Chemosphere, 41, 345–352.
Bankar, A., Winey, M., Prakash, D., Kumar, A., Gosavi, S., Kapadnis, B., Zinjarde, S. (2012). Bioleaching of fly ash by the tropical marine yeast, Yarrowia lipolytica NCIM 3589. Applied Biochemistry and Biotechnology, 168, 2205–2217.
Seidel, A., Zimmels, Y., & Armon, R. (2001). Mechanism of bioleaching of coal fly ash by Thiobacillus thiooxidans. Chemical Engineering Journal, 83, 123–130.
Brombacher, C., Bachofen, R., & Brandl, H. (1998). Development of a laboratory-scale leaching plant for metal extraction from fly ash by Thiobacillus strains. Applied and Environmental Microbiology, 64, 1237–1241.
Bosshard, P., Bachofen, R., & Brandl, H. (1996). Metal leaching of fly ash from municipal waste incineration by Aspergillus niger. Environmental Science and Technology, 30, 3066–3070.
Singh, P., Singh, A., Kumar, A., & Singh, M. (2012). Mixed bacterial consortium as an emerging tool to remove hazardous trace metals from coal. Fuel, 102, 227–230.
Roy, S., Vanbroekhoven, K., Dejonghe, W., & Diels, L. (2006). Immobilization of heavy metals in the saturated zone by sorption and in situ bioprecipitation processes. Hydrometallurgy, 83, 195–203.
Wu, H., & Ting, Y. (2006). Metal extraction from MSW incinerator fly ash-Chemical leaching and fungal bioleaching. Enzyme and Microbial Technology, 38, 839–847.
Jadhav, U., & Hocheng, H. (2013). Extraction of silver from spent silver oxide-zinc button cells by using Acidithiobacillus ferrooxidans culture supernatant. Journal of Cleaner Production, 44, 39–44.
Hocheng, H., Chang, J., & Jadhav, U. (2012). Micromachining of various metals by using Acidithiobacillus ferrooxidans 13820 culture supernatant experiments. Journal of Cleaner Production, 20, 180–185.
Yang, J., Wang, Q., Wang, Q., & Wu, T. (2009). Heavy metals extraction from municipal solid waste incineration fly ash using adapted metal tolerant Aspergillus niger. Bioresource Technology, 100, 254–260.
Xu, T., & Ting, Y. (2004). Optimization on bioleaching of incinerator fly ash by Aspergillus niger use of central composite design. Enzyme and Microbial Technology, 35, 444–454.
Xu, T., Ramanathan, T., & Ting, Y. (2014). Fungal bioleaching of incineration fly ash: Metal extraction and modeling growth kinetics. Biotechnology Reports, 3, 8–14.
Singh, L., & Siddiqui, Z. (2003). Effects of fly ash and Helminthosporium oryzae on growth and yield of three cultivars of rice. Bioresource Technology, 86, 73–78.
Gupta, M., Kumar, A., & Yunus, M. (2000). Effect of fly-ash on metal composition and physiological responses in Leucaena leucocephala (LAMK.) DE. WIT. Environmental Monitoring and Assessment, 61, 399–406.
Pandey, V., Abhilash, P., Upadhyay, R., & Tewari, D. (2009). Application of fly ash on the growth performance and translocation of toxic heavy metals within Cajanus cajan L.: Implication for safe utilization of fly ash for agricultural production. Journal of Hazardous Materials, 166, 255–259.
Jadhav, U., & Hocheng, H. (2014). Use of Aspergillus niger 34770 culture supernatant for tin metal removal. Corrosion Science, 82, 248–254.
Ure, A. (1995). Heavy metals in soils (pp. 58–102). London: Blackie Academic and Professional.
Tam, N., & Tiquia, S. (1994). Assesing toxicity of spent sawdust pig-litter using seed germination technique. Resources, Conservation and Recycling, 11, 261–264.
Tiquia, S. (2010). Reduction of compost phytotoxicity during the process of decomposition. Chemosphere, 79, 506–512.
Jadhav, U., Kadu, S., Thokal, N., Padul, M., Dawkar, V., Chougale, A., et al. (2011). Degradation of tannic acid by cold-adapted Klebsiella sp NACASA1 and phytotoxicity assessment of tannic acid and its degradation products. Environmental Science and Pollution Research, 18, 1129–1138.
Wang, K., Chiang, K., Lin, K., & Sun, C. (2001). Effects of a water-extraction process on heavy metal behavior in municipal solid waste incinerator fly ash. Hydrometallurgy, 62, 73–81.
Brandl, H., Bosshard, R., & Wegmann, M. (2001). Computer-munching microbes: metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy, 59, 319–326.
Xiang, Y., Wu, P., Zhu, N., Zhang, T., Liud, W., Wu, J., et al. (2010). Bioleaching of copper from waste printed circuit boards by bacterial consortium enriched from acid mine drainage. Journal of Hazardous Materials, 184, 812–818.
Wang, Q., Yang, J., Wang, Q., & Wu, T. (2009). Effects of water-washing pretreatment on bioleaching of heavy metals from municipal solid waste incinerator fly ash. Journal of Hazardous Materials, 162, 812–818.
Saito, C., Okada, H., Titus, M., Yoshioka, T., & Mizoguchi, T. (2007). Leaching of heavy metals from fly ash generated from gasification and melting furnace for municipal solid wastes by organic acids. Japan Society of Waste Management Expert, 18, 157–166.
Ishigaki, T., Nakanishi, A., Tateda, M., Ike, M., & Fujita, M. (2005). Bioleaching of metal from municipal waste incineration fly ash using a mixed culture of sulfur-oxidizing and iron-oxidizing bacteria. Chemosphere, 60, 1087–1094.
Vollmer, A., Turner, F., Straughan, I., & Lyons, C. (1982). Effects of coal precipitator ash on germination and early growth of desert annuals. Environmental and Experimental Botany, 2, 409–413.
Teaca, C., & Bodirlau, R. (2008). Assessment of toxicity of industrial waste using crop plant assays. Bioresources, 3, 1130–1145.
Kumari, V. (2009). Physicochemical properties of fly ash from thermal power station and its effect on vegetation. Global Journal of Environmental Research, 3, 102–105.
Singh, S., Kulshreshtha, K., & Ahmed, K. (1997). Impact of fly ash soil amendment on seed germination, seedling growth and metal composition of Vicia faba L. Ecological Engineering, 9, 203–208.
Acknowledgments
The current research is supported by the National Science Council of Taiwan under contract 100-2221-E-007-015-MY3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jadhav, U.U., Hocheng, H. Analysis of Metal Bioleaching from Thermal Power Plant Fly Ash by Aspergillus niger 34770 Culture Supernatant and Reduction of Phytotoxicity During the Process. Appl Biochem Biotechnol 175, 870–881 (2015). https://doi.org/10.1007/s12010-014-1323-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1323-2