Abstract
Tannase production by newly isolated Penicillium purpurogenum PAF6 was investigated by ‘one variable at a time’ (OVAT) approach followed by response surface methodology (RSM). Tannin-rich plant residues were used as supporting solid substrate and sole carbon source and, among them, tamarind seed was found to be the most favorable substrate than haritaki, pomegranate, tea leaf waste and arjun fruit. Physicochemical parameters were initially optimized using OVAT methodology and some important factors like incubation time, incubation temperature, substrate:moisture ratio as well as carbon, nitrogen and phosphate concentrations were verified with Box–Behken design of response surface methodology. Phosphate source, nitrogen source and temperature were found as the most favorable variables in the maximization of production. Tannase production was enhanced from 1.536 U/g to 5.784 U/g using tamarind seed OVAT optimization and further enhancement up to 6.15 U/g following RSM. An overall 3.76- and 4.0-fold increases in tannase production were achieved in OVAT and RSM, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tannin-acyl-hydrolase, commonly known as tannase (E.C. 3.1.1.20), has wide applicability in the field of biotechnology and industry. It catalyzes the hydrolysis of ester and depside bonds in hydrolysable tannins releasing glucose and gallic acid. The enzyme is found to be useful in the manufacture of gallic acid, instant tea, acron wine, coffee-flavored soft drinks and clarification of beer and fruit juices [1–4]. The enzyme also has applications in the treatment of wastewater contaminated with polyphenolic compounds [5, 6]. Application of tannase for detannification of foods and feeds can improve their palatability, digestibility and nutritive value [2, 7]. Besides that, the hydrolytic product of tannin, i.e., gallic acid is also a primary anti-inflammatory, cardioprotective agent found in wine, tea and cocoa and also used for the synthesis of propyl gallate, trimethoprim and photosensitive resin in semiconductor production [8–10].
Though some fungi and bacteria are able to synthesize tannase, commercial production is hampered due to higher production cost, unavailability of novel substrate, medium composition and fermentation conditions. In this respect, tannin-rich plant residues could be cost-effective substrate in solid substrate fermentation (SSF) and are advantageous for countries where these tannin-rich materials are generated in abundance (Table 1).
Among the microbial resources, filamentous fungi have the intrusion power for penetrating deep into the intercellular space for better utilization of plant material. SSF is a bioprocess in which microorganisms are in tight contact with the insoluble substrate and, in the absence of free flowing water, thus, to achieve the maximum utilization of the substrate to get maximum growth and product formation under optimized conditions [26].
The optimization of fermentation conditions is an important problem in the development of economically feasible bioprocesses. The conventional method of optimization involves changing one variable at a time (OVAT), keeping the others at fixed levels. Being single dimensional, this laborious and time-consuming method often does not guarantee determination of optimal conditions [27]. In addition, carrying out experiments with every possible factorial combination of the test variables is impractical because of the large number of experiments required. However, designing an appropriate fermentation condition is of significant importance because it influences product concentration, yield and volumetric productivity [28]. Optimizing the parameters by a statistical method (response surface methodology, RSM) reduces time and expense. RSM consists of a group of empirical techniques devoted to the evaluation of relations existing between a cluster of controlled experimental factors and the measured responses according to one or more selected criteria.
Considering these, the present study was undertaken for the enhancement of extracellular tannase production by Penicillium purpurogenum PAF6 under SSF using tannin-containing plant residues like tamarind seed (Tamarindus indica), tea (Camellia sinensis) waste, haritaki (Terminalia chebula), pomegranate (Punica granatum) residues and arjun (Terminalia arjuna) fruit. We report the optimization of physicochemical parameters for the production of tannase by P. purpurogenum PAF6 under a classical technique (OVAT) and also with Box–Behken RSM statistical design.
Materials and Methods
Chemicals
All chemicals used in this study were of analytical grade and procured from Himedia, India and Merck, India.
Selection of Solid Substrate
Tamarind seed, arjun fruit, pomegranate residue, haritaki and tea waste were collected from the local area of the Midnapore, West Bengal, India. All the substrates were dried at 60 °C and powdered in a grinder of desired mesh size. These substrates were used as solid media for tannase production without any pretreatment.
Determination of Tannin Content of Substrates
Total tannin content of the substrates was determined by Folin–Denis method [29].
Microorganism
A number of tannase-producing fungi were screened by a serial dilution technique from the soil of Gurguripal sal (Sorea robusta Gaertn. f.) forest, Midnapore, West Bengal, India using selective tannic acid agar medium containing (g l−1): tannic acid, 5.0; NH4Cl, 3.0; KH2PO4, 0.5; MgSO4, 0.5; glucose, 0.1 and agar, 30.0. Medium was inoculated with serially diluted soil sample and incubated at 30 °C for 72 h. Appearance of a clear transparent zone around the colony was selected as a tannase-producing organism. According to the clear zone to colony size ratio (cz/cs), best fungus was selected and designed as PAF6. The strain was subcultured on Czapek's agar medium and their morphological and structural features (mycelium, spore) were examined microscopically and identified as P. purpurogenum. The strain was preserved at 4 °C for further use and subcultured at a regular interval on tannic acid agar slant.
Inoculum Preparation
The fully sporulated 1-week-old agar slant culture was flooded with 10 ml of sterile distilled water with 0.1% (v/v) Tween-80. The spores were dislodged using a sterile inoculation loop under strict aseptic conditions and the number of spores in the suspension was determined using Neubauer chamber. The volume of 1 ml of the prepared spore suspension contains around 3.13 × 106 spores.
Moistening Agent
A salt solution with 1% (w/v) urea and 0.1% (w/v) (NH4)2HPO4 was used as the moistening medium for SSF. Final pH of the medium was adjusted to 5.0.
Solid State Fermentation
Five grams of studied solid substrate was taken in 250 ml of Erlenmeyer flasks, moistened with 10 ml of moistening agent, autoclaved at 121 °C for 15 min, cooled to room temperature and inoculated with 1 ml of the fungal spore suspension. The contents were mixed thoroughly and incubated at 30 °C in an environmental chamber (REMI-CHM-6S) for the desired period. The influencing parameters of SSF like moisture ratio (1:1/2–1:4), incubation period (24–120 h), incubation temperature (25 °C–40 °C), nitrogen source, phosphorus sources, metal salt, supplementation with additional tannic acid concentration (0.1%–10%) and inoculum volume (5%–40%) are optimized.
Extraction of Tannase from Fermented Matter
The fermented solid mass mixed with extractants, viz. distilled water, aqueous mixture (10%, v/v) of glycerol, acetone methanol, Tween 80 and acetate buffer (0.2 M pH 5.0) and agitated through a rotary shaker at 150 rpm for 1 h at room temperature (25 °C). The slurry was then squeezed through cheesecloth followed by centrifugation at 10,000 × g for 10 min at 4 °C to remove the insoluble matters. The clear supernatant was used for the tannase assay.
Enzyme Assay
Tannase activity in the fermented medium was determined by the colorimetric method of Mondal et al. [30].
One unit of the tannase was defined as the amount of enzyme, which is able to hydrolyse 1 μmol of ester linkage of tannic acid in 1 min at specific condition.
SEM Studies
The structure of P. purpurogenum PAF6 cells and the substrate morphology after fermentation were studied (Fig. 1) under scanning electron microscopy (VEGA TESCAN, USA). After the desired period of fermentation and before fermentation, samples were fixed in 10–100% of gradient acetone solution and, finally, with 5% glutaraldehyde solution. The fixed cells were dried to a critical point by a drying apparatus and kept in desiccators until use. Samples were then fixed onto a graphite stub and kept in an autosputter coater (E5200, Bio-Rad) under vaccum for gold coating up to 150 s.
RSM Experimental Design and Statistical Analysis
The first step in this study was to identify the levels of process parameters which have high influence on enzyme production (response). To explore the effect of variables on the response in the region of investigation, a Box–Behnken factorial design was employed in the optimization of culture conditions for tannase production. An experimental design was created on Minitab 15 statistical software. Previously optimized important six factors (incubation time, incubation temperature, substrate:moisture ratio, tannic acid, nitrogen and phosphate concentration) and their effective three-level (−1, 0 and +1) design (Table 2) were selected and a total of 54 experiments were conducted in this study. The response was tannase activity (U/g). All independent variables were taken at the central-coded value considered as zero. The minimum and maximum ranges of variables were used and a full experimental design is listed in coded form in Table 3. Each set of experiment were conducted in triplicate to reduce the error level. The relation between the coded value and actual values were described as in the following Eq. 1
where Xi is the independent variable coded value, xi is the independent variable actual value, x0 is the independent variable actual value on the center point and ∆xi is the step change value. The statistical significance of this equation was determined by Fisher's test. The quality of the model was checked by the coefficient of determination R 2 value. The 3D graphs were generated to understand the effect of selected variables individually and in combination to determine their optimum level for maximal production of tannase.
Results
Optimization of SSF Components Following OVAT Methodology
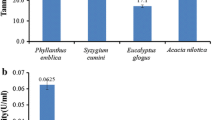
The selection of substrate for large-scale enzyme production by fermentation depends upon its cost and availability. Haritaki was found to be a maximum tannin-containing substrate among the other studied substrates (1.27 U/g). Among the different substrates used, tamarind seed powder was found as the best substrate for tannase production (1.536 U/g) followed by haritaki and pomegranate, and it can be arranged in the following order: tamarind seed > haritaki > pomegranate > tea leaf waste > arjun fruit (Fig. 2). The further optimization of tannase production was carried out using tamarind seed powder as solid substrate.
It was found that an incubation period of 48 h and temperature of 30 °C and substrate:moisture ratio of 1:3 were best (Fig. 3) for maximum tannase production (1.836 U/g). Figure 4 depicts that among all the nitrogen sources, urea was the most effective. The maximum enzyme yield of (2.88 U/g) was obtained with 1% (w/v) urea in tamarind seed substrate. Among the studied phosphate sources (Fig. 5), diammonium hydrogen orthophosphate with 0.1% (w/v) concentration maximized tannase production (3.828 U/g). Different metal ions were added to study their effects on enzyme biosynthesis. It has been observed that metal ions have not showed any observable alteration in tannase production using tamarind seed powder (Fig. 6). Maximum tannase of 5.706 U/g was obtained with additional 4% (w/v) tannic acid supplementation. Beyond this level, tannase synthesis was comparatively lower (Fig. 6). The spores of the experimental organism were used as the inoculums at different volumes to maximize enzyme production and solid substrate utilization. The 20% inoculum (1 ml contains around 3.13 × 106 spores) volume was optimum for tannase production (Fig. 7). The enzyme extraction from fermented substrate was optimized employing various extractants. The amount of the extracted enzyme from the fermented substrate was maximum when distilled water used as solvent at 1:6 ratio (w/v) (Fig. 7).
Effect of different nitrogen sources for tannase production by Penicillium purpurogenum PAF6. CL control, BE beef extract, YE yeast extract, TRP tryptone, PEP peptone, PN potassium nitrate, AN ammonium nitrate, AC ammonium chloride, AS ammonium sulphate, UR urea, SN sodium nitrate. In the inset is the effect of different concentrations (0.1%–5%) of urea
Statistical Optimization and Model Validation
A Box–Behnken factorial design was performed for further enhancement of enzyme production. The experimental and predicted values of yields of tannase are given in Table 2. It was observed that the predicted values for tannase production were in good agreement with RSM plots.
All the predicted and observed responses related with tannase production summarized in Table 2. The adequacy of the model and fitness were evaluated by ANOVA (analysis of variance), and regression coefficients for the experimental design were used (Table 4). The highest R 2 value in response could be explained by the following second order equation
where A = incubation time, B = temperature, C = substrate:moisture ratio, D = carbon concentration, E = nitrogen concentration and F = phosphate concentration.
From the ANOVA study, it was clear that tannase production by P. purpurogenum PAF6 in SSF was mostly influenced by a phosphate source other than nitrogen. Three-dimensional plots were drawn to illustrate the main and interactive effects of the independent variables on the dependent ones. Response surface three-dimensional plots were generated by plotting the response on the z-axis against any two independent variables while keeping the other four variables at their experimental level.
The three-dimensional surface plot (Fig. 8a) depicts the interaction between nitrogen concentration and phosphate concentration while keeping the other variable at zero level. The result demonstrated that with increased phosphate and nitrogen concentration up to 0.1% and 1.0%, respectively, tannase production increased steadily and, thereafter, declined sharply. Figure 8b showed the effect of phosphate concentration and incubation temperature on tannase production, keeping others at zero. The graph showed the maximum tannase production temperature of 30 °C and phosphate concentration of 0.1%, which is similar with Fig. 8a. Figure 8c explains the same responses with the previous result.
Practical Verification of Software-Predicted Result
Further, to support the optimized data as given by statistical modeling under optimized condition, the confirmatory experiments were conducted with the parameters as suggested by the model (incubation time, 70 h; temperature, 28.5 (°C); substrate: moisture ratio, 1:2; carbon concentration, 3.89 (%); nitrogen concentration, 0.99 (%) and phosphate concentration, 0.09 (%)). Under these conditions, confirmation experiments were conducted in five replicates. The optimized process condition yielded tannase production (6.15 U/g) that was closer to the predicted tannase production (6.20 U/g).
Discussion
From the SEM study, it is clear that a regular granular structure was found in the broken tamarind seed materials (Fig. 1a) and the same was fermented by P. purpurogenum PAF6. The seed kernel was extensively wrapped by the mycelium with explicating the sporal structure [Fig. 1b].
Production of tannase by P. purpurogenum PAF6 was determined in the presence of different available natural tannin-rich agricultural residues through SSF, and the best supporter of tannase induction and production was studied. Among the different substrates used, tamarind seed was found as the best substrate for maximum tannase production though its posses less tannin contents as compared to haritaki and pomegranate. It may be due to the presence of condensed tannin in haritaki and pomegranate which was difficult for the experimental organism to utilize [19].
Duration of cultivation has an immense value in the production of metabolites. P. purpurogenum PAF6 produced the maximum tannase after 48 h of cultivation. Thereafter, the enzyme production decreased. This is also due to accumulation of gallic acid, the major end product of tannic acid degradation. Both (residual tamarind seed tannin and gallic acid) lead to an acidic environment [31]. Decreased enzyme yield on prolonged incubation could also be due to inhibition and denaturation of the enzyme. Arulpandi et al. [13] reported tannase was formed during the primary phase of growth and, thereafter, the activity decreases either due to the decrease in production or due to enzyme degradation.
The incubation temperature of 30 °C was the maximum for tannase production. Above this, there was a decrease in enzyme production. With an increase in temperature, sporulation is induced, thereby, hampering mycelia growth. Supportively, there are many reports that described optimum temperature for tannase from 30 °C to 35 °C [2, 32].
The solid substrate and moisture ratio of 1:2 (w/v) was found to be the best for enzyme (1.836 U/g) production. Above or below this, the enzyme production was found to be decreased. As fermentation proceeds, heat is generated causing subsequent evaporation. Relative moisture helps to maintain the water content of the fermentation media. Intraparticular solute and moisture gradients developed during fermentation affects the physiology of microorganisms and overall process productivity. The higher production at lower moisture ratio might be due to low water activity as required by fungi. Further increase in humidity resulted in the decrease in enzyme activity, which may be due to low oxygen supply and osmotic imbalance inside the cell, resulting in cell lysis. Holker et al. [33] reported that filamentous fungi are known to grow at water-deficient substrates like bark of trees, dry leaves, etc.
Among the supplemented nitrogen source, urea was the most effective (2.88 U/g tannase). Elemental nitrogen is a very essential component for growth of the microbes and production of secondary metabolites. Different workers reported an optimal tannase production in the presence of different inorganic nitrogen sources in the medium [11, 24, 34]. Organic nitrogen sources showed relatively lower tannase activity because of tannic acid complexes with proteins and are able to inhibit the growth of microorganisms. Sherief et al. [23] reported a 2.52-fold increase in tannase production in the presence of peptone.
The supplementations of 4% (w/v) tannic acid with tamarind seed powder maximize tannase activity by about 1.5-fold higher than without tannic acid supplementation. Each and every microorganism requires a specific tannin concentration for maximum tannase production. In this study, the concentration (0.48 U/g) of phenolic compounds decreased as the fermentation time increased. This decrease was likely caused by the action of the enzyme on the tannic acid present in tamarind. So, the additional tannic acid is required for activity maximization. However, tannic acid at a higher concentration (>4%) produces a complex with membrane protein of the organism and inhibits growth and enzyme production. The actual mode of tannase induction in a particular concentration of tannin has not been properly explained until now. Srivastava and Kar [14], and Yee et al. [24] observed an improvement of tannase production when tannic acid was added to culture medium. Rodrigues et al. [11] reported that an addition of 2% (w/w) tannic acid in cashew apple bagasse maximizes tannase production.
It was observed that diammonium hydrogen orthophosphate with 0.1% concentration is found to be best for tannase production. Most of the microbial metabolism is regulated by the phosphorylation pattern of their respective proteins. So, phosphate is necessary in the culture medium. Yee et al. [24] reported the addition of phosphorus to the fermentation media (tannin-rich R. apiculata bark powder) promotes an increase in enzyme yield and productivity.
The addition of divalent cations had shown reduction in enzyme production. The ions like Hg+2 and Zn+2 had shown harm by reducing tannase production. Arulpandi et al. [13] have also reported the negative effect of metal salt in tamarind seed SSF in the production of tannase.
The inoculum level was also a determining factor for microbial fermentation process. A 20% (w/v) inoculum was found most suitable for the highest tannase production by P. purpurogenum PAF6. An increase in inoculum size (above 20%) resulted in the decline of enzyme production. The high inoculum size was related to the high biomass, which is responsible for the reduction in enzyme biosynthesis under nutrient-limited conditions in the fermentation masses [35]. Nutan et al. [26] reported a specific quantity of inoculum is very essential for fungal and bacterial enzyme production under SSF.
Enzyme extraction process from fermented solid substrate is an important step in downstream processing as it occupied a major cost of industrial enzyme production. The secreted enzyme is generally bound with fungal mycelia by means of different noncovalent bonds. Therefore, a suitable solvent system and an optimized condition are most favorable for enzyme extraction. Among the different solvents, distilled water extraction process was most suitable and effective. This also revealed indirectly that the secreted enzyme is highly polar and hydrophilic in nature. Ahmed et al. [36] also reported distilled water-mediated extraction from the fermented masses.
The volume of leaching solvent and the time of shaking are limiting factors to extract concentrate and adequate enzyme from the fermented solid mass. It was found that the fermented solid to solvent ratio of 1:6 was optimum for the extraction of tannase. In low volume of added solvent, less amount of enzyme was extracted and this might be due to the deficiency of the solvent to penetrate the fermented substrate. When the amount of solvent in many folds increased, the total volumetric activity of the enzyme remains constant.
The ANOVA for the quadratic model was highly significant with an F-value of 51.26 as shown by Fisher's F-test, along with a very low probability value (P model > F = 0.001), which was significant at 95% confidence interval. The lack of fit F-value of 42.29 implies the lack of fit is significant. The goodness of fit of the model was checked by the determination coefficient (R 2) that was indicating 98.15% of variability in the response could be accounted by the model (Table 4). The R 2 value is always closer to 1.0 and always lies between 0 and 1 resulting in a stronger model which better predicts the response [37]. The predicted R 2 of 90.38% is in reasonable agreement with the adjusted R 2 of 96.23%. Further, a high similarity was observed between the predicted and experimental results. Thus, it can be concluded that the model is statistically sound.
This study showed that tannase yield using OVAT optimization was 5.784 U/g that is 3.76-fold higher than the unoptimized condition. Using response surface methodology (Box–Behnken design), tannase production efficiency increased to 6.15 U/g that is 4.0 times higher than the unoptimized condition. Sharma et al. [38] and Naidu et al. [39] reported that the tannase production by Aspergillus niger and A. foetidus under submerged fermentation was elevated by 2.0-fold using RSM. Das Mohapatra et al. [40] and Saxena and Saxena [16] reported 2.18- and 2.4-fold increases in tannase production than unoptimized condition using Taguchi DOE methodology and RSM, respectively. On the contrary, Sivashanmugam and Jayaraman [41] reported 6.5 times higher tannase recovered from K. pneumoniae using Central composite and Box–Behnken design.
This is the first report of P. purpurogenum PAF6 in tannase production. The experimental organism produced a higher amount of tannase as compared to previously reported Penicillium sp. [42]. Whereas P. atramentosum [21] produced higher tannase than PAF6, it requires additional salt and maltose as secondary carbon source. The optimum fermentation time of 70 h is quite lower than A. aculeatus (96 h), A. awamori (120 h) and A. niger (144 h), which make it restricted for industrial-scale tannase production. The best nitrogen source (urea) is also more cost-effective than the other nitrogen sources like peptone and ammonium nitrate. In comparison to A. oryzae [11] and A. niger [24], P. purpurogenum PAF6 indicate the superiority using low amount tannin-rich plant residue with higher tannase activity.
Tamarind seed is successfully used as solid substrate for tannase production by P. purpurogenum PAF6; the process parameters lead to the proper utilization of agroindustrial residues into value-added products and minimize waste disposal-related problems where accumulation of agricultural residues is a serious problem. The experimental organism withstands high percentage of tannic acid toxicity and can be directly utilized for tannin removal from tannin-rich tannery effluent.
References
Das Mohapatra, P. K., Mondal, K. C., & Pati, B. R. (2007). Journal of Applied Microbiology, 102, 1462–1467.
Lekha, P. K., & Lonsane, B. K. (1997). Advances in Applied Microbiology, 44, 215–260.
Chandini, S. K., Jaganmohan, R. L., Gowthaman, M. K., Haware, D. J., & Subramanian, R. (2011). Food Chemistry, 127, 1039–1045.
Selwal, M. K., Yadav, A., Selwal, K. K., Aggarwal, N. K., Gupta, R., & Gautam, S. K. (2011). Brazilian Journal of Microbiology, 42, 374–387.
Aguilar, C. N., & Sanchez, G. (2001). Food Science and Technology International, 7, 373–382.
Lekha, P. K., & Lonsane, B. K. (1994). Process Biochemistry, 29, 497–503.
Archambault, J., Lacki, K., & Duvnjak, Z. (1996). Biotechnology Letters, 18, 771–774.
Muir, R. M., Ibanez, A. M., Uratsu, S. L., Ingham, E. S., Leslie, C. A., McGranahan, G. H., Batra, N., Goyal, S., Joseph, J., Jemmis, E. D., & Dandekar, A. M. (2011). Plant Molecular Biology. doi:10.1007/s11103-011-9739-3.
Das Mohapatra, P. K., Mondal, K. C., & Pati, B. R. (2006). Polish Journal of Microbiology, 55, 297–301.
Hadi, T. A., Banerjee, R., & Bhattacharya, B. C. (1994). Bioprocess Engineering, 11, 239–243.
Rodrigues, T. H. S., Pinto, G. A. S., & Gonçalves, L. R. B. (2008). Biotechnology and Bioprocess Engineering, 13, 571–576.
Lokeswari, N. (2010). Rasayan Journal of Chemistry, 3, 32–37.
Arulpandi, I., Sangeetha, R., & Kalaichelvan, P. T. (2010). Zaffius Biotechnology, 3, 1–7.
Srivastava, A., & Kar, R. (2009). Brazilian Journal of Microbiology, 40, 782–789.
Hota, S. K., Dutta, J. R., & Banerjee, R. (2007). Indian Journal of Biotechnology, 6, 200–204.
Saxena, S., & Saxena, R. K. (2004). Biotechnology and Applied Biochemistry, 39, 99–106.
Selwal, M. K., Yadav, A., Aggarwal, N. K., Kumar, K., & Kumar, A. (2008). World Journal of Microbiology and Biotechnology, 24, 3023–3030.
Mukherjee, G., & Banerjee, R. (2006). World Journal of Microbiology and Biotechnology, 22, 207–212.
Mahapatra, K., Nanda, R. K., Bag, S. S., Banerjee, R., Pandey, A., & Szakacs, G. (2005). Process Biochemistry, 40, 3251–3254.
Panda, B. P., Mazumdar, R., & Banerjee, R. (2009). Journal of Pharmacy and BioAllied Sciences, 1, 37–42.
Selwal, M. K., & Selwal, K. K. (2011). Annals of Microbiology. doi:10.1007/s13213-011-0238-1.
Lokeswari, N., & Reddy, D. S. R. (2010). Pharmacophore, 1, 112–122.
Sherief, A. A., EL-Tanash, A. B., & Nour, A. (2011). Biotechnology. doi:10.3923/biotech.2011.
Yee, T. W., Prabhu, N. G., Jain, K., & Ibrahim, D. (2011). African Journal of Biotechnology, 10, 13147–13154.
Ordonez, R. M., Colombo, I., Alberto, M. R., & Isla, M. I. (2011). World Journal of Microbiology and Biotechnology. doi:10.1007/s11274-011-0699-1.
Nutan, D., Ulka, S. P., Kulbhusan, B. B., Jayant, M. K., & Digamber, V. G. (2002). Process Biochemistry, 38, 715–721.
Box, G. E. P., Hunter, W. G., & Hunter, J. S. (1978). Statistics for experiments: An introduction to design, data analysis, and model building. New York: Wiley.
Haaland, P. D. (1989). Statistical problem solving in experimental design in biotechnology. New York: Marcel Dekker.
Schanderi, S. H. (1970). Methods in food analysis. New York: Academic Press.
Mondal, K. C., Banerjee, D., Jana, M., & Pati, B. R. (2001). Analytical Biochemistry, 295, 168–171.
Kar, B., Banerjee, R., & Bhattacharyya, B. C. (1999). Journal of Industrial Microbiology and Biotechnology, 23, 173–177.
Kumar, R. A., Gunasekaran, P., & Lakshmanan, M. (1999). Journal of Basic Microbiology, 39, 161–168.
Holker, U., Hofer, M., & Lenz, J. (2004). Applied Microbiology and Biotechnology, 64, 175–186.
Sabu, A., Pandey, A., Daud, M. J., & Szakacs, G. (2005). Bioresource Technology, 96, 1223–1228.
Raimbault, M., & Alazardd, D. (1980). European Journal of Applied Microbiology and Biotechnology, 9, 199–209.
Ahmed, W. B., Haq, I., & Iqbal, J. (2002). Biosynthesis of xylanase by UV-treated mutant strain of Aspergillus niger GCBMX-45. Biotechnology, 1, 10–14.
Rajasimman, M., & Subathra, S. (2010). International Journal of Chemistry and Biomolecular Engineering, 3, 1–7.
Sharma, S., Agarwal, L., & Saxena, R. K. (2007). Indian Journal of Microbiology, 47, 132–138.
Naidu, R. B., Saisubramanian, N., Sivasubramanian, S., Selvakumar, D., Janardhanan, S., & Puvanakrishnan, R. (2008). Current Trends in Biotechnology and Pharmacy, 2, 523–530.
Das Mohapatra, P. K., Maity, C., Rao, R. S., Pati, B. R., & Mondal, K. C. (2009). Food Research International, 42, 430–435.
Sivashanmugam, K., & Jayaraman, G. (2011). African Journal of Microbiology Research, 5, 3611–3615.
Batra, A., & Saxena, R. K. (2005). Process Biochemistry, 40, 1553–1557.
Acknowledgement
Financial support offered by the University Grant Commission, New Delhi, India is thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jana, A., Maity, C., Halder, S.K. et al. Tannase Production by Penicillium purpurogenum PAF6 in Solid State Fermentation of Tannin-Rich Plant Residues Following OVAT and RSM. Appl Biochem Biotechnol 167, 1254–1269 (2012). https://doi.org/10.1007/s12010-012-9547-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9547-5