Abstract
In this study, a fungal and two yeast β-galactosidases were immobilized using alginate and chitosan. The biochemical parameters and lactose hydrolysis abilities of immobilized enzymes were analyzed. The pH optima of immobilized fungal β-galactosidases shifted to more acidic pH compared to free enzyme. Remarkably, the optimal temperature of chitosan-entrapped yeast enzyme, Maxilact, increased to 60 °C, which is significantly higher than that of the free Maxilact (40 °C) and other immobilized forms. Chitosan-immobilized A. oryzae β-galactosidase showed improved lactose hydrolysis (95.7%) from milk, compared to the free enzyme (82.7%) in 12 h. Chitosan-immobilized Maxilact was the most efficient in lactose removal from milk (100% lactose hydrolysis in 2 h). The immobilized lactases displayed excellent reusability, and chitosan-immobilized Maxilact hydrolyzed > 95% lactose in milk after five reuses. Compared to free enzymes, the immobilized enzymes are more suitable for cost-effective industrial production of low-lactose milk due to improved thermal activity, lactose hydrolysis efficiencies, and reusability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
β-Galactosidases (EC 3.2.1.23) are enzymes that hydrolyze the (1 → 4) linkage of lactose [galactosyl (1 → 4) glucose] to glucose and galactose and are one of the most widely used enzymes in the food industry. They are mainly used for the production of low-lactose milk for consumption by lactose-intolerant individuals who cannot digest lactose [1]. These enzymes are also used in the food and dairy industry for preventing crystallization of lactose and increasing the creamy texture of ice creams, making artificial sweeteners, production of galacto-oligosaccharides which are used as prebiotics, and for bio-remediation by utilizing cheese-whey which is an environmental pollutant [1,2,3,4,5].
In the recent years, isolation of novel microbial β-galactosidases from bacteria, yeast, and fungi has generated tremendous interest since they exhibit favorable biochemical and catalytic properties [6,7,8,9]. Several microbial β-galactosidases have shown potential for lactose hydrolysis in milk and whey [4, 10, 11]. However, there exist several bottlenecks which limit the direct application of these enzymes at industrial scale. The major bottle necks in their industrial applications are high cost, low solubility, poor recovery, and low operational stability [12,13,14]. The use of immobilized enzymes is considered as a better alternative for industrial applications since it circumvents the problems caused by the use of soluble enzyme. Immobilized enzymes offer favorable properties such as reusability, better operational stability, easy separation of the enzymes from products which eliminates microbial contamination, and low cost of operation [12, 15].
Immobilization of enzymes can be performed using suitable matrices by several different methods such as physical adsorption, ionic linkages, and stable covalent bonds. These techniques produce immobilized enzymes of varying stability due to changes in the surface microenvironment and degree of multipoint attachment [16]. β-galactosidases immobilized on various supports have been applied for lactose hydrolysis in milk and cheese whey [12,13,14]. Although different immobilization techniques for lactose hydrolysis have been reported, only a few were successfully scaled up and applied at an industrial scale. This could be due to the fact that the immobilization matrices were too costly, complex, or non-biocompatible for use in food industry. Therefore, for successful application of immobilized enzyme in industries, it is imperative that the immobilization method is simple and makes use of inexpensive matrices for cost-effective processes [17]. Other criteria for safe use in food industry include biocompatibility and non-toxicity of the supports. Presently, β-galactosidases from GRAS micro-organisms such as yeast (Kluyveromyces marxians, Kluyveromyces lactis, and Kluyveromyces fragilis) and fungi (Aspergillus niger and Aspergillus oryzae) are mainly used in the food industry since they are considered as safe for human use [8]. The microbial source often determines the biochemical properties of the enzyme, and thereby its intended applications. Fungal β-galactosidases are commonly used for acid whey hydrolysis due to their acidic pH optima, while the yeast enzymes are suitable for lactose hydrolysis in milk and sweet whey since they are active at neutral pH [4, 8, 18]. The yeast enzymes, however, have moderate temperature optima and hence, cannot be used for lactose removal at high temperatures in the food industry [10].
In this study, with the aim of improving their pH and temperature activities and lactose hydrolysis properties, three β-galactosidases from the food-grade fungus, Aspergillus oryzae and yeast, Kluyveromyces lactis (Maxilact and Lactozym) were immobilized by entrapment in barium alginate and chitosan macrospheres. In the present study, two natural polymers, chitosan and alginate, were selected as immobilization matrices since they are biocompatible, chemically inert, and biodegradable. Most importantly, since these supports are inexpensive and non-toxic, they are advantageous for a cost-effective process in the food industry. The biochemical properties of the immobilized enzymes were analyzed and compared with that of the free enzymes. Furthermore, comparative analysis of the immobilized enzymes for hydrolysis of lactose from milk was performed in order to select the most efficient and robust immobilization method for production of low-lactose milk in the food industry.
Materials and Methods
Materials
The β-galactosidase enzyme from Aspergillus oryzae was bought from Shanghai Rui Yong Biological Company (Shanghai, China). Two different commercially available lactases from the yeast, Kluyveromyces lactis, were used viz. Lactozym Pure 3000L (Novozymes Biotechnology Co., Ltd., Beijing, China) and Maxilact (DSM, Shanghai, China). The substrate, o-nitrophenyl-D-galactopyranoside (ONPG), was from Shanghai Baoman Biological technology Co. Ltd. (Shanghai, China). Chitosan was from Sangon (Shanghai, China) and sodium alginate was purchased from Shanghai Ryon Biological Technology Co. Ltd. (Shanghai, China). All other chemicals and reagents were of analytical grade.
Enzyme Activity Assay
To 1.7 mL of 0.05 M sodium acetate buffer, pH 4.5, 0.1 mL suitably diluted enzyme, and 0.2 mL of 20 mM ONPG were added and incubated at 40 °C for 15 min. The reaction was stopped by adding 2.0 mL of 2.0 M sodium carbonate solution. The OD at 410 nm was measured. One unit of β-galactosidase activity is defined as the amount of enzyme that liberates 1.0 μmol of o-nitrophenol per min under standard assay conditions.
Immobilization of β-Galactosidases on Alginate
To 2 mL Maxilact enzyme/lactozym/β-galactosidase from A. oryzae, 8-mL of 2.5% sodium alginate solution was added. The mixture was taken in 10-mL syringe with needle and added drop wise to 0.2 M BaCl2 solution (prepared in distilled water) while stirring. The beads were allowed to harden for 4 h. The beads were separated on filter paper, washed extensively with distilled water, then stored in appropriate buffer viz. 0.1 M phosphate buffer (pH 6.5) for Maxilact enzyme/Lactozym and 0.1 M acetate buffer (pH 4.5) for β-galactosidase from A. oryzae.
Immobilization of β-Galactosidases on Chitosan
Chitosan (1.5%) was dissolved in 1.5% acetic acid by heating at 60 °C with continuous stirring. This solution was taken in a syringe and allowed to fall in 100-mL solution of 1 M KOH with constant stirring. The beads were washed with distilled water several times, then with either 0.1 M phosphate buffer (pH 6.5) or 0.1 M acetate buffer (pH 4.5), and the enzymes were immobilized on the beads as follows. Five milliliters of Maxilact/Lactozym enzyme was added to 5 g (wet weight) beads and 15-mL phosphate buffer (pH 6.5) and were shaken gently at 10 °C for 16 h, then filtered and washed several times with phosphate buffer (pH 6.5). Similarly, 5 mL A. oryzae enzyme (20 mg dissolved in 5-mL acetate buffer, pH 4.5) was added to 5 g (wet weight) beads and 15-mL acetate buffer (pH 4.5) and immobilized in the same way as above. The beads were washed several times with 0.1 M acetate buffer (pH 4.5) and stored at 4 °C.
Analysis of pH Profiles of Free and Immobilized β-Galactosidases
The effect of pH on free and immobilized β-galactosidases was carried out at different pH range (pH 3.0–8.5) at 50 °C using oNPG as the substrate. The buffers used were sodium citrate (pH 3.0–4.0), sodium acetate (pH 4.5–5.5), sodium phosphate (6.0–7.0), and Tris–HCl (pH 8.0–8.5).
Analysis of Temperature Profiles of Free and Immobilized β-Galactosidases
The effect of temperature on soluble and immobilized β-galactosidases was studied by measuring the activity of the enzyme preparations at various temperatures ranging from 30 to 80 °C in 0.1 M sodium acetate buffer, pH 4.5 for fungal lactase, and 0.1 M sodium phosphate buffer, pH 6.5 for yeast lactases.
Hydrolysis of Lactose in Milk
Twenty units of free and enzyme-immobilized beads were added to 20 mL milk and kept in a shaker at 200 rpm at 40 °C for 12 h. Samples were withdrawn every 2 h (2, 4, 6, 8, 10, and 12 h) and kept in boiling water bath for 10 min to stop the enzyme reaction. To 1 mL of milk sample, 1 mL of distilled water was added and mixed, and then, HCl was added to decrease the pH to 3. The mixture was shaken for 10 min, and then centrifuged at 10,000 rpm for 15 min. The supernatant was removed and an equal volume of acetonitrile was added to it, mixed and shaken for 10 min. The sample was centrifuged at 10,000 rpm for 15 min. The clear supernatant was analyzed by HPLC as described below. Control sample was prepared in the same manner as above except that no enzyme was added.
HPLC Analysis
Lactose hydrolysis in milk treated with free and barium-alginate and chitosan immobilized fungal and yeast β-galactosidases were analyzed on Sugar-D column (4.5 mm × 250 mm) (Cosmosil) by HPLC system (Agilent, CA, USA). A mobile phase of acetonitrile/water at 75:25 (v/v) at a flow rate of 1 mL/min was used for column equilibration and for elution of the sugars. Twenty microliters of each sample was injected into the column and the sugars were detected with a refractive index detector. Authentic sugars such as lactose, galactose, and glucose were also injected on the column and used as standards.
Study of Reusability of Immobilized β-Galactosidases
The reusability of barium-alginate and chitosan-immobilized β-galactosidases from A. oryzae and K. lactis (Maxilact and Lactozym) was performed for five consecutive batches. Lactose hydrolysis was performed by independently adding 20 units of either alginate-immobilized enzymes or chitosan-immobilized enzymes into 20 mL of milk and kept in a shaker (200 rpm) for 4 h at 40 °C. At the end of each batch, the immobilized enzymes were filtered, washed thoroughly with distilled water, and then transferred to 20 mL of fresh milk. Aliquots were removed after each batch, boiled to stop the enzymatic reaction, and the samples were prepared for HPLC as described before. Samples from all the batches were analyzed on Sugar D column by HPLC. Lactose hydrolysis of 1st batch was regarded as 100%. All the experiments were carried out in triplicate.
Results and Discussion
Immobilization on Alginate
The properties and functions of immobilized enzymes are dependent mainly on several factors which include enzymatic biochemical properties, the chemical and mechanical characteristics of immobilization matrices, and immobilization methods [19]. Therefore, selection of suitable immobilization matrix and method is of utmost importance for industrial processes. In the present study, three β-galactosidases from food-grade micro-organisms such as Aspergillus oryzae and Kluyveromyces lactis (Maxilact and Lactozym) were immobilized onto suitable supports. For immobilizing the enzymes, two different matrices were selected (barium alginate and chitosan) due to their favorable characteristics such as biocompatibility, non-toxicity, and cost-effectiveness. Chitosan dissolves in dilute organic acids forming a viscous solution which precipitates in alkaline solution to form water insoluble complexes. In our study, neutralization method was used for preparing spherical porous beads by drop-wise addition of chitosan solution to a solution of KOH, followed by immobilization of β-galactosidase on the hardened macrospheres. For entrapment within barium-alginate beads, the enzyme-alginate mixture was allowed to form into spheres by dropping into barium chloride solution.
Analysis of pH Profiles of Free and Immobilized β-Galactosidases
In several instances, immobilization of enzymes on suitable matrices has been known to drastically affect and sometimes enhance enzymatic properties such as the optimal ranges of pH and temperature [20,21,22]. Therefore, in the present study, the effect of pH on the activities of β-galactosidases from A. oryzae and K. lactis (Maxilact and Lactozym) entrapped in barium-alginate and chitosan was investigated and compared with those of the free enzymes. As seen in Fig. 1a, pH optima of chitosan-immobilized and alginate-immobilized enzymes shifted to more acidic pH values of pH 3.0 and pH 4.0, respectively, as compared to that of the free enzyme (pH 4.5). On the other hand, immobilization did not greatly affect the optimal pH of the yeast (K. lactis) enzymes. It is noteworthy that although the pH optima of free and immobilized Lactozym were similar at pH 7.0, chitosan-immobilized enzyme retained significantly higher activities (> 60%) in the pH range of 5.5–8.5. In the case of another yeast β-galactosidase (Maxilact), the free and chitosan-immobilized enzymes were both optimally active at pH 7.0, while the alginate-immobilized enzyme was active at pH 8.0. The free enzyme showed a sharp decline in its activity at other pH values, whereas remarkably, both the immobilized enzymes displayed high activities in a wide pH range (pH 6.0–8.5), retaining more than 50% activities. Thus, entrapment within the porous beads appears to confer protection of the enzyme tertiary structure against pH changes. The above results suggest that pH changes may induce changes in the micro-environment of the enzyme active site. Changes in pH have been shown to affect the microenvironment of the immobilized enzymes to different extents [23, 24].
Analysis of pH-activity profiles of various free and immobilized β-galactosidases. The enzyme activity of free and immobilized β-galactosidases from A. oryzae (a), Lactozym (b), and Maxilact (c) was measured in 0.1 M buffers of various pH values at 50 °C. CIA, AIA, and FA indicate chitosan-immobilized A. oryzae, alginate-immobilized A. oryzae, and free A. oryzae β-galactosidase, respectively. CIL, FL, and AIL indicate chitosan-immobilized Lactozym, free Lactozym, and alginate-immobilized Lactozym, respectively. AIM, CIM, and FM indicate alginate-immobilized Maxilact, chitosan-immobilized Maxilact, and free Maxilact, respectively
Effect of Immobilization on Temperature Optima of β-Galactosidase
From the perspective of industrial applications, enzymes that are active at high temperatures are desirable, since high operational temperatures prevent microbial contamination and favor catalysis. β-Galactosidases from yeast are known to be active at lower temperatures as compared to the fungal enzymes. Therefore, in our study, the effect of immobilization on temperature optima of β-galactosidases from A. oryzae and K. lactis (Maxilact and Lactozym) was studied (Fig. 2a). As seen in Fig. 2a, the free as well as chitosan and alginate-immobilized enzymes from A. oryzae were maximally activity at 50 °C, indicating that immobilization did not exert any effect on the temperature activities of the fungal β-galactosidase. Similarly, the thermal activities of the yeast enzyme (Lactozym) were unaffected by immobilization as indicated by similar temperature optima (40 °C) of free and immobilized enzymes (Fig. 2b). However, remarkably, the optimal temperature of chitosan-immobilized Maxilact enzyme from K. lactis increased to 60 °C, which was significantly higher than that of the free and alginate-immobilized Maxilact enzymes (temperature optima of 40 °C) (Fig. 2c). Moreover, chitosan-immobilized Maxilact enzyme displayed high activity at temperatures ranging from 50 to 70 °C. The increase in activity at higher temperatures may imply that chitosan offers protection of the active site of Maxilact enzyme against heat-induced denaturation. Enzymes active at high temperatures are useful from the point of view of industrial applications since high temperatures can prevent microbial contamination during industrial processes. Therefore, the chitosan-immobilized Maxilact enzyme is more suitable for lactose hydrolysis in the food industries as compared to the free enzyme and other immobilized preparations.
Lactose Hydrolysis by Immobilized β-Galactosidases
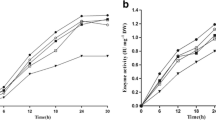
It is critical to evaluate the performance of immobilized enzymes for industrial applications. Hence, the free and immobilized β-galactosidases from fungal and yeast sources were tested for hydrolysis of lactose in milk, by batch mode. The lactose hydrolysis efficiencies of free enzymes and enzymes immobilized by chitosan and barium alginate were compared by incubating milk with enzymes for different time intervals up to 12 h. Lactose and other sugars were analyzed on SugarD column using HPLC system and the percentage of lactose hydrolysis was calculated by considering lactose present in milk (without enzyme) as 100%. As shown in Fig. 3a, all the enzymes (free and immobilized) from A. oryzae displayed similar lactose hydrolysis (~ 60%) up to 6 h. After that, the rate of lactose hydrolysis of chitosan-immobilized enzyme increased rapidly and was much higher than that of the free and alginate-immobilized enzymes. After 12 h, chitosan-immobilized enzyme hydrolyzed 95.7% lactose while the free and alginate-immobilized enzymes hydrolyzed 82.7% and 70.9% lactose, respectively. Thus, among the three forms of A. oryzae β-galactosidases, the chitosan-immobilized enzyme was found to be superior since it was capable of almost complete lactose removal from milk.
Comparison of lactose hydrolysis efficiencies of various free and immobilized β-galactosidases. Lactose hydrolysis was performed by incubating milk with free as well as barium-alginate and chitosan-entrapped preparations of β-galactosidases from A. oryzae (a), Lactozym (b), and Maxilact (c) in independent experiments. Aliquots at various time intervals were analyzed by HPLC for the amount of residual lactose
β-Galactosidases from yeast are frequently used for lactose removal from milk; hence, the lactose hydrolysis potential of two commercial enzymes from K. lactis viz. Maxilact and Lactozym was compared to study the effect of immobilization. Among the three forms of Maxilact enzymes, chitosan-immobilized enzyme was found to be the best since it hydrolyzed 100% lactose from milk within a short time (2 h), while the free enzyme and alginate-immobilized enzymes hydrolyzed 82% and 40% lactose, respectively, in the same duration (Fig. 3b). The results from lactose hydrolysis using another yeast enzyme, Lactozym, were somewhat different from the above findings. As shown in Fig. 3c, free and alginate-immobilized Lactozym rapidly and completely hydrolyzed lactose (100% hydrolysis) in 2 h as opposed to chitosan-immobilized enzyme which hydrolyzed 64% lactose in 2 h. Nevertheless, all the enzymes were capable of complete lactose hydrolysis after 12 h (Fig. 3c). Previously, Lactozym immobilized on cellulose beads has been used for the hydrolysis of milk lactose (60% conversion) in 5 h [25].
From our findings, it is evident that the performance of the enzyme depends on various parameters like tertiary structure and characteristics of the enzyme, immobilization methods, and matrix used. In our study, with respect to lactose hydrolysis potential, chitosan-immobilized Maxilact was found to be the best among all the various free and immobilized forms of β-galactosidases. In a previous report, K. lactis β-galactosidase immobilized on cotton fabric was used at a pilot-scale for hydrolysis of lactose in whole milk and 95% of lactose conversion was observed after 2 h of batch operation [26]. Since the chitosan-immobilized Maxilact enzyme, in our study, exhibited 100% lactose hydrolysis in 2 h, it is highly suitable for application in the food industry for production of low-lactose milk.
Analysis of Reusability and Storage of Immobilized Enzymes
Reusability can be a deciding factor and important criteria for selecting immobilized enzymes for industrial applications, since enzyme reuse can greatly reduce the operational cost [27]. Hence, the reusability of alginate and chitosan-immobilized β-galactosidases was evaluated for five consecutive batches of lactose hydrolysis in milk. After every batch, the enzyme-immobilized beads were washed with water and then added to fresh milk and samples from each batch were analyzed for their lactose content by HPLC. For A. oryzae β-galactosidase, chitosan proved to be a robust matrix for immobilization since the chitosan-immobilized enzyme retained 80% hydrolytic activity after five uses. In comparison, the lactose hydrolysis efficiency of the alginate-entrapped fungal β-galactosidase decreased to 60% after five uses. The loss of hydrolytic activity may be attributed to leaching of the enzyme from the alginate matrix upon repeated use. The phenomenon of leaching of enzymes from entrapped preparations has been previously reported [20, 28]. The yeast β-galactosidases (Maxilact and Lactozym) showed excellent reusability even after five uses. While the alginate-immobilized Maxilact enzyme showed 73% lactose hydrolysis (Fig. 4a), other immobilized preparations displayed > 95% lactose hydrolysis after five batches of reuse (Fig. 4a, b). These results suggest that due to their efficient reusability, the immobilized preparations can successfully reduce the cost of operation during lactose hydrolysis in industrial processes and they can be better alternatives than free enzymes.
Analysis of reusability of the immobilized β-galactosidases. The immobilized enzymes from K. lactis viz. Maxilact (a) and Lactozym (b) as well as the fungal enzyme from A. oryzae (c) were incubated with milk at 40 °C for 4 h for five batches each. After every cycle, the sugars in the hydrolyzed samples were analyzed by HPLC. The lactose hydrolysis of batch 1 was regarded as 100%
Conclusions
In the present study, three β-galactosidases from the food-grade micro-organisms, A. orzae and K. lactis, were immobilized by entrapment within the porous matrices of barium alginate and chitosan. The effect of immobilization on pH and temperature optima of β-galactosidases was studied. Our results indicate that pH optima of fungal β-galactosidase was shifted to more acidic pH values while that of the yeast enzymes remained largely unaffected. Remarkably, the yeast enzymes retained activity in a wide pH range as compared to the free enzymes. Immobilization did not alter the temperature optima of fungal and yeast β-galactosidases. However, the optimal temperature of Maxilact enzyme from K. lactis markedly increased from 40 to 60 °C after immobilization on chitosan, which is significantly higher than that of the free enzyme as well as other immobilized forms. The chitosan-immobilized Maxilact enzyme was also highly active at high temperatures up to 70 °C, which makes it suitable for applications in the food industries. The lactose hydrolysis abilities of the different immobilized fungal and yeast β-galactosidases were compared. For β-galactosidase from A. oryzae, immobilization on chitosan was found to be the best method, since chitosan-immobilized enzyme showed maximum lactose hydrolysis (95.7%) from milk in 12 h. The β-galactosidase from K. lactis was found to be more efficient and rapid in hydrolyzing lactose from milk. Among all the various free and immobilized forms of β-galactosidases tested, the chitosan-immobilized Maxilact was found to be the most superior since it displayed 100% lactose hydrolysis in 2 h. Moreover, reusability studies of immobilized preparations indicate that the immobilized enzymes from A. oryzae and K. lactis could be reused five times, and the chitosan-immobilized Maxilact showed > 95% lactose hydrolysis after five batches of reuse. Thus, in this study, using simple and inexpensive matrices, fungal and yeast β-galactosidases could be successfully immobilized, which led to an improvement in their biochemical properties and lactose hydrolysis efficiencies. Furthermore, due to their excellent reusability, the immobilized enzymes can be applied at industrial scale for cost-effective production of low-lactose milk.
References
Mlichova, Z., & Rosenberg, M. (2006). Current trends of beta-galactosidase application in food technology. Journal of Food and Nutrition Research, 45(2), 47–54.
Bosso, A., Morioka, L. R. I., dos Santos, L. F., & Suguimoto, H. H. (2016). Lactose hydrolysis potential and thermal stability of commercial β-galactosidase in UHT and skimmed milk. Food Science and Technology (Campinas), 36(1), 159–165. https://doi.org/10.1590/1678-457X.0085.
Demirhan, E., Apar, D. K., & Özbek, B. (2010). A modelling study on hydrolysis of whey lactose and stability of β-galactosidase. Korean Journal of Chemical Engineering, 27(2), 536–545. https://doi.org/10.2478/s11814-010-0062-5.
Katrolia, P., Yan, Q., Jia, H., Li, Y., Jiang, Z., & Song, C. (2011). Molecular cloning and high-level expression of a β-galactosidase gene from Paecilomyces aerugineus in Pichia pastoris. Journal of Molecular Catalysis B: Enzymatic, 69(3–4), 112–119. https://doi.org/10.1016/j.molcatb.2011.01.004.
Martínez-Villaluenga, C., Cardelle-Cobas, A., Corzo, N., Olano, A., & Villamiel, M. (2008). Optimization of conditions for galactooligosaccharide synthesis during lactose hydrolysis by β-galactosidase from Kluyveromyces lactis (Lactozym 3000 L HP G). Food Chemistry, 107(1), 258–264. https://doi.org/10.1016/j.foodchem.2007.08.011.
Katrolia, P., Zhang, M., Yan, Q., Jiang, Z., Song, C., & Li, L. (2011). Characterisation of a thermostable family 42 β-galactosidase (BgalC) family from Thermotoga maritima showing efficient lactose hydrolysis. Food Chemistry, 125(2), 614–621. https://doi.org/10.1016/j.foodchem.2010.08.075.
Niu, D., Tian, X., Mchunu, N. P., Jia, C., Singh, S., Liu, X., Prior, B. A., & Lu, F. (2017). Biochemical characterization of three aspergillus Niger β-galactosidases. Electronic Journal of Biotechnology, 27, 37–43. https://doi.org/10.1016/j.ejbt.2017.03.001.
Oliveira, C., Guimarães, P. M. R., & Domingues, L. (2011). Recombinant microbial systems for improved β-galactosidase production and biotechnological applications. Biotechnology Advances., 29(6), 600–609. https://doi.org/10.1016/j.biotechadv.2011.03.008.
Park, A.-R., & Oh, D.-K. (2010). Galacto-oligosaccharide production using microbial beta-galactosidase: Current state and perspectives. Applied Microbiology and Biotechnology, 85(5), 1279–1286. https://doi.org/10.1007/s00253-009-2356-2.
Dutra Rosolen, M., Gennari, A., Volpato, G., & Volken De Souza, C. F. (2015). Lactose hydrolysis in Milk and dairy whey using microbial β-galactosidases. Enzyme Research, 2015, 1–7. https://doi.org/10.1155/2015/806240.
Kumar Mukesh, D. J., Sudha, M., Devika, S., Balakumaran, M. D., Ravi Kumar, M., & Kalaichelvan, P. T. (2012). Production and optimization of β-galactosidase by Bacillus Sp. MPTK 121, isolated from dairy plant soil. Annals of. Biological Research, 3(4), 1712–1718.
Grosová, Z., Rosenberg, M., & Rebroš, M. (2008). Perspectives and applications of immobilised β-galactosidase in food industry - a review. Czech Journal of Food Sciences., 26(1), 1–14.
Husain, Q. (2010). β galactosidases and their potential applications: A review. Critical Reviews in Biotechnology, 30(1), 41–62. https://doi.org/10.3109/07388550903330497.
Panesar, P. S., Kumari, S., & Panesar, R. (2010). Potential applications of immobilized β-galactosidase in food processing industries. Enzyme Research, 2010(16), 1–16. https://doi.org/10.4061/2010/473137.
DiCosimo, R., McAuliffe, J., Poulose, A. J., & Bohlmann, G. (2013). Industrial use of immobilized enzymes. Chemical Society Reviews, 42(15), 6437. https://doi.org/10.1039/c3cs35506c.
Mohamad, N. R., Marzuki, N. H. C., Buang, N. A., Huyop, F., & Wahab, R. A. (2015). An overview of technologies for immobilization of enzymes and surface analysis techniques for immobized enzymes. Biotechnology and Biotechnological Equipment., 29(2), 205–220. https://doi.org/10.1080/13102818.2015.1008192.
Zhang, D., Yuwen, L., & Peng, L. (2013). Parameters affecting the performance of immobilized enzyme. Journal of Chemistry, 2013(Article ID 946248), 7. https://doi.org/10.1155/2013/946248.
Ansari, S. A., & Satar, R. (2012). Recombinant β-galactosidases - past, present and future: A mini review. Journal of Molecular Catalysis B: Enzymatic., 81, 1–6. https://doi.org/10.1016/j.molcatb.2012.04.012.
Datta, S., Christena, L. R., & Rajaram, Y. R. S. (2013). Enzyme immobilization: An overview on techniques and support materials. 3 Biotech, 3(1), 1–9. https://doi.org/10.1007/s13205-012-0071-7.
Bibi, Z., Qader, S. A. U., & Aman, A. (2015). Calcium alginate matrix increases the stability and recycling capability of immobilized endo-β-1,4-xylanase from Geobacillus stearothermophilus KIBGE-IB29. Extremophiles, 19(4), 819–827. https://doi.org/10.1007/s00792-015-0757-y.
Garcia-Galan, C., Berenguer-Murcia, Á., Fernandez-Lafuente, R., & Rodrigues, R. C. (2011). Potential of different enzyme immobilization strategies to improve enzyme performance. Advanced Synthesis and Catalysis., 353(16), 2885–2904. https://doi.org/10.1002/adsc.201100534.
Ladero, M., Santos, A., & García-Ochoa, F. (2000). Kinetic modeling of lactose hydrolysis with an immobilized β- galactosidase from Kluyveromyces fragilis. Enzyme and Microbial Technology, 27(8), 583–592. https://doi.org/10.1016/S0141-0229(00)00244-1.
Chen, W., Chen, H., Xia, Y., Yang, J., Zhao, J., Tian, F., Zhang, H. P., & Zhang, H. (2009). Immobilization of recombinant thermostable β-galactosidase from Bacillus stearothermophilus for lactose hydrolysis in milk. Journal of Dairy Science, 92(2), 491–498. https://doi.org/10.3168/jds.2008-1618.
Gürdaş, S., Güleç, H. A., & Mutlu, M. (2012). Immobilization of aspergillus oryzae β-galactosidase onto Duolite A568 resin via simple adsorption mechanism. Food and Bioprocess Technology, 5(3), 904–911. https://doi.org/10.1007/s11947-010-0384-7.
Roy, I., & Gupta, M. N. (2003). Lactose hydrolysis by Lactozym ™ immobilized on cellulose beads in batch and fluidized bed modes. Process Biochemistry, 39(3), 325–332. https://doi.org/10.1016/S0032-9592(03)00086-4.
Li, X., Zhou, Q. Z. K., & Chen, X. D. (2007). Pilot-scale lactose hydrolysis using β-galactosidase immobilized on cotton fabric. Chemical Engineering and Processing: Process Intensification, 46(5), 497–500. https://doi.org/10.1016/j.cep.2006.02.011.
Norouzian D.. (2003). Enzyme immobilization: The state of art in biotechnology. Iranian Journal of Biotechnology, 1(4).
Zhang, Z., Zhang, R., Chen, L., & McClements, D. J. (2016). Encapsulation of lactase (β-galactosidase) into k-carrageenan-based hydrogel beads: Impact of environmental conditions on enzyme activity. Food Chemistry, 200(June), 69–75. https://doi.org/10.1016/j.foodchem.2016.01.014.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 31401628).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Katrolia, P., Liu, X., Li, G. et al. Enhanced Properties and Lactose Hydrolysis Efficiencies of Food-Grade β-Galactosidases Immobilized on Various Supports: a Comparative Approach. Appl Biochem Biotechnol 188, 410–423 (2019). https://doi.org/10.1007/s12010-018-2927-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2927-8