Abstract
The synthesis of galacto-oligosaccharides (GOS) by β-galactosidase immobilized in both polyvinyl alcohol (PVA) lenses and sol–gel carriers was studied and compared with the performance of the free enzyme. PVA-immobilized β-galactosidase retained 95 % of the initial activity after seven repeated uses and retained 51 % of the initial activity after 3 months of storage, while sol–gel-immobilized β-galactosidase only retained 39 % of the initial activity under storage. Lactose conversion takes place at a higher rate in the PVA-immobilized β-galactosidase, while the lowest rate of lactose conversion was noticed with immobilized β-galactosidase in sol–gel. Continuous production of GOS from either lactose or whey, with PVA-immobilized β-galactosidase, was performed in a packed-bed reactor. A maximum GOS production of 30 % of total sugars was attained for a 40-% lactose feed solution with a feed rate of 10.8 ml/h, at pH 4.5 and 40 °C, corresponding to a productivity of 117 g/l h. The maximum GOS productivity of 344 g/l h was obtained at a flow rate of 28.7 ml/h. 3-OS and 4-OS were the major types of GOS formed. Conversion of whey in continuous mode resulted in GOS production of 15 % of total sugars and formation of 45 % 3-OS, 40 % 4-OS, and 15 % 5-OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Galacto-oligosaccharides (GOS) have been established as prebiotic ingredients after in vitro and in vivo dedicated studies. GOS, nondigestible oligosaccharides, are not hydrolyzed or absorbed in the upper intestinal tract, but for a minor part (less than 10 %); they instead pass into the colon where they are fermented selectively by beneficial intestinal bacteria. GOS have a generally recognized as safe (GRAS) status in the USA and a non-novel food status in the EU and are regarded as foods for specific health use in Japan [1]. Besides the prebiotic effects, GOS have low cariogenicity, low caloric values, and low sweetness [2]. GOS were shown to be very stable under exposure to high temperatures and to low pH environments. Therefore, GOS can be used in a variety of products including fermented milk products, breads, jams, confectionery, and beverages [3, 4]. GOS occur naturally in breast and cow milk, in honey, and in a variety of fruits and vegetables, but only in trace amounts [2]. As a result, the development of chemical or enzymatic production of GOS is necessary.
Synthesis of GOS can be performed either by highly regio- and stereo-specific glycosyl transferases (EC 2.4) or by glycoside hydrolases (EC 3.2.1). The former are not readily available, and they require sugar nucleotides as substrates, making their application in industrial production of GOS cost-prohibitive [1, 5]. Glycoside hydrolases, although less stereo-specific then glycosyl transferases, are more readily available and are therefore preferred for the industrial production of GOS [1, 5]. Several microbial glycoside hydrolases have been proposed for the synthesis of GOS from lactose [6]. In this context, the amount and structure of GOS produced are dependent on the source of the enzyme, which also conditions the range of pH and temperature for the synthesis reaction [5, 7]. Among the glycoside hydrolases used for GOS synthesis, ß-galactosidases are clearly prominent [5, 6]. These enzymes catalyze not only the hydrolysis of lactose to d-galactose and d-glucose but also the transgalactosylation reaction to produce GOS [8]. In the latter reaction, the enzyme transfers the galactose unit resulting from hydrolysis to the d-galactose moiety of the remaining lactose molecules instead of to the hydroxyl group of water, leading to the production of oligosaccharides with a higher degree of polymerization [5, 6]. The conversion of lactose into GOS by β-galactosidases is thus a kinetically controlled reaction, where competition between hydrolysis and transgalactosylation occurs. During conversion, the hydrolysis of lactose, which is favored thermodynamically, competes with the transferase activity that generates a complex mixture of various galactose-based di- and oligosaccharides of different structures [5]. Thus, the composition of the product mixture in the reaction medium typically varies notably with the time course of reaction [6]. Therefore, a thorough knowledge of the time course of the reaction (or of lactose conversion) is critical to determine the point of maximum yield of the desired product [1]. In the cheese industry, whey lactose is a waste, which causes several economical and environmental problems. Approximately 47 % of the whey produced annually worldwide is disposed off [9]. Therefore, conversion of lactose into a highly valuable product such as GOS is of high interest to the food industry. In the last 20 years, the immobilization of ß-galactosidase (EC 3.2.1.23) on different surfaces has gained a lot of attention in the food industry [10]. Many different techniques have been reported for the immobilization of ß-galactosidase, including cross-linking [11], covalent binding [12, 13], entrapment [14], and adsorption [15]. However, most of these methods were developed for application in processes for lactose hydrolysis instead of GOS formation. Also, many different supports have been used related with these immobilization methods. The main drawbacks of these methods are the use of toxic reactants, expensive supports, and the difficulty to scale up for industrial proposes due to high-pressure drop. Indeed during the immobilization process, factors like the nature of the enzyme carrier, the chemical reactants, and the foreseen role of the resulting biocatalyst have to be considered so that easy recovery of the immobilized enzyme and low cost, non-hazardous operation can be achieved and thus have potential for industrial-scale application.
One promising method for the immobilization of enzymes is sol–gel technique, whereby enzymes are confined within a chemically inert sol–gel support that is prepared by the hydrolysis and polycondensation of organometallic precursors [16]. The silica matrixes are chemically inert, hydrophilic, and inexpensive to synthesize. They also exhibit higher mechanical strength, enhanced thermal stability, and negligible swelling in organic solvents compared to most organic polymers. On the other hand, a recently developed immobilization method uses polyvinyl alcohol (PVA) hydrogel, which has several advantages compared to other matrices such as low toxicity, mechanical and good long-term stability, low biodegradability, and no side effects for enzyme reactions [17].
This study focuses on the development of a process for GOS production by immobilized β-galactosidase in sol–gel matrix as well as in lens-shaped PVA hydrogel capsules. The β-galactosidase from Aspergillus oryzae was chosen as a model enzyme for this study. The selected PVA-immobilized β-galactosidase was further used for continuous GOS synthesis using either lactose or whey as substrates in a continuous packed-bed reactor.
Materials and Methods
Materials
Commercial ß-galactosidase (EC 3.2.1.23) from A. oryzae (12 U/mg solid, 125,000 U), lactose, tetramethoxysilane (TMOS), and sodium dioctyl sulfosuccinate (AOT) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Lentikats® were obtained from Genialab (Braunschweig, Germany). All other reagents were of analytical grade from different sources.
Enzyme Immobilization in Sol–gel
The technique for enzyme immobilization was based on the work of Bernardino et al. [18]. Briefly, a solution containing 100 μL TMOS and 40 μL HCl (10 mM) was sonicated in a Transsonic T 460 sonicating water bath for 10 min until the hydrolysis reaction was completed. A total of 160 μL of enzyme solution (10 mg/ml in pH 4.5 acetate buffer 100 mM) was suspended in the sol solution. To obtain micro-particles, 300 μL of the sol–gel solution with enzyme was immediately added to 6 ml of 150 mM AOT/isooctane solution before gelation. The resulting mixture was vortexed for 1 min, washed twice with acetate buffer, and aged at room temperature under controlled water activity (a w = 0.75) during 1 week. The micro-particles obtained were suspended in 1 ml of the acetate buffer and stored at 4 °C until use.
Enzyme Immobilization in PVA
Immobilization in PVA was performed according to the protocol provided by GeniaLab (http://www.genialab.de/download/tt-english.pdf) by adding 0.2 ml of the diluted enzyme solution (10 mg/ml) to 1 ml LentiKat® liquid. The resulting solution was extruded to Petri dishes. After dehydration, under 30 °C, to 30 % (w/w) of the original weight, to allow for gelation, the lenses were incubated in 100 ml of a 15-g/l solution of LentiKat® stabilizer for 2 h at room temperature. The lenses were then washed and stored in 100 mM acetate buffer pH 4.5 at 4 °C until use.
Enzyme Activity
For ß-galactosidase activity assay, the immobilized enzyme was added to lactose solution and samples were collected with time. The product released in the assay, glucose, was determined spectrophotometrically using an enzymatic colorimetric assay kit from NZYTech (Lisboa, Portugal). One ß-galactosidase unit (U) was defined as the amount of immobilized enzyme catalyzing the release of 1 μmol of glucose per minute per milligram of protein at pH 4.5 and 40 °C. Immobilization efficiency was defined as:
Batch runs with free enzyme were performed using a ß-galactosidase concentration of 0.067 mg/lreaction medium. Batch runs with immobilized enzyme were performed using biocatalyst concentrations corresponding to 0.15 mg ß-galactosidase/lreaction medium and 0.18 mg ß-galactosidase/lreaction medium, for PVA and sol–gel carriers, respectively. Continuous operation was performed using a biocatalyst load corresponding to 55.7 mg ß-galactosidase.
Storage Stability
Immobilized ß-galactosidase was stored at 4 °C in 100 mM acetate buffer pH 4.5 for 9 months. The aliquots of each preparation were taken in triplicates at 30-day gaps and were then analyzed for the remaining activity. The activity determined on the first day was taken as control (100 %) for the calculation of the remaining percent activity.
Reusability of the Immobilized ß-Galactosidase
Immobilized ß-galactosidase was incubated with lactose solution in acetate buffer (10 % w/v) at pH 4.5 in triplicates for assaying the activity of enzyme. After each reaction cycle, the pellet containing the immobilized enzyme was recovered by centrifugation at 10,000 × g at 4 °C for 5 min and reused for another reaction cycle under the same conditions.
Batch Conversions
Production of GOS from lactose was studied with free and immobilized enzyme in sol–gel and PVA carriers. The reaction kinetics was studied at different lactose concentrations (10–60 % w/v). The lactose solution was prepared by dissolving lactose in 100 mM acetate buffer at pH 4.5. Samples were taken at appropriate time intervals and analyzed for sugar content by high-performance liquid chromatography (HPLC).
Continuous Conversions
A packed-bed reactor with a total volume of 10 ml was used. The enzyme reactor was operated at 40 °C and using lactose solution (initial lactose concentration 40 % w/v) or whey dissolved in acetate buffer (pH 4.5). The feed solution was continuously fed into the reactor at given flow rates until equilibrium of the reaction was achieved. Samples were taken at appropriate time intervals and analyzed for sugar content by HPLC. Skimmed whey was prepared according to Roy and Gupta [19]. The milk was skimmed by centrifuging the cold milk at 8,000 × g for 20 min. The fat layer was removed and whey was prepared from the skimmed milk by acidifying with HCl until the pH reached 4.8. Casein was removed by centrifugation. Prepared whey was stored at 4 °C for further use. The reactor productivity was calculated from the final GOS concentration (g/l) times the feed rate and divided by the reactor volume.
Analytical Methods
Protein concentration was determined by the Bradford assay using bovine serum albumin as standard [20]. The amount of immobilized protein was calculated by the difference between the amount of protein offered to the support for immobilization and that found in the supernatant and the washing buffer. Carbohydrate content was determined by HPLC. Twenty microliters of sample was filtered through 0.45-μm filters and injected into Agilent 1200 HPLC (USA) fitted with a Zorbax carbohydrate analysis column (4.6 × 150 mm, 5 μm particle size) and a Zorbax NH2 guard column (4.6 × 12.5 mm) (Agilent 1200, USA). Detection of sugars was performed using a refractive index detector (Agilent 1200, USA). The mobile phase was 75/25 (v/v) acetonitrile/water and the flow rate was kept constant at 1.5 ml/min. The column temperature was kept at 30 °C. Appropriate dilutions of a solution containing each of the carbohydrates were used as the calibration standards.
Results and Discussion
Immobilization Efficiency
In this study, β-galactosidase from A. oryzae was immobilized into lens-shaped PVA hydrogel capsules without pre-treatment and into sol–gel particles. These carriers were tested in order to obtain a suitable preparation for effective oligosaccharide synthesis. The immobilization efficiency of either immobilized formulation is given in Table 1. As can be seen from the results, the PVA enzyme system shows higher immobilization efficiency (88.5 %) for the immobilized β-galactosidase when compared to the sol–gel enzyme system (73.8 %). On the other hand, the activity of the β-galactosidase was higher in sol–gel formulation. This may be a consequence of the different amounts of immobilized enzyme being used per gram of carrier. It should be noted that the leakage of the enzyme from the sol–gel was higher (up to approximately fivefold higher) than that observed in the PVA carrier.
Activity Dependence of Free and Immobilized β-Galactosidase on Temperature and pH
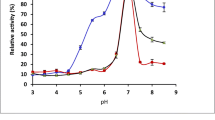
The dependence of catalytic activity of the immobilized enzyme preparations on pH was compared with that of the free enzyme. The effect of pH was examined in the pH range of 3.5 to 6.5 at 40 °C and the results are presented in Fig. 1. The optimum pH of the enzyme was 4.5. The pH profile of the immobilized β-galactosidase suggests an improved stability at the extreme pH values tested, particularly at the higher values and for sol–gel immobilization, in comparison to that in the free form. Such pattern has been reported previously and tentatively related to diffusion limitations or to secondary interactions between the enzyme and the support. In the particular case of sol–gel, the protective microenvironment may partly result from a decrease in the pH of the inner matrix as compared to the bulk outer phase that becomes increasingly more noticeable as the pH shifts towards less acidic values [21, 22]. The temperature activity profiles of the free and immobilized β-galactosidase are displayed in Fig. 2. The temperature optimum for free and immobilized enzyme in PVA was 50 °C. No further runs at higher temperatures were performed with PVA Lentikats since 55 °C was shown to provide a boundary for operation with these capsules—for at this temperature the capsules tend to melt [23]. Moreover, leakage of β-galactosidase at 50 °C was also reported [10]. The immobilization of the enzyme in sol–gel particles changed the temperature optimum to 60 °C. In addition, the sol–gel-immobilized β-galactosidase retained higher fractions of the catalytic activity at higher temperatures (63 % of the maximum enzyme activity was observed at 70 °C). Similar findings were reported when β-galactosidase from A. oryzae was immobilized in concanavalin A-cellulose [24]; increased activity retention at higher temperatures for β-galactosidase immobilized into polysiloxane–polyvinyl alcohol magnetic composite (mPOS–PVA) was also reported [25]. This behavior can be due to limitations in the thermal movement of the enzyme molecule as an outcome of immobilization [22, 26]. On the other hand, sol–gel-immobilized enzyme displayed lower activity at lower temperatures as compared to free and PVA-immobilized β-galactosidase. The poorer performance of the sol–gel biocatalyst could be the result of diffusion limitations and again to restrictions to the thermal movement of the enzyme, which are aggravated at lower temperatures.
Reusability of the Immobilized Enzyme
One of the limitations associated with the industrial application of enzymes is the high cost and instability under operational conditions. The overall process becomes cost-effective if the preparation shows high efficiency and reusability. The reusability of the immobilized β-galactosidase is shown in Fig. 3. PVA- and sol–gel-entrapped enzyme displayed retention of 95 and 61 % of the initial activity, respectively, after their seventh repeated use. Such results suggest that the PVA-immobilized biocatalyst is a better candidate for application in a realistic process. These results are comparable or best data reported in the literature, at the same temperature. An immobilized β-galactosidase on mPOS–PVA preparation was successively reutilized for 10 cycles, retaining approximately 84 % of its initial activity [27]. Pan et al. were able to reuse β-galactosidase covalently coupled onto magnetic Fe3O4–chitosan nanoparticles for 15 consecutive cycles, of 2 h each, using a 60-% lactose solution as substrate. At the final cycle, the biocatalyst retained about 90 % of the initial value [28]. On the other hand, Feng et al. reported a significant decrease in the yield in GOS throughout four consecutive batch cycles when assessing the feasibility of using β-galactosidase immobilized in calcium-alginate gel beads for the production of GOS from lactose [29].
Storage Stability of the Enzyme Preparations
The immobilized β-galactosidase in PVA and sol–gel carriers were stored for 90 days and the remaining enzyme activity was measured. The immobilized enzyme in PVA carriers exhibits higher storage stability than the immobilized β-galactosidase in sol–gel. After 1 month of storage, the remaining activity of the β-galactosidase immobilized in PVA was 83 %, while that of the β-galactosidase immobilized in sol–gel was only 68 %. Furthermore, after 3 months of storage, the activity loss of the β-galactosidase immobilized in PVA was 49 %, while the activity loss of the β-galactosidase immobilized in sol–gel was 61 %. Alongside with the natural time-dependent loss of enzyme activity, the activity decay of the immobilized biocatalysts may be ascribed to enzyme leakage from the carrier throughout the 3 months of storage period, a drawback that has been shown to occur when immobilized biomaterials are addressed [30, 31] . This drawback is more marked in the case of the sol–gel carrier, according to the results obtained under incubation in the presence of buffer, where enzyme leakage in this type of carrier was approximately fivefold higher than in the PVA carrier.
Process Development for GOS Production
Effect of Lactose Concentration on GOS Formation
GOS production after 1 h of incubation in the presence of free and immobilized β-galactosidase at different initial lactose concentrations (10–60 % w/v) is shown in Fig. 4. It was found that the production of GOS increased with increasing lactose concentrations from 10 to 40 %, at which point GOS production reached a maximum. In particular, the highest GOS concentration (marginally over 30 %) was obtained with β-galactosidase immobilized in PVA lenses. Further increase in lactose concentration resulted in the reduction of GOS production. This behavior can be related to spontaneous lactose precipitation for the concentrations of 50 and 60 %, where the solution was unstable, therefore reducing the concentration of lactose in solution [32]. On the other hand, at low lactose concentration, the rate of transgalactosylation is lower than that of hydrolysis since the amount of hydroxyl groups of carbohydrates is scarce, which results in a higher amount of glucose and galactose and lower amount of GOS in the reaction solution. Thus, to optimize transgalactosylation, careful selection of initial lactose concentration is critical [1]. Figure 5 shows lactose hydrolysis and production of GOS with free and immobilized β-galactosidase in PVA and sol–gel. Generally, a high rate of transgalactosylation was accompanied with a rapid decrease in lactose concentration at the beginning of the reaction. As can be seen in Fig. 5a, lactose consumption takes place at a higher rate in the PVA immobilized β-galactosidase, reaching 50 % conversion after 1 h of the reaction. On the other hand, the lowest rate of lactose consumption was noticed with sol–gel immobilized β-galactosidase, where 50 % lactose was consumed at the third hour of reaction. This suggests that immobilized β-galactosidase in PVA provided a more accessible form of biocatalyst which does not impose any mass transfer limitations on GOS formation from lactose. Similar results were obtained with β-galactosidase from A. oryzae immobilized on cotton cloth [12].
GOS production for free and immobilized β-galactosidase in PVA and sol–gel at different levels of lactose conversion is presented in Fig. 5b. A maximum production of GOS obtained with the free β-galactosidase was of 22 % of total carbohydrates at 50 % conversion of lactose. As lactose was continuously depleted from the reaction, the GOS formation rate decreased. GOS production by immobilized β-galactosidase in PVA lenses increased with increasing lactose conversion, until a maximum of 30 % of total sugars was reached at 45 % conversion. On the other hand, for the immobilized β-galactosidase in sol–gel particles, there was a slight shift in the overall reaction pattern, and a maximum yield of GOS (27 % of total carbohydrates) was reached at 75 % of lactose conversion, possibly as a result of mass transfer hindrance. Overall, however, these results are in accordance with data in the literature that relate GOS production kinetics to lactose conversion [33, 34]. Thus, as the reaction starts, lactose is naturally the predominant sugar that can act as a galactosyl acceptor. However, as lactose conversion proceeds, there is an increase of monosaccharides (glucose and galactose) and, through transgalactosylation, other disaccharides are formed. Moreover, the GOS produced also act as galactosyl acceptors, resulting in a complex mixture [35].
The ratio of glucose to galactose in the reaction mixture has been acknowledged as a suitable indicator of transgalactosylation activity [35, 36]. The higher this ratio, the more galactose has been transferred to suitable acceptors. Figure 5c is illustrative of the higher transgalactosylation activity of PVA-immobilized β-galactosidase as compared to the remaining formulations, as well as of the evolution of the said ratio with lactose conversion. β-Galactosidase immobilized in sol–gel particles produced large amounts of glucose and galactose, indicating a strong hydrolytic activity. Typically, the glucose/galactose ratio is larger than 1 under conditions where GOS are formed as it reflects the transfer of galactose to suitable acceptor molecules. It changes over the course of lactose conversion reaction and becomes 1 when the hydrolysis of lactose and oligosaccharides is complete [35].
Batch GOS Production
GOS composition as a result of the catalytic action of free and PVA- and sol–gel-immobilized β-galactosidase at maximal GOS concentration is shown in Fig. 6. The total GOS yield was 23, 31, and 26 % for the free and PVA- and sol–gel-immobilized β-galactosidase, respectively. These maximal values correspond to lactose conversion of 51, 44, and 67 % for the free and PVA- and sol–gel-immobilized β-galactosidase, respectively. Figure 6 shows that the maximum amount of GOS was produced with immobilized β-galactosidase in PVA lenses, where the composition of the different types of GOS was as follows: 25 % 3-OS and 6 % 4-OS. As shown in Fig. 6, 3-OS dominate over the other types of GOS formed in the reaction. Our results were similar to those observed for β-galactosidase of A. oryzae [12, 27], Lactobacillus reuteri [35], and Thermus aquaticus YT-1 [37]. They all demonstrated that 3-OS were the main GOS synthesized by β-galactosidase. Larger GOS, 5-OS, were produced only with immobilized β-galactosidase in sol–gel. On the other hand, high glucose and galactose yields were observed with sol–gel immobilized β-galactosidase, which suggests that hydrolysis reaction dominated the transgalactosylation reaction.
Continuous GOS Production
The production of GOS from lactose in the continuous reactor with PVA-immobilized β-galactosidase at pH 4.5 and 40 °C is shown in Fig. 7. It should be noted that in most of the published works related to the production of GOS, reaction conditions are such that temperatures are usually below 50 °C. Higher temperatures have an increasingly noticeable negative impact on the operational stability of the biocatalyst. In the present work, the reactor performance was very stable, and there was no apparent decrease in the level of GOS or lactose conversion except when feed rate was changed (Fig. 7a). With 40 % in the feed at 10.8 ml/h, 40 % of lactose conversion was attained (Fig. 7b) and the outlet product stream presented a GOS titer corresponding to 30 % of total sugars with an average reactor productivity of 117 g/l h. When the feed rate increased to 28.7 ml/h, lactose conversion decreased to 20 %, with small changes in the GOS content, while the productivity increased from 127 to 344 g/l h. When the feed rate decreased to 10.8 ml/h, 30 % lactose conversion was obtained with 28 % GOS in the final product and reactor productivity of ~120 g/l h. Residence time of 1 h was achieved with the feed rate of 10.8 ml/h. Longer residence times increase the risk of microbial contamination in long-term continuous process at moderate temperature. Suitable residence time prevents enzymatic hydrolysis of the product back to the mono- and disaccharides [38]. Overall, there was no noticeable loss of enzyme activity or reactor productivity over the entire 135-h period studied with lactose as a substrate and additionally 80 h of lactose conversion using whey as a substrate.
Aiming for alternatives to further lower the process costs, a cheap lactose source such as whey was tentatively assayed. The reaction was carried out throughout 80 h of operation. Lactose hydrolysis was higher in whey as a substrate, 70 to 80 %, than in pure lactose solution, where 40 to 50 % was observed. This may be due to a different composition of salts and to the presence of whey proteins because the salts can influence enzyme activity [27]. Furthermore, the concentration of lactose is four times lower in whey than in pure lactose solution, a feature that is detrimental to the transgalactosylation reaction. In the case of whey, GOS production was 10–20 % of total sugars, while in the case of lactose the concentration of GOS was ~30 % of total sugars. Another striking difference was the content of oligosaccharides, where 3-OS and 4-OS was almost same (~7 % of total sugars, respectively), and 5-OS was produced only in the case of whey as substrate in a concentration of 2.5 % of total sugars. On the other hand, when lactose was used as a substrate, the concentration of 3-OS was 16.2 % of total sugars, while 4-OS was 10.8 % of total sugars.
The operational stability displayed by the bioconversion system developed in the present work is promising when compared to reports in the literature of similar setups, also operating at 40 °C. Albayrak and Yang reported a stable process for GOS synthesis for about 3 days of continuous operation, while feeding a 40-% lactose solution to a packed-bed reactor filled with β-galactosidase aggregates immobilized on cotton cloth [33]. Botelho-Cunha et al. also reported of stable operation using β-galactosidase immobilized in Duolite A-568 in a continuous flow stirred tank reactor for a 6-h period, using as substrate a 15-% lactose solution [39]. Chockchaisawasdee and co-workers reported of stable, continuous operation of an ultra-filtration bioreactor, but only for 4 h and for lactose concentration of 2.5 % [40].
Table 2 shows the comparison of maximum GOS content achieved in various studies reported in the literature. The productivity obtained in this study was higher than of the reported values, which can be attributed to the good properties of the PVA as support matrices in the packed-bed reactor.
Conclusion
β-Galactosidase from A. oryzae was immobilized by entrapment in sol–gel capsules and PVA lenses and, alongside with free enzyme formulation, challenged to perform the kinetically controlled synthesis of GOS from lactose. PVA-immobilized β-galactosidase proved to be the best biocatalyst in terms of lactose conversion and operational stability. PVA as the support matrix for enzyme immobilization neither imposed any significant diffusion limitation nor affected the GOS formation characteristics of the enzyme. Moreover, FDA has approved the use of PVA in the production of food ingredients. Maximum GOS production, corresponding to 31 % of total sugars, was obtained out of a 40-% lactose solution with β-galactosidase immobilized in PVA. Within GOS, tri-saccharides (3-OS) were predominant, corresponding to 80 % of total GOS, while tetra-saccharides (4-OS) accounted for 20 % of the total GOS produced. Having selected PVA as carrier, a continuous process was carried out in a packed-bed reactor for GOS production, from either pure lactose or whey as substrate. The continuous enzyme reactor had high productivity and displayed a stable long-term performance. GOS production corresponded to 30 % of total sugars with a feed rate of 10.8 ml/h lactose solution and a GOS productivity of 117 g/l h.
References
Torres, D. P. M., Goncalves, M. P. F., Teixeira, J. A., & Rodrigues, L. R. (2010). Galacto-oligosaccharides: Production, properties, applications, and significance as prebiotics. Comprehensive Review in Food Science and Food Safety, 9, 438–454.
Sako, T., Matsumoto, K., & Tanaka, R. (1999). Recent progress on research and applications of non-digestible galacto-oligosaccharides. International Dairy Journal, 9, 69–80.
Czermak, P., Ebrahimi, M., Grau, K., Netz, S., Sawatzki, G., & Pfromm, P. H. (2004). Membrane-Assisted Production of Galactosyl-Oligosaccharides in a Continuous Process. Journal of Membrane Science, 232(1–2), 85–91.
Charalampopoulos, D., & Rastall, R. A. (2012). Prebiotics in foods. Current Opinion in Biotechnology, 23, 187–191.
Tzortzis, G., & Vulevic, J. (2009). In D. Charalampopoulos & R. A. Rastall (Eds.), Galacto-oligosaccharide prebiotics. Prebiotics and probiotics science and technology (pp. 207–244). New York: Springer.
Otieno, D. O. (2010). Synthesis of β-galactooligosaccharides from lactose using microbial β-galactosidases. Comprehensive Reviews in Food Science and Food Safety, 9, 471–482.
Mahoney, R. R. (1998). Galactosyl-oligosaccharide formation during lactose hydrolysis: a review. Food Chemistry, 63, 147–154.
Park, A. R., & Oh, D. K. (2010). Galacto-oligosaccharide production using microbial ß-galactosidase: current state and perspectives. Applied Microbiology and Biotechnology, 85, 1279–1286.
Novalin, S., Neuhaus, W., & Kulbe, K. D. (2005). A new innovative process to produce lactose reduced skim milk. Journal of Biotechnology, 119, 212–218.
Grosova, Z., Rosenberg, M., Rebros, M., Sipocz, M., & Sedlackova, B. (2008). Entrapment of β-galactosidase in polyvinylalcohol hydrogel. Biotechnology Letters, 30, 763–767.
Sheu, D. C., Li, S. Y., Duan, K. J., & Chen, C. W. (1998). Production of galactooligosaccharides by β-galactosidase immobilized on glutaraldehyde-treated chitosan beads. Biotechnology Techniques, 12, 273–276.
Albayrak, N., & Yang, S. T. (2002). Production of galacto-oligosaccharides from lactose by Aspergillus oryzae beta-galactosidase immobilized on cotton cloth. Biotechnology and Bioengineering, 77, 8–19.
Di Serio, M., Maturo, C., De Alteriis, E., Parascandola, P., Tesser, R., & Santacesaria, E. (2003). Lactose hydrolysis by immobilized ß-galactosidase: the effect of the supports and the kinetics Catalysis Today, 80, 333–339.
Mammarella, E. J., & Rubiolo, A. C. (2005). Study of the deactivation of ß-galactosidase entrapped in alginate-carrageenan gels. Journal of Molecular Catalysis B:Enzymatic, 34, 7–13.
Husain, Q., Ansari, S. A., Alam, F., & Azam, A. (2011). Immobilization of Aspergillus oryzae β-galactosidase on zinc oxide nanoparticles via simple adsorption mechanism. International Journal of Biological Macromolecules, 49, 37–43.
Avnir, D., Braun, S., Lev, O., & Ottolengi, M. (1994). Enzymes and other proteins entrapped in sol gel materials. Chemistry of Materials, 6, 1605–1614.
Lozinsky, V. I., & Plieva, F. M. (1998). Polyvinylalcohol cryogels employed as matrices for cell immobilization. 3. Overview of recent research and developments-Basics and applications. Enzyme and Microbial Technology, 23, 227–242.
Bernardino, S., Fernandes, P., & Fonseca, L. (2009). A new biocatalyst: Penicillin G acylase immobilized in sol-gel. Biotechnology Journal, 4, 695–702.
Roy, I., & Gupta, M. N. (2003). Lactose hydrolysis by LactozymTM immobilized on cellulose beads in batch and fluidized bed modes. Process Biochemistry, 39, 325–332.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Bhatia, R. B., Brinker, C. J., Gupta, A. K., & Singh, A. K. (2000). Aqueous Sol-Gel Process for Protein Encapsulation. Chemistry of Materials, 12, 2434–2441.
Santa, G. L., Bernardino, S. M., Magalhães, S., Mendes, V., Marques, M. P., Fonseca, L. P., et al. (2011). From inulin to fructose syrups using sol-gel immobilized inulinase. Applied Biochemistry and Biotechnology, 165, 1–12.
Rebros, M., Rosenberg, M., Mlichova, Z., & Kristofikova, L. (2007). Hydrolysis of sucrose by invertase entrapped in polyvinyl alcohol hydrogel capsules. Food Chemistry, 102, 784–787.
Ansari, S., & Husain, Q. (2010). Lactose hydrolysis by β-galactosidase immobilized on concanavalin A-cellulose in batch and continuous mode Journal of Molecular Catalysis B: Enzymatic, 63, 68–74.
Neri, D. F. M., Balcão, V. M., Carneiro-da-Cunha, M. G., Carvalho, L. B., & Teixeira, J. A. (2008). Immobilization of β-galactosidase from Kluyveromyces lactis onto a polysiloxane–polyvinyl alcohol magnetic (mPOS–PVA) composite for lactose hydrolysis. Catalysis Communications, 9, 2334–2339.
Wei, W., Wan, W. L. H., & Wang, S. (1999). Continuous preparation of fructose syrups from Jerusalem artichoke tuber using immobilized intracellular inulinase from Kluyveromyces sp. Y-85. Process Biochemistry, 34, 643–646.
Neri, D. F. M., Balcao, V. M., Costa, R. S., Rocha, I., Ferreira, E., Torres, D. P. M., et al. (2009). Galacto-oligosaccharides production during lactose hydrolysis by free Aspergillus oryzae beta-galactosidase and immobilized on magnetic polysiloxane-polyvinyl alcohol. Food Chemistry, 115, 92–99.
Pan, C., Hu, B., Li, W., Sun, Y. I., Ye, H., & Zeng, X. (2009). Novel and efficient method for immobilization and stabilization of β-D-galactosidase by covalent attachment onto magnetic Fe3O4-chitosan nanoparticles. Journal of Molecular Catalysis B: Enzymatic, 61, 208–215.
Feng, Y., Chang, X., Wang, W., & Ma, R. (2010). Artificial cells. Stabilities of Immobilized β-galactosidase of Aspergillus sp. AF for the Optimal Production of Galactooligosaccharides from Lactose. Blood Substitutes and Biotechnology, 38, 43–51.
Ma, L., & Wen, J. (2008). Biocomposite of double-walled carbon nanotube-doped alginate gel for biomaterial immobilization. Composites Science and Technology, 68, 1297–1303.
Arabaci, G., & Usluoglu, A. (2012). Immobilization of dill (Anethum Graveolens L.) catalase and its properties. Asia-Pacific Journal of Chemical Engineering, 7(Suppl. 3), S296–S300.
Vera, C., Guerrero, C., Conejeros, R., & Illanes, A. (2012). Synthesis of galacto-oligosaccharides by β-galactosidase from Aspergillus oryzae using partially dissolved and supersaturated solution of lactose. Enzyme and Microbial Technology, 50, 188–194.
Albayrak, N., & Yang, S. T. (2002). Immobilization of β-Galactosidase on Fibrous Matrix by Polyethylenimine for Production of Galacto-Oligosaccharide from Lactose. Biotechnology Progress, 18, 240–251.
Neri, D. F. M., Balcao, V. M., Dourado, F. O. Q., Oliveira, J. M. B., Carvalho, L. B., & Teixeira, J. A. (2009). Galactooligosaccharides production by beta-galactosidase immobilized onto magnetic polysiloxane-polyaniline particles. Reactive and Functional Polymers, 69, 246–251.
Splechtna, B., Thu-ha, N., Steinbck, M., Kulbe, K. D., Lorenz, W., & Haltrich, D. (2006). Production of Prebiotic Galacto-Oligosaccharides from Lactose Using β-Galactosidases from Lactobacillus reuteri. Journal of Agricultural and Food Chemistry, 54(14), 4999–5006.
Splechtna, B., Thu-ha, N., Zehetner, R., Lettner, H. P., Lorenz, W., & Haltrich, D. (2007). Process development for the production of prebiotic galactooligosaccharides from lactose using β-galactosidase from Lactobacillus sp. Biotechnology Journal, 2, 480–485.
Berger, J. L., Lee, B. H., & Lacroix, C. (1995). Immobilization of β-galactosidases from Thermus aquaticus YT-1 for oligosaccharides synthesis. Biotechnology Letters, 9, 601–606.
Chen, C. S., Hsu, C. K., & Chiang, B. H. (2002). Optimization of the enzymatic process for manufacturing low-lactose milk containing oligosaccharides. Process Biochemistry, 38, 801–808.
Botelho-Cunha, V. A., Mateus, M., Petrus, J. C. C., & de Pinho, M. N. (2010). Tailoring the enzymatic synthesis and nanofiltration fractionation of galacto-oligosaccharides. Biochemical Engineering Journal, 50, 29–36.
Chockchaisawasdee, S., Athanasopoulos, V. I., Niranjan, K., & Rastall, R. A. (2005). Synthesis of galacto-oligosaccharide from lactose using β-galactosidase from Kluyveromyces lactis: studies on batch and continuous UF membrane-fitted bioreactors. Biotechnology and Bioengineering, 89, 434–443.
Gaur, R., Pant, H., Jain, R., & Khare, S. K. (2006). Galacto-oligosaccharide synthesis by immobilized Aspergillus oryzae β-galactosidase. Food Chemistry, 97(3), 426–430.
Matella, N. J., Dolan, K. D., & Lee, Y. S. (2006). Comparison of galactooligosaccharide production in free-enzyme ultrafiltration and in immobilized-enzyme systems. Journal of Food Science, 71(7), C363–C368.
Chen, S. X., Wei, D. Z., & Hu, Z. H. (2001). Synthesis of galacto-oligosaccharides in AOT/isooctane reverse micelles by beta-galactosidase. Journal of Molecular Catalysis B:Enzymatic, 6, 109–114.
Leiva, M. H. L., & Guzman, M. (1995). Formation of oligosaccharides during enzymatic-hydrolysis of milk whey permeates. Process Biochemistry, 30, 757–762.
Acknowledgments
Ruzica Jovanovic-Malinovska gratefully acknowledge professor Luis Fonseca for the opportunity to work in the laboratory of Institute for Biotechnology and Bioengineering, Centre for Biological and Chemical Engineering, and Department of Bioengineering, Instituto Superior Técnico, Universidade Técnica de Lisboa. Pedro Fernandes acknowledges Programa Ciência 2007 from Fundação para a Ciência e a Tecnologia (Portugal) for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jovanovic-Malinovska, R., Fernandes, P., Winkelhausen, E. et al. Galacto-oligosaccharides Synthesis from Lactose and Whey by β-Galactosidase Immobilized in PVA. Appl Biochem Biotechnol 168, 1197–1211 (2012). https://doi.org/10.1007/s12010-012-9850-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9850-1