Abstract
Using both polar and low polar organic solvents (DMSO and toluene) as screening stress, a solvent-stable bacterium Burkholderia cepacia RQ3 was newly isolated. An organic solvent-stable lipase from strain RQ3 was purified in a single step with 50.1 % recovery by hydrophobic chromatography. The purified lipase was homogenous on SDS-PAGE and had an apparent molecular mass of 33 kDa. The gene of lipase RQ3 with an open reading frame of 1095 bp encoding 364-amino acid residues was cloned. The optimal pH and temperature for lipase activity were 9.0 and 40 °C. The lipase was stable in a wide pH range of 6.0–10.0 and at temperature below 50 °C. Strikingly, all the tested hydrophilic and hydrophobic organic solvents significantly extended the half-life of lipase RQ3 compared with that in a solvent-free system, which indicated that lipase RQ3 showed a broad solvent tolerance to various organic solvents. The lipase demonstrated excellent enantioselective transesterification toward the (S)-1-phenylethanol with a theoretical conversion yield of 50 % and ee p of 99.9 %, which made it an exploitable biocatalyst for organic synthesis and pharmaceutical industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipases constitute one of the most important groups of industrial enzymes, which possess not only the natural ability to hydrolyze triacylglycerols in aqueous environment, but also to catalyze esterification and transesterification in non-aqueous media [1]. Especially, there are numbers of advantages of conducting enzymatic conversions in organic solvents such as shifting of thermodynamics equilibrium in favor of synthesis, improving the solubility of substrates and controlling substrate specificity by solvent engineering [2]. Solvent-stable lipases are obligatory in biotechnological applications, specifically in the industrial scale production of chiral compounds as building blocks for the synthesis of pharmaceuticals and agrochemicals [3]. Unfortunately, most enzymes, including lipases, are inactivated or give very low reaction rates in organic solvent [4]. Several strategies have been performed to overcome this limitation such as molecular modification, immobilization and protein engineering [5, 6]. Alternatively, it has been proposed that instead of modifying enzyme to increase solvent stability, it would be more desirable to screen for naturally stable enzymes for industrial application [1, 7]. To data, although several solvent-tolerant lipases have been purified and characterized from various microbes, such as Pseudomonas and Bacillus, most of these lipases were found to be destabilizing in polar solvents [8–10]. Another, organic reactions performed by lipases are enormous. So far, the number of solvent-tolerant lipases is inadequate, thus it becomes crucial to explore novel lipases with high activity in different types of organic solvents to expand their application in practical catalysis.
In this study, a bacterium producing a lipase with broad solvent stability was screened from soil samples and identified as Burkholderia cepacia RQ3. The characteristics of this lipase, especially its stability in the presence of various organic solvents, were investigated. In addition, the application of the lipase for chiral resolution of 1-phenylethanol in non-aqueous system was also investigated.
Materials and methods
Isolation of solvent-stable lipase-producing strains
Soil samples were collected from oil- and chemical-contaminated areas. Toluene or dimethyl sulfoxide (DMSO) was added to the medium at a concentration of 20 % (v/v) as selective pressure. The screening procedure and culture conditions followed the method described by Ji [9]. The strain secreting the lipase with broad organic solvent tolerance was selected and identified based on the analysis of the Microlog Microbial Identification System (Biolog, USA) and 16S rDNA sequence BLAST in the Genebank Data Library.
Culture conditions for producing lipase
The isolated strain was cultured in lipase-producing medium consisting of (w/v) 1.0 % soya bean powder, 0.5 % sucrose, 0.2 % KH2PO4, 0.05 % MgSO4, 0.05 % (v/v) Triton X-100 and 0.5 % (v/v) sunflower oil, with a pH of 8.0. The incubations were carried out at 30 °C with shaking at 180 rpm. After 36 h of incubation, the culture supernatant was collected by centrifugation and used as the crude lipase.
Assay of lipase activity and protein concentration
Lipase activity was measured using p-nitrophenyl palmitate as the substrate as described by Cao [7]. One unit of lipase activity was defined as the amount of enzyme that produced 1 µmol of p-nitrophenol under standard assay conditions.
The protein concentration was determined by the Bradford dye method using bovine serum albumin as a standard [11].
Purification and cloning of the lipase
The culture supernatant was dialyzed in 50 mmol/L sodium phosphate buffer (pH 7.0, containing 0.8 mol/L (NH4)2SO4) overnight. The dialyzed enzyme solution was loaded onto a Phenyl-Sepharose FF column (1.2 cm × 10 cm) equilibrated with the same buffer. The bound proteins were eluted with an ethyl alcohol gradient from 20 to 50 % in 50 mmol/L sodium phosphate buffer. Fractions with lipase activity were collected. Then, the purified lipase was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to the method of Laemmli [12].
The coomassie-stained band of the purified lipase RQ3 was excised from the gel and submitted to the National Center of Biomedical Analysis for LC/MS–MS analysis. The sequences of fragments were submitted to the Mascot program for possible identity matching. Since some fragments of lipase RQ3 were identical to the lipase from Burkholderia sp. MC16-3 [13], the forward primer 5′-GAGTCGTGATTCACTCCCGCATT-3′ and the reverse primer 5′-AGCCCCACGACACCATAGACCA-3′ were designed based on the nucleotide sequence of Bukholderia sp. MC16-3 (NCBI Accession NO. AAV34203.1). The PCR amplification of the lipase was carried out using the genomic DNA of strain RQ3 as a template. The PCR product was purified and ligated into a PMD-18T simple vector and then sequenced. Database homology search of obtained amino acid sequences was carried out using BLAST in GenBank at NCBI. Multiple sequence alignment analysis was performed using Clustal X2 program.
Effect of temperature and pH on activity and stability of lipase RQ3
The effect of temperature on lipase activity was studied by carrying out the enzyme reactions at different temperatures in the range of 20–60 °C at pH 7.0. The thermostability of lipase was measured by incubating lipase solutions at various temperatures ranging from 30 to 60 °C at pH 7.0 for 1 h and the remaining activity was measured by the method in lipase assay.
To investigate the optimum pH, lipase activity was determined at 40 °C at various pH values (6.0–10.0). The stability of the lipase in the range pH 3.0–12.0 was examined by incubating solutions of the enzyme for 1 h at different pH values and the residual activity was measured according to the pNPP method described above.
Effects of metal ions on the activity of the lipase
To study the effects of metal ions on enzyme activity, purified lipase was pre-incubated with 1 or 10 mmol/L of various metal ions and EDTA for 1 h at 40 °C. The residual activity was assayed using the standard method.
Substrate specificity of the lipase
Substrates p-nitrophenyl fatty acid esters of varying chain length (C2, C4, C8, C10, C14, C16, and C18) were used as substrates at the final concentration of 0.3 mg/mL and the lipase activity was measured under standard assay conditions [7].
Effect of organic solvents on lipase RQ3
The effects of organic solvents at 25 % concentration on the stability of the lipase were investigated following the method defined by Ogino [14]. The purified enzyme dissolved in 50 mmol/L sodium phosphate buffer (pH 7.0) was filter sterilized (using cellulose acetate membrane, 0.22 μm). One milliliter of organic solvent was added into 3 mL of filtrate in a sealed glass vial. The mixture was incubated at 40 °C with shaking at 150 rpm. The residual lipase activity was determined under standard assay conditions.
Kinetic resolution of racemic 1-phenyl ethanol by the lipase
The culture supernatant containing extracellular lipase was obtained by centrifugation at 4 °C and 10,000×g for 20 min. The supernatant was collected in a glass breaker and to it chilled acetone was added slowly, with continuous stirring, up to 50 % (v/v) concentration and kept at 4 °C for 2 h. The precipitates were harvested by centrifugation at 4 °C and 10,000×g for 30 min. The precipitates were then dried by air and used for kinetic resolution of racemic 1-phenyl ethanol.
The transesterification reaction of racemic 1-phenyl ethanol was carried out in a 4 ml of shaking flask and incubated on orbital shaker (200 rpm) at 40 °C. The reaction mixture consisted of 1 ml n-hexane, 300 mmol/L vinyl acetate, 200 mmol/L racemic 1-phenylethanol and 20 mg lipase power. After the completion (20 h) of reaction, an aliquot of sample was collected and analyzed by HPLC equipped with a chiracel OJ column (250 mm × 4.6 mm) at 35 °C [7].
Results and discussion
Isolation of a solvent-stable lipase-producing strain
Solvent-tolerant bacteria are a relatively novel group of extremophilic microorganisms adapted to living in environments polluted by organic solvents. Therefore, some of the enzymes from these microbes are naturally stable in organic solvents, especially extracellular enzymes such as proteases and lipases. However, in the process of screening solvent-tolerant microorganisms, polar organic solvent (Log P < 1) had rarely been used for enrichment due to its extreme toxicity to cells and enzymes [8, 15]. In this study, we selected both hydrophilic (DMSO, Log P −1.35) and hydrophobic solvents (toluene, Log P 2.5) as screening stress to obtain the lipase with broad solvent stability. Sixty strains producing organic solvent-stable lipases were obtained from more than 200 soil samples. Of these, strain RQ3 was selected for further research as it produced the lipase with highest activity and the crude lipase was quite stable in hydrophobic and hydrophilic organic solvents. On the basis of the Biolog identification, strain RQ3 was identified as Burkholderia cepacia (the value of Sim 0.58). The strain RQ3 was also identified by sequencing of 16S rDNA gene (GeneBank Accession No. KP064036). BLAST search revealed that strain RQ3 exhibited 99 % sequence homology with complete 16S rDNA sequence of B. cepacia SE-1. Combining with the results of Biolog and 16S rDNA analysis, strain RQ3 was identified as Burkholderia cepacia and deposited in CCTCC (ID M2010330). Recently, lipases from Burkholderia sp. have attracted much attention due to its remarkable properties like broad substrate adaptability and high enantioselectivity [15–18]. However, only a few have been reported to exhibit high solvent stability in polar solvent. Therefore, we further purified and characterized the lipase with good solvent stability from B. cepacia RQ3 for its application in organic catalysis.
Purification and cloning of the lipase
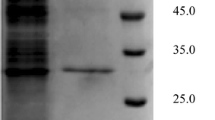
The extracellular lipase from strain RQ3 was purified to homogeneity simply by one-step Phenyl-Sepharose FF hydrophobic chromatography (Table 1). Approximately, 15.5-fold purification with 50.1 % recovery was achieved. Compared with most other purified lipases, the purification of lipase RQ3 was simple and with high recovery. Ogino et al. reported purification of P. aeruginosa LST-03 lipase by four successive steps with 12.6 % recovery [14]. B. ambifaria YCJ01 lipase was purified to 24.2-fold with 12.5 % recovery using anion exchange and hydrophobic interaction chromatography [15]. Relative low purification yields of 4.8 % for lipase from B. cepacia ATCC 25416 [19] and 0.96 % for lipase from B. multivorans V2 [2] were also reported. Rahman et al. succeeded in getting higher recovery of 52 % of the lipase from Pseudomonas sp. strain S5, but they employed affinity chromatography in combination with ion-exchange chromatography [20]. Simple purification steps and high recovery will be conducive to the lager-scale application of lipase RQ3. The apparent molecular weight of lipase RQ3 was estimated to be 33 kDa by SDS-PAGE analysis (Fig. 1), which was close to many known lipases from Burkholderia sp. with 29–35 kDa molecular weights [15–19].
The internal fragments of lipase RQ3 were identified by trypsin digestion and sequencing. These sequences, such as YPIILVHGLSGTDK, and VNLVGHSQGGLSSRYVAAVAPDLVASVTTIGTPHR and WNHLDEINQLLGVRGAYAEDPVAVIR, showed high identity to that of the lipase from Burkholderia sp. MC16-3 [13]. Based on the sequence of the lipase from strain MC16-3, the gene encoding lipase RQ3 was cloned. The full-length open reading frame of lipase RQ3 was composed of 1095 bp and encoded 364 amino acids including an N-terminal signal sequence of 44 amino acids (Fig. 2). The conserved region of mature lipase included the penta-peptide motif, Gly-His-Ser-Gln-Gly containing an active serine residue (Ser 87). Furthermore, Ser 87, Asp 264 and His 286 formed a catalytic center, as shown in other Burkholderia sp. lipases [13, 19, 21]. In addition, the lipase RQ3 showed one residue (156 A/T) and two residues (156 A/T, 281 N/S) difference with that of Burkholderia sp. MC 16-3 and B. cepacia; however, the characteristics of the lipases from strains MC16-3 and B. cepacia were not reported [13, 22]. Although there are only six residues’ differences (128 A/T, 150 T/A, 156A/T, 256 I/V, 274 Y/F, 311 H/R) between lipase RQ3 and LipA, the optimal pH and temperature of LipA from strain ATCC 25416 were observed at pH 11 and 60 °C [19]. On the other hand, high-polar organic solvents such as various alcohols inhibited the LipA [19], and the biochemical properties were distinct to those of lipase RQ3.
Multiple alignment of amino acid sequences of lipase RQ3 with several other lipases. Abbreviations and accession numbers of lipases are as follows: lipase RQ3 from B. cepacia RQ3, lipase MC16-3 from Burkholderia sp. MC16-3 (accession no. AAV34203.1), lipase Lips from B. cepacia (accession no. WP 027791104.1), lipase 99-2-1 from Burkholderia sp. 99-2-1 (accession no. AAV34204.1), lipase LipA from B. cepacia ATCC 25416 (accession no. ADT80785.1). Active-site residues are indicated by stars (*)
Effects of temperature and pH on activity and stability of lipase RQ3
The optimal temperature of lipase RQ3 was 40 °C (Fig. 3), similar to that of many other organic solvent-stable lipases. The lipase LST-03 exhibited a maximum activity at 37 °C [14]. The optimal temperature of lipases from P. aeruginosa PseA and B. cepacia ATCC 25609 was 40 °C [8, 17]. Dandavate showed lipase V2 having optimum activity at 45 °C [2]. However, the optimum temperature of lipase YCJ01 and lipase LipA was 60 °C [15, 19]. The lipase RQ3 was stable below 50 °C and retained more than 85 % initial activity after incubation at 50 °C for 1 h (Fig. 3). The lipase from B. cepacia ATCC25609 showed lower stability at 50 °C with a half-life of 54 min [17].
Effect of temperature on lipase activity and stability. The effect of temperature on lipase activity (filled square) was studied by performing the enzymatic reaction at different temperatures in the range 20–60 °C in 50 mmol/L sodium phosphate buffer (pH 7.0). Thermostability (filled circle) was measured by incubating the lipase in 50 mmol/L sodium phosphate buffer (pH 7.0) at various temperatures for 1 h
The lipase RQ3 showed relatively high activity under alkaline conditions, and exhibited optimum activity at pH 9.0 (Fig. 4). The enzyme was fairly stable over a wide pH range of 6.0–10.0 for 1 h and retained 62 % of its original activity after treatment with pH 11.0 buffer. Most microbial lipases exhibited narrow pH stabilities. The lipases from B. cepacia ATCC 25609 and LP08 were stable in the pH range of 9.0–10.0 and 8.0–9.0, respectively [17, 23]. Yao reported the stability of lipase YCJ01 was in the pH range of 6.0–8.0 [15]. Lipases LST-03 and PseA showed high stability at pH 5.0–8.0 and 6.5–8.0 [8, 14]. It was clearly evident that lipase RQ3 showed excellent stability across a broad pH range, which would be beneficial to the application of this lipase in a complex industrial environment.
Effect of pH on lipase activity (solid) and stability (hollow). Lipase activity was measured at different pH values at 40 °C. Lipase stability was evaluated following incubation at 40 °C for 1 h in different pH buffers. The buffer systems were as follows: 50 mmol/L citric acid/sodium citrate (pH 3.0–6.0) (traingle), 50 mmol/L Na2HPO4/KH2PO4 (pH 6.0–8.5) (square, filled square), 50 mmol/L Gly/NaOH (pH 8.6–10.6) (circle, filled circle) and 50 mmol/L Na2HPO4/NaOH (pH 10.0–12.0) (inverted triangle)
Interestingly, lipase RQ3 showed only six amino acids’ difference with lipase LipA from B. cepacia ATCC 25416. But they exhibited different optimal temperature and pH values, 40 °C and 9.0 for lipase RQ3 while 60 °C and 11.0 for lipase LipA [19].
Effects of metal ions on the activity of lipase RQ3
The effect of different metal ions on the activity of the lipase is shown in Fig. 5. Among the tested metal ions, lipase activity was significantly enhanced by Ca2+ ion, followed by Mg2+. Many lipases have been found to display enhanced activity in the presence of Ca2+. A possible explanation was that Ca2+ built a stable catalytic enzyme structure as a result of calcium ions binding to the internal structure of the enzyme, thereby changing the solubility and behavior of the ionized fatty acids at interfaces. Fe2+, Cu2+ and Zn2+ ions significantly inhibited the activity of the lipase, which was in agreement with the finds of lipases from B. multivorans V2, Pseudomonas sp. DMVR46, and B. ambifaria YCJ01 [1, 2, 15]. The metal chelator EDTA had a little influence on the activity of lipase, which suggested that lipase RQ3 might not be a metalloenzyme. The activity of lipases from P. stutzeri LC2-8 and B. ambifaria YCJ01 had also been reported to be unaffected by EDTA [7, 15].
Substrate specificity
Lipase RQ3 exhibited highest hydrolytic activity on p-nitrophenyl myristate (C14) (Fig. 6). This enzyme also had high hydrolytic activity toward p-nitrophenyl palmitate (C16) and p-nitrophenyl stearate (C18). A trend of preferential specificity towards longer chain length substrates is clearly evident. Most lipases, especially from Pseudomonas sp., prefer short- or medium-chain fatty acid esters [8, 10]. Nevertheless, most lipases from Burkholderia sp. showed specificity for longer chain fatty acid substrates [1, 15, 19].
Organic solvent stability of the lipase RQ3
Employing lipases for bioconversions in organic solvents is advantageous from biotechnological point of view, hence activity and stability in solvents are considered as novel attributes in a lipase. Effects of various organic solvents at 25 % concentration on stability of lipase RQ3 are shown in Table 2. All the tested hydrophilic and hydrophobic organic solvents significantly extended the half-life of lipase RQ3. The lipase half-life was more than twofold longer in organic solvents than in buffer system. Generally, polar solvents are considered to be extremely toxic to enzymes because essential water molecules present on the enzyme surface can easily be replaced with polar solvent molecules [8]. Although solvent stability has been reported for other lipases, most of these lipases were inhibited by polar solvents like methanol, ethanol and isopropanol. Dandavate reported that lipase from B. multivorans V2 was inhibited by dimethyl fluoride and dimethyl sulfoxide [2]. Lipase from B. cepacia S31 retained only 43 and 71 % residual activity in 25 % ethanol and acetonitrile after incubation for 6 h [24]. Lipase from Pseudomonas sp. DMVR46 was inhibited by polar organic solvents such as butanol and chloroform [1]. Strikingly, there are only six residues’ differences between lipase RQ3 and LipA; however, these two lipases showed great difference in solvent stability. Polar organic solvents such as ethanol, isopropanol and butanol inhibited lipase LipA activity [19]. From the structures of lipase RQ3 and LipA modeled using lipase from B. cepacia (PDB ID 2NW6) as the template (99 % identity to lipase RQ3), five of these six residues (128A, 150T, 156A, 256I and 311H of lipase RQ3) were located at the surface of the enzyme. It was found that the amino acids located at the surface of an enzyme played an important role in its organic solvent stability [25]. Therefore, the difference in 5-amino acids might contribute to the polar organic solvent stability of lipase RQ3. Further studies on this lipase could provide an insight into the structure–function relationship of its solvent tolerance. The remarkable stability of lipase RQ3 to various hydrophobic and hydrophilic solvents made it ideal for using as a biocatalyst in non-aqueous media.
Kinetic resolution of racemic 1-phenylethanol in hexane
1-phenylethanol is one of the most important chiral blocks used in cosmetics and pharmaceutical industry [7]. Lipase-catalyzed resolution is an important approach of preparing chiral 1-phenylethanol. However, these approaches were frustrated by poor organic solvent stability, relative stereo selectivity of lipase and the low solubility of substrates. Ivana reported transesterification of racemic 1-phenylethanol, catalyzed by lipase from Streptomyces rimosus, proceed with partial R-enantioselectivity (E-value only 2.3) [26]. Xue reported that commercial P. cepacia lipase was used for the transesterification resolution racemic 1-phenylethanol with e.e. >99 at 50 % conversion, while only 7 % conversion was obtained with the free form [27]. Lipase LC2-8 was found to be high activity towards (R)-1-phenylethanol. The conversion reached 52.4 %, and the residual substrate, (S)-1-phenylethanol, remained with the yield of 47.6 % [7]. In this study, the ability of lipase RQ3 to catalyze the kinetic resolution of racemic 1-phenyl ethanol in n-hexane with vinyl acetate as acyl donor was investigated. The time course of transesterification showed that conversion of (S)-1-phenylethanol to s-ester was 50 % with an almost theoretical yield after 20 h reaction (Fig. 7). The enantiomeric excess ee p of (S)-1-phenylethanol reached excellent values of 99.9 %, giving an E value over 200. This result suggested that lipase RQ3 in free form could efficiently perform chiral resolution of racemic 1-phenylethanol. As well as its solvent stability, lipase RQ3 is an alternative efficient biocatalyst for resolution of chiral alcohols.
Time course of conversion (square) and enantioselectivity (eep filled circle) of 1-phenyl ethanol using lipase RQ3 at 40 °C. The transesterification was carried out in 1 ml n-hexane containing 200 mmol/L (R, S)-1-phenylethanol and 300 mmol/L vinyl acetate catalyzed by 20 mg lipase RQ3 powder at 40 °C, 200 rpm. The results shown represent averages from three independent experiments. Error bars represent the standard deviation
Conclusion
In summary, using DMSO and toluene as screening stress, an extracellular lipase from a newly isolated solvent-tolerant bacterium Burkholderia cepacia RQ3 was purified and identified. The lipase exhibited broad stability to the various hydrophilic and hydrophobic solvents. Furthermore, the lipase showed good enantioselectivity toward racemic 1-phenylethanol. These indicated that lipase RQ3 would be a very attractive enzyme for potential application in biocatalysis in non-aqueous media.
References
Patel V, Nambiar S, Madamwar D (2014) An extracellular solvent stable alkaline lipase from Pseudomonas sp. DMVR46: partial purification, characterization and application in non-aqueous environment. Process Biochem 49:1673–1681
Dandavate V, Jinjala J, Keharia H, Madamwar D (2009) Production, partial purification and characterization of organic solvent tolerant lipase from Burkholderia multivorans V2 and its application for ester synthesis. Bioresour Technol 100:3374–3381
Baharum SN, Salleh AB, Razak CNA, Basri M, Rahman MBA, Rahman R (2003) Organic solvent tolerant lipase by Pseudomonas sp. Strain S5: stability of enzyme in organic solvent and physical factors affecting its production. Ann Microbiol 53:75–83
Zhao LL, Xu JH, Zhao J, Pan J, Wang ZL (2008) Biochemical properties and potential applications of an organic solvent-tolerant lipase isolated from Serratia marcescens ECU010. Process Biochem 43:626–633
Klibanov AM (2001) Improving enzymes by using them in organic solvents. Nature 409:241–246
Li W, Wu H, Liu BG, Hou XD, Wan DJ, Lou WY, Zhao J (2015) Highly efficient and regioselective synthesis of dihydromyricetin esters by immobilized lipase. J Biotechnol 199:31–37
Cao Y, Zhuang Y, Yao CJ, Wu B, He BF (2012) Purification and characterization of an organic solvent-stable lipase from Pseudomonas stutzeri LC2-8 and its application for efficient resolution of (R, S)-1-phenylethanol. Biochem Eng J 64:55–60
Gaur R, Gupta A, Khare SK (2008) Purification and characterization of lipase from solvent tolerant Pseudomonas aeruginosa PseA. Process Biochem 43:1040–1046
Ji QC, Xiao SJ, He BF, Liu XL (2010) Purification and characterization of an organic solvent-tolerant lipase from Pseudomonas aeruginosa LX1 and its application for biodiesel production. J Mol Catal B-enzym 66:264–269
Chen SX, Qian LL, Shi BZ (2007) Purification and properties of enantioselective lipase from a newly isolated Bacillus cereus C71. Process Biochem 42:988–994
Bradford MM (1976) Rapid and sensitive method for quantization of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254
Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature 227:680–685
Park OJ, Lee SH (2005) Stereoselective lipase from Burkholderia sp., cloning and their application to preparation of methyl (R)-N-(2,6-dimethylphenyl)alaninate, a key intermediate for (R)-metalaxyl. J Biotechnol 120:174–182
Ogino H, Nakagawa S, Shinya K, Muto T, Fujimura N, Yasuda M, Ishikawa H (2000) Purification and characterization of organic solvent-stable lipase from organic solvent tolerant Pseudomonas aeruginosa LST-03. J Biosci Bioeng 89:451–457
Yao CJ, Cao Y, Wu SS, Li S, He BF (2013) An organic solvent and thermally stable lipase from Burkhoideria ambifaria YCJ01: purification, characteristics and application for chiral resolution of mandelic acid. J Mol Catal B-enzym 85:105–110
Yang J, Guo D, Yan Y (2007) Cloning, expression and characterization of a novel thermal stable and short-chain alcohol tolerant lipase from Burkholderia cepacia strain G63. J Mol Catal B-enzym 45:91–96
Dalal S, Singh PK, Raghava S, Rawat S, Gupta MN (2008) Purification and properties of alkaline from Burkholderia cepacia ATCC 25609. Biotechnol Appl Biochem 51:23–32
Park DS, Oh HW, Heo SY, Jeong WJ, Shin DH, Bae KS, Park HY (2007) Characterization of an extracellular lipase in Burkholderia sp. HY-10 isolated from longicorn beetle. J Microbiol 45:409–417
Wang XQ, Yu XW, Xu Y (2009) Homologous expression, purification and characterization of a novel high-alkaline and thermal stable lipase from Burkholderia cepacia ATCC 25416. Enzyme Microb Tech 45:94–102
Rahman RNZRA, Baharum SN, Salleh AB, Basri M (2006) S5 lipase: an organic solvent tolerant enzyme. J Microbiol Biotechnol 44:583–590
Kim KK, Song HK, Shin DH, Hwang KY, Suh SW (1977) The crystal structure of a triacylglycerol lipase from Pseudomonas cepacia reveals a highly open conformation in the absence of a bound inhibitor. Structure 5:173–185
Jorgensen S, Skov KW, Diderichsen B (1991) Cloning, sequence and expression of a lipase gene from Pseudomonas cepacia: lipase production in heterologous hosts requires two Pseudomonas genes. J Bacteriol 173:559–567
Wang HK, Liu RJ, Lu FP, Qi W, Shao J, Ma HJ (2009) A novel alkaline and low-temperature lipase of Burkholderia cepacia isolated from Bohai in china for detergent formulation. Ann Microbiol 59:105–110
Lu Y, Lu F, Wang X, Bie X, Sun H, Wuyundalai LuZ (2009) Identification of bacteria producing a thermophilic lipase with positional non-specificity and characterization of the lipase. Ann Microbiol 59:565–571
Ogino H, Uchiho T, Doukyu N, Yasuda M, Ishimi K, Ishikawa H (2007) Effect of exchange of amino acid residues of the surface region of the PST-01 protease on its organic splvent-stability. Biochem Biophys Res Commun 358:1028–1033
Ivana LA, Bojana V, Maja ME, Wolfram S, Marija A (2001) Substrate specificity and effects of water-miscible solvents on the activity and stability of extracellular from Streptomyces rimosus. Enzyme Microb Tech 29:548–553
Xue P, Yan XH, Wang Z (2007) Lipase immobilized on HOOC-MCF: a highly enantioselective catalyst for transesterification resolution of (R, S)-1-phenylethanol. Chin Chem Lett 18:929–932
Acknowledgments
Financial supports for this research from the National Program on Key Basic Research Project (2011CBA00807), the National High Technology Program Research and Development Program of China (2012AA022205) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, C., Wu, B., Qin, S. et al. A lipase with broad solvent stability from Burkholderia cepacia RQ3: isolation, characteristics and application for chiral resolution of 1-phenylethanol. Bioprocess Biosyst Eng 39, 59–66 (2016). https://doi.org/10.1007/s00449-015-1489-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1489-1