Abstract
Tannase catalyzes the de-esterification of tannins into gallic acid and glucose. This enzyme has extensive value in removing tannins from tea, beer, and wine. For its benefit, innovative tannase-producing organisms continue to be reported in the literature. In this study, a novel tannase-producing fungal strain with a high tolerance to tannin was isolated from corn cobs and identified as Penicillium commune HS2. Four variables, i.e., initial pH, temperature, potato peel, and tannin concentrations, were evaluated to optimize their effects on tannase production. Using central composite design (CCD) of response surface methodology (RSM) for the optimization of tannase production on potato peels, a 4.62-fold upsurge was successfully achieved. The maximum productivity of 288.48 U from 1 g of dry potato peels was obtained under solid-state fermentation (SSF) at pH 5.0, 25.1 °C, in a medium containing 1.13% and 9.99% of potato peel and tannin, respectively. The purified enzyme had a molecular weight (Mw) of 35 kDa and showed maximal activity at 40–50 °C and a pH range of 4–5, as well as a half-life of 70 min at 40 °C. Using the tannic acid as a substrate, the enzyme had a Km value of 0.217 mM and Vmax of 8.08 U/ml/min. The purified enzyme successfully reduced 33.89% of total tannin content in lemon tea after 2 h at 45 °C. It can be concluded that Penicillium commune is a potentially high-tolerant tannin fungus that may be produced commercially on potato peel waste at a low cost and has promising applications in the food sector.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tannase (EC 3.1.1.20), tannin acyl hydrolase, is an inducible and extracellular glycoprotein esterase that catalyzes the de-esterification of tannins to gallic acid and glucose [1]. Tannase is used for various applications, including removing the tannins from instant tea, wine, beer, and coffee-flavored soft drinks, as well as producing gallic acid [2]. It is also employed for treating wastewater contaminated with polyphenolic compounds and as a clarifying agent to reduce haze and bitterness in the case of beer and fruit juices [3, 4].
Tannase can be acquired from various sources, including tannin-rich plants; however, microbial sources are favored for industrial production [5]. Most research work of tannin-degrading microbes has been focused on filamentous fungi of the Aspergillus and Penicillium genera. However, many bacterial species, such as Klebsiella pneumoniae, Staphylococcus lugdunensis, and Lactobacillus pentosus, have also been studied [3, 6, 7], and it has been recently identified in the yeast Rhodosporidium diobovatum [2].
Potatoes are grown and consumed all year round, and their production quantity increased according to the most recent Food and Agriculture Organization statistics, from 361 million tons in 2012 to 370 million tons in 2019 [8]. With the harvesting of these quantities every year, a major problem in managing potato peel waste has arisen. Such waste is worthless to the potato industry, accounting for 100 million tons per year, as it is reaching in average 27% of the potato weight [9, 10]. Owing to their moisture content, potato peel wastes are prone to quick microbial spoilage, and they are usually used in animal feeding [10]; hence, their valorization should be of interest to the food sector. Although whole potatoes are a relatively poor source of polyphenols, numerous studies have shown that these secondary metabolites are abundant in the peels of most cultivars. This is not surprising given their role in the tuber as an allelochemical to prevent fungi and other microbes from attacking it [11]. Potato peel is a respected source of phenolic acids such as chlorogenic (90% of the phenolic compounds), caffeic, gallic, and protocatechuic acid [12]. This nutrient-rich waste has received attention in enzyme production, as it was used as a substrate for α-amylase [13], co-production of amylase and protease [14], and thermostable laccase [15], but not tannase.
Traditional methods for optimizing cultural conditions by changing one factor at a time and keeping the other factors constant are time-consuming and costly. Additionally, these methods fail to provide information about the interaction between the various variables. Response surface methodology (RSM) has been applied to overcome these limitations. It has been widely used to optimize tannase production conditions as well as to evaluate the interactions between the nutritional and physiological dependent variables. It offers many advantages, such as estimating the appropriate pH, temperature, agitation speed, and tannic acid content, as well as their combination [5, 16, 17]. In all these studies, tannic acid concentration in the production medium ranged between 1 and 5%, and no strain exceeded the range of 8%, as tannic acid is a potent microbial inhibitor. This study aims to investigate the optimization of tannase production by central composite design (CCD) of response surface methodology using an isolating fungus that tolerates tannin under solid-state fermentation. Furthermore, the promising fungus tannase was purified, characterized, and tested to remove tannins from lemon tea.
2 Materials and methods
2.1 Chemicals and raw materials
Gallic acid, rhodanine, methanol, tannic acid, tannin, Sepharose 4-B gel, and Bradford reagent were purchased from Sigma Aldrich Co. (Germany). All the reagents and kits used in molecular identification and enzyme purification were of analytical grade and imported from Thermo Scientific (Lithuania). The potato peel (88.85% moisture content) was supplied by the International Food and Consumable Goods Company, 6 October, Egypt. It was dried for 20 h at 60 °C until the moisture content was less than 5%. Later, it was milled to ≤ 0.5 mm by the Ultra Centrifugal Mill ZM200 of Retch and stored at 4 °C until use.
2.2 Tannase activity determination
Tannase activity was determined by a modified version of Sharma procedure [18] by determining the produced gallic acid. In this experiment, 100 µl of enzyme extract and 500 µl of tannic acid, 0.3 mM, in sodium acetate buffer (10 mM, pH 5) were mixed. After 20 min at 30 °C, 1500 µl of methanolic rhodanine (0.667%) was added and left for 5 min before adding 500 µl KOH (0.5 M). To bring the volume up to 3 ml, sodium acetate buffer was used. After 5 min, the absorbance was measured by a spectrophotometer (Unico, UV-2000, USA) set to 520 nm. The standard curve was drawn using a freshly prepared gallic acid solution (5–50 nM) in the previous buffer. The amount of tannase that liberates 1 nM gallic acid in 1 min/g substrate (dw) or ml solution was defined as one unit (U).
2.3 Screening of the tannase-producing fungi
2.3.1 Isolation of the tannase-producing fungi
The main sources of isolated fungi were various wet moldy wastes (i.e., black tea leaves, corn cobs, and pomegranate peels) and distilled water containing 5% tannin. Using a sterile needle, the isolate was spread onto acidified potato dextrose agar medium. Only fungi were collected and transferred to tannase-producing (TP) medium (%): 1 yeast extract, 0.5 NaCl, 1 sucrose, and 0.5 tannin (filter sterilized by a 0.45-µm membrane filter, Millipore, Bedford, MA, USA) at pH 6.5 and incubated for 48 h at 30 °C [19]. After incubation, the diameter of the clear zone formed around the colonies was measured. Surviving fungi with a high hydrolysis zone were re-streaked on TP medium until a pure culture was obtained. All isolates were preserved on TP medium in slant tubes and reactivated monthly. The spore suspension of each isolate was prepared by pouring 5 ml of a solution (0.85% NaCl and 0.01% Tween 80 v/v) onto a slant full of spores (7-day-old) [20].
2.3.2 Screening of the highest tannase-producing fungus
All potential isolated tannase-producing fungi were screened according to tannase activity as follows. The 250-ml flasks containing 0.5 g (dw) of potato peels were autoclaved for 15 min at 121 °C. Fifty milliliters of a solution containing (%) 1 yeast extract, 0.5 NaCl, and 0.5 tannin were filter-sterilized and then added. The flasks were inoculated with 1 ml of spore suspension (30 × 107 CFU/ml) and incubated for 4 days (the stationary phase) at 30 °C under shaking (110 rpm). Finally, the enzyme-containing supernatant was filtered through Whatman No. 1 paper, and the activity was determined.
2.4 Molecular identification of the highest tannase-producing isolate (T-5)
2.4.1 DNA extraction and isolation
Genomic DNA was isolated from a 3-day old isolate (T-5) using the Gene JET genomic DNA purification kit following the manufacturer’s protocol. Briefly, the cells were first digested by proteinase K. After incubation at 56 °C for 45 min, the RNase A solution was added and further incubated for 10 min at room temperature. After obtaining a homogeneous mixture, ethanol (50%) was added and vortexed. The prepared lysate was transferred to a Gene JET Genomic DNA Purification Column to elute genomic DNA, and then the isolation procedure was completed according to the producer’s protocol [21]. The Nanodrop spectrophotometer and agarose gel electrophoresis were used to check DNA yields and purity.
2.4.2 Molecular identification of the isolate T-5
ITS gene sequencing was applied to identify the isolate T-5 at the molecular level. The universal primer pair ITS-1 (5′-CTT GGT CAT TTA GAG GAA GTA GA-3′) and ITS-4 (5′-TCC TCC GCT TGA TAT GC-3′) was used to perform ITS-PCR on pure DNA. The amplification step was executed using a thermal cycler PCR (Bio-Rad T100, USA). The PCR yields were verified via agarose gel electrophoresis, purified using a gel extraction kit, and sequenced by Macrogen (Republic of Korea). Phylogenetic analysis was completed by the neighbor-joining approach with 1000 bootstrap resamplings using the MEGA 6 program [22].
2.5 Optimization of tannase production under solid-state fermentation using RSM
The effect of four parameters, namely initial pH, temperature, potato peel, and tannin concentration (%, w/v), was statistically screened and optimized for their interaction impact by the central composite design (CCD) of the response surface methodology [23] by Design-Expert V7 software. Each independent factor was evaluated at four different levels: − α, − 1, + 1, and + α (Table 1). Based on 30 experimental runs, tannase activity was monitored as a response.
2.6 Solid-state fermentation
Tannase of the fungal strain Penicillium commune HS2 was produced under solid-state fermentation as follows. In a 250-ml Erlenmeyer flask, a defined mass of potato peel (5 g), as a support material, was moistened with the same volume of a solution (filter sterilized, 0.45-µm filter membrane) containing 0.5% NaCl, 1% yeast extract, and different pure tannin concentrations. Each flask was inoculated with 1 ml of the spore inoculum (30 × 107 CFU/ml), mixed thoroughly, and incubated for 4 days at the specified temperature [24]. Tannase was extracted from the culture medium by adding 20 ml of distilled water and then determined.
2.7 Tannase purification and molecular weight determination
At the end of the optimized cultivation period (4 days), the crude enzyme of Penicillium commune HS2 was extracted with sodium acetate buffer (10 mM, pH 5) and then purified in a two-step procedure using ammonium sulfate and gel filtration chromatography. Firstly, it was concentrated by ammonium sulfate precipitation (40–60% saturation) method. In the sodium acetate buffer (pH 5.0), the precipitate was dissolved and dialyzed for 24 h at 4 °C with continuous stirring [25]. Tannase was further purified by the Sepharose 4-B column in the following manner: the Sepharose 4-B gel was washed with the same buffer before being poured into a column (1 × 32 cm) and then equilibrated with the same buffer. The crude enzyme (0.5 ml) was then loaded and eluted at 4 °C with the same buffer at a flow rate of 0.15 ml/min. Fractions of 2 ml were collected, and both tannase activity and protein content at 280 nm were determined offline [26]. The Bradford method was applied for protein quantification, and bovine serum albumin was used as a standard [27]. The enzyme’s purity and molecular weight were assessed using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis [28].
2.8 Characterization of the purified tannase
2.8.1 Effect of temperature and pH on tannase activity and stability
The effect of temperature on tannase activity was analyzed at various temperatures (10, 20, 30, 40, 50, 60, 70, 80, and 90 °C). The reaction mixture was incubated for 20 min at the above-mentioned temperatures, and then enzyme activity was measured under standard assay conditions (0.3 mM tannic acid and pH 5.0). The pH effect on tannase activity was assayed by performing the reaction at different pH values (3.0–10.0) at 30 °C for 20 min. Buffer systems such as acetate buffer (pH 3.0–5.0), phosphate buffer (pH 6.0–7.0), Tris–HCl (pH 8.0–9.0), and carbonate (pH 10.0) at 10 mM concentration were used. To assess tannase stability, the pure enzyme was incubated at different temperatures (40, 50, and 60 °C) in sodium acetate buffer, pH 5.0, for 2 h. In terms of pH stability, it was assessed by incubating the purified enzyme at various pH values ranging from 3.0 to 10.0 for 24 h at 4 °C [29].
2.8.2 Effect of additives
The metal ions’ effect on tannase activity was analyzed by incubating purified P. commune tannase with metal ions such as K+, Na+, Cu2+, Ca2+, Ni2+, Mn2+, Al3+, Ba2+, and Mg2+ for 5 min at room temperature at two concentrations (1 and 10 mM). On the other side, the effect of different additives was studied by incubating the enzyme with ethylene diamine tetraacetic acid (EDTA) and many surfactants (i.e., sodium dodecyl sulfate (SDS), Tween 80, β-mercaptoethanol, and Triton-X 100) at 1% for 5 min at room temperature. The residual tannase activity was determined under the optimum conditions (45 °C and pH 5.0). The pure enzyme in the absence of any additive (control) was used to calculate the relative activity [6].
2.8.3 Determination of kinetic constants
The Km and Vmax values of P. commune tannase were determined by various concentrations of tannic acid from 0.1 to 0.8 mM. The assay conditions were similar to the standard assay conditions. The data was fitted, and the constants were calculated from the Lineweaver–Burk plot [17].
2.9 Application of the purified enzyme
The purified enzyme was applied to remove tannins from lemon black tea. Firstly, tea extract was prepared by boiling 1 g of tea powder with 90 ml of distilled water for 5 min then filtered [30]. The pure extract was cooled, and the volume was brought to 100 ml with 10 ml of freshly squeezed lemon juice. Purified P. commune tannase (53.3 U/ml) was added and incubated for 2 h at 45 °C. The tannin content in the samples was determined by the protein precipitation method as described by de Lima [30] where tannic acid was applied to draw the standard curve, whereas gallic acid content was determined by the rhodanine method as mentioned above.
2.10 Statistical analysis
The experimental design to maximize tannase production was created and statistically analyzed by Design-Expert V7 software (Stat-Ease, Minneapolis, MN, USA). CoStat statistical software was used to compare the tannase activity in all experiments using analysis of variance, ANOVA one way. The mean of three replicates was compared using Duncan’s test [31] at a significant level of p ≤ 0.05.
3 Results and discussion
3.1 Isolation and screening of tannase-producing isolates
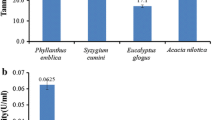
Eight colonies competent for sustainable growth on tannin-containing medium were successfully isolated in this investigation, and the morphology of each one is presented in Table 2. For further analysis, all pure strains were screened for the highest tannase-producing fungus by measuring the clear zone and their tannase activity (Fig. 1). There are significant differences (p ≤ 0.05) in tannase production among the isolated fungi. Among these eight potential isolates, isolate T-5 exhibited significantly (p ≤ 0.05) the maximum clear zone (8 mm) and tannase activity (64.31 U/g/min) and was chosen for further study.
3.2 Molecular identification of fungal isolate T-5
ITS gene sequence analysis by BLASTN of the fungal isolate T-5 showed that it belongs to the Penicillium genus with a high similarity percentage of more than 99%. The ITS sequence was identified as Penicillium commune strain HS2 and deposited in the Gene Bank under the Accession No. of MT084577. The bootstrap value shows similarity to Penicillium expansum strain DUCC5734 and Penicillium commune SW23 isolates with a 95% bootstrap value (Fig. 2).
3.3 Optimization of tannase production using central composite design (CCD)
Temperature, pH, and tannic acid have been identified as the most significant variables in the production of tannase [3]. Therefore, four independent factors (i.e., initial pH, temperature, potato peel, and tannin concentrations (w/v)) were selected to find the optimum conditions for tannase production by CCD of RSM analysis. A total of 30 experiment runs were conducted with various combinations of these variables, where a quadratic model was suggested (Table 3). The following quadratic equation was derived from the multiple regression analysis of the observed response:
where A, B, C, and D are the coded values of pH, temperature, potato peel, and tannin concentrations, respectively.
According to the ANOVA table (Table 4), the F-value of 26.78 for the model suggests that it is significant. Due to noise, there is only a 0.01% chance that a “Model F-value” this large will occur. The determination coefficient (R2) was used to verify the model’s best fit. Here, R2 = 0.9615 implies that 96.15 of the total variation in the tannase yield is attributed to the independent variables. The predicted R2 of 0.7784 agrees with the adjusted R2 of 0.9256 sensibly. That indicates the reliability of the experiment to predict precise conditions, implying that the model’s accuracy is greater than 95%. Values of Prob > F less than 0.0500 indicate that model terms are significant. In this design, the four variables (A, B, C, and D) and the quadratic AD, BC, BD, as well as the squared term B2 of the model were significant, suggesting that tannase production strongly depends on the interactions between those factors. Adequate precision also evaluates the signal-to-noise ratio. A ratio of more than 4 is anticipated. As the ratio is 19.512, which indicates that this model could be used to navigate the design.
The 3D response plot for the final tannase activity (U/g/min) represents the interaction of two parameters at a time when the other parameters are held at zero level (Fig. 3 A–F). Figure 3A demonstrated a decreasing pattern in tannase production as the temperature and pH parameters were increased. This effect is due to thermal denaturation of tannase at high temperatures and basic pH, protonation, or deprotonation of its amino acids and active sites, as well as conformational changes caused by amino acid ionization [3]. Figure 3 B, C, and D show an escalating pattern, where tannase activity positively increased when potato peel and tannin concentrations were increased. This may be attributed to the fact that most tannase-producing microbes require tannic acid as an inducer. Alternatively, although potato peels are rich in chlorogenic acid, which is resistant to tannase, they also contain gallic acid and caffeic acid, which may enhance tannase production [32].
3.4 Numerical optimization
A series of validation experiments were conducted for verification, based on the conditions provided by CCD. The numerical optimization was calculated, and the best conditions to maximize tannase production were chosen under the following criteria: minimum pH, temperature, potato peel, and maximum tannin concentrations (Table 5). The optimized critical culture components derived from the analysis were directly applied in solid-state fermentation. Based on the best solution, the highest tannase activity (288.48 U/g/min) was obtained with an initial pH of 5.00, a temperature of 25.10 °C, a potato peel content of 1.13% (w/v), and a tannin concentration of 9.99% (w/v). The CCD design applied in this study resulted in a 4.62-fold increase in tannase production as it enhanced enzyme titers from 62.44 (under un-optimized conditions) up to 288.48 U/g dry potato peels. Alternatively, the RSM-mediated statistical approach in the study of Lekshmi [33] showed a nine-fold higher tannase activity than the un-optimized medium for Bacillus velezensis TA3 grown in SSF with pomegranate peels as a substrate. In the presence of 1.546% tannic acid, Thiyonila and coauthors [34] obtained a 4.46 fold of tannase from Serratia marcescens IMBL5 higher than their minimal medium by CCD.
Because of the low energy requirements, the low incubation temperature for P. commune HS2 could be a technical advantage for industrial tannase production. Additionally, a low initial pH value represents a practical benefit because it eliminates the need to adjust the pH value with alkalis, thereby reducing the preparation time and the possibility of contamination. The isolated fungus in this study has the most important advantage of being tannin tolerant, which could be used to remove tannins from highly tannin-contaminated wastewater, especially in acidic conditions. A similar acidic pH has also been reported for RSM-mediated tannase production by Aspergillus awamori and bacteria Klebsiella pneumoniae and Bacillus cereus M1GT [1, 3, 35] (Table 6). However, RSM recognized the optimum concentration of tannic acid at 3.5% in the culture medium for the maximum tannase production by the fungus Penicillium montanense [36]. Meanwhile, the highest accepted tannin concentration recorded to date was 7.49%, as RSM indicated for maximum Aspergillus tubingensis tannase production (245 U/g dry tea stalk) [16], which is lower than that tolerated by our strain.
To date, no study has optimized tannase production using potato peel as the sole carbon source. The studies focused on fungal tannase production via SSF have used agro-industrial wastes as substrates, such as tea stalks, pomegranate peels, and black plum leaves (Table 6). Regarding their tannase yield under the optimized conditions, it was lower than that obtained in our study. That indicates the remarkable application potential of potato peel and this enzyme for commercial production.
3.5 Purification and molecular weight identification of P. commune HS2 tannase
The extracellular tannase of Penicillium commune HS2 was purified by two steps of purification (Table 7), and only a single band on SDS-PAGE was visualized (Fig. 4 A and B). It was obtained with a high recovery yield of 73.45%. The chromatogram of Sepharose 4-B gel filtration confirmed four protein peaks and one tannase peak from 32 fractions collected. A single band with a molecular weight of 35 kDa was detected on SDS-PAGE, making it the smallest known active tannase to date (Table 8). It is close to the Mw of Serratia marcescens IMBL5 and Herbaspirillum camelliae tannases, which were resolved at 39 and 40 kDa, respectively [34, 38].
3.6 Characterization of the purified enzyme
3.6.1 Effect of temperature and pH on tannase activity
The purified tannase exhibited the highest activity at 40 and 50 °C and pH range of 4.0 and 5.0, with no significant difference (Fig. 5 A and B). Above 60 °C and pH 5.0, tannase showed lower activities, whereas at ≥ 80 °C or in the carbonate buffer system, it lost its activity. Our results are comparable to those of the commercial tannase (Table 8), which has a temperature optimum of 30 °C. The optimum temperature of P. commune tannase is also higher than that reported for fungal tannases (33–40 °C) and near the optimum temperature of bacterial strains (20–50 °C). Furthermore, most fungal tannases were most active at pH values ranging from 4.1 to 6.0, whereas bacterial enzymes were most active at alkali pH values of 6–9 (Table 8).
The stability of tannase is one of the important factors that demonstrate the cost-effective possibility of preventing them from degrading in tannery effluent and maintaining their action under harsh conditions and throughout industrial operations. The results (Fig. 5 C and D) showed that the pure tannase is slightly stable. The enzyme retained 70% of its activity after 1 h at 40 °C and 34.5% at 50 °C. At 60 °C, it lost 95% of its activity after 40 min. The half-life at 40 °C was 70 min, whereas a value of 40 min was recorded at 50 °C. Enzymes with high thermal stability are particularly important for biotechnological applications, and this enzyme may be considered a promising option. Compared with the commercial tannase of Aspergillus ficuum, it maintained 75% at 40 °C and 60% at 50 °C after 1 h [30].
Like the thermal stability, P. commune tannase was stable at pH values close to the optima (4.0 and 5.0). On the other side, it maintained at least 55% of its initial activity for 24 h in buffers with pH 3.0 and 6.0 values. Similarly, Penicillium rolfsii tannase was stable at the optimum pH 4.0 for 6 h [39]. Although P. commune tannase showed the same optimum pH range as the fungal tannases, it was more stable since it maintained 100% of its initial activity for 24 h.
3.6.2 Effect of additives on tannase activity
Since several enzymes need metal ions as cofactors, the impact of different metal ions on tannase activity was tested (Table 9). All the cations selected for this study, except Na+, have negatively affected the activity, particularly Cu2+ and Al2+. Higher levels of inhibition were observed in the presence of high ion concentrations. This might be attributed to an increase in the ionic strength of the solution [6]. Tannase retained 68.36 to 75.68% of its initial activity in the presence of K+, Ca2+, Ba2+, Mn2+, and Mg2+, with no significant differences (p ≤ 0.05). Furthermore, tannase activity was enhanced by Na+ ion addition by 9.44% and 14.02% at 1 mM and 10 mM, respectively. A reduction in the tannase of Enterobacter cloacae and Penicillium rolfsii CCMB 714 in the presence of Cu2+, Ba2+, Mn2+, and Mg2+ at 1 mM was also reported [41]. On the other side, yeast tannase of Sporidiobolus ruineniae A45.2 was not affected by Na+ and K+ [17].
The tested detergents and the chelator, EDTA, listed in Table 9 had different significant effects (p ≤ 0.05) on the purified tannase. Among the detergents, only Tween 80 at a concentration of 1% completely inhibited tannase. It lost 90.84% of its activity in the existence of β-mercaptoethanol, which also inhibited many tannases [6, 38]. SDS and Triton-X 100 decreased the activity by 59.23% and 64.6%, respectively. These findings suggest that the surfactants may interfere with the hydrophobic interaction of the protein, leading to partial activity loss of the obtained enzyme [41]. The activity of an enzyme that requires metal ions as cofactors drastically decreases in the presence of EDTA. Here, P. commune tannase is inhibited by this chelator, suggesting that this enzyme needs metal ions as cofactors. A previous study showed that tannase was inactivated by EDTA [41], whereas Tween 80 inhibited 19.5 and 24% after 5 min and 1 h of incubation, respectively.
3.6.3 Kinetic constants of the purified tannase
The Km value of the purified tannase was 0.217 mM tannic acid, whereas Vmax was 8.08 U/ml as calculated from the Lineweaver–Burk plot (Fig. 5E). It indicates that this enzyme exhibited a higher affinity towards tannic acid than that of Penicillium notatum [44] (3.13 mM) and Enterobacter cloacae [41] (3 mM). Furthermore, many fungal strains reported higher Km values for methyl gallate [38, 42]. The Vmax of the pure investigating enzyme is two-fold the velocity recorded by Govindarajan [41] of Enterobacter cloacae tannase in the presence of tannic acid (4.4 U/ml), suggesting that P. commune tannase could be a novel and promising fungal member of the tannase family.
3.7 Tannins removal from lemon black tea
The purified tannase obtained in this study was applied to reduce the tannin content of lemon tea. Among the tea types, lemon tea is used in medicine for colds, influenza, diarrhea, and infection treatments [45]. Tea products with high tannin content, on the other hand, can result in an unmarketable turbid product with an astringent flavor. As a result, tannin removal is desired not only to reduce the astringent taste of tea but also to improve its clarity and its medicinal benefits [30].
Figure 6 illustrates the tannin and gallic acid content in lemon black tea before and after tannase treatment. Tannin content was reduced in the final product by 33.89%, whereas gallic acid increased by 69.42% by the action of tannase. This rise in gallic acid concentration is predictable as it is the final product of hydrolyzable tannin hydrolysis, consistent with early studies [46]. De Lima [30] successfully removed 22% of tannins from boldo tea in 2 h at 40 °C using 170 U/ml of free Aspergillus ficuum tannase (the commercial enzyme). Aharwar and Parihar [25], on the other hand, used immobilized Talaromyces verruculosus tannase at 60 °C to achieve the highest tannin reduction percentage (78.02%) in black tea.
4 Conclusions
The production efficiency of tannase and gallic acid is limited by the microbial inhibition caused by high concentrations of tannic acid, a potent microbial inhibitor. The reliability of statistical optimization of external factors in increasing the tannase production by Penicillium commune HS2 was demonstrated in the current work. For maximum tannase production by this strain, high tannin content and acidic pH were evidenced. Furthermore, the ability of this fungus to utilize potato peel waste makes this isolate an excellent choice for low-cost commercial tannase production. Taking into consideration the characteristics of the purified enzyme, e.g., thermal and pH stability, it was applied to reduce tannins and their astringency in one of the tea products, lemon tea. Further research into the characteristics of its immobilized form, as well as finding new applications in the food sector, is encouraged.
Data availability
Data can be obtained from the corresponding author.
Change history
12 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s13399-022-02451-y
References
Saeed S, Aslam S, Mehmood T et al (2021) Production of gallic acid under solid-state fermentation by utilizing waste from food processing industries. Waste Biomass Valorization 12:155–163. https://doi.org/10.1007/s12649-020-00980-z
Pan J, Wang NN, Yin XJ et al (2020) Characterization of a robust and pH-stable tannase from mangrove-derived yeast Rhodosporidium diobovatum Q95. Mar Drugs 18:546. https://doi.org/10.3390/md18110546
Kumar M, Rana S, Beniwal V, Salar RK (2015) Optimization of tannase production by a novel Klebsiella pneumoniae KP715242 using central composite design. Biotechnol Rep 7:128–134. https://doi.org/10.1016/j.btre.2015.06.002
de Lima JS, Cabrera MP, de Souza Motta CM et al (2018) Hydrolysis of tannins by tannase immobilized onto magnetic diatomaceous earth nanoparticles coated with polyaniline. Food Res Int 107:470–476. https://doi.org/10.1016/j.foodres.2018.02.066
Kumar M, Singh A, Beniwal V, Salar RK (2016) Improved production of tannase by Klebsiella pneumoniae using Indian gooseberry leaves under submerged fermentation using Taguchi approach. AMB Express 6:46. https://doi.org/10.1186/s13568-016-0217-9
Chaitanyakumar A, Anbalagan M (2016) Expression, purification and immobilization of tannase from Staphylococcus lugdunensis MTCC 3614. AMB Express 6:89. https://doi.org/10.1186/s13568-016-0261-5
Kanpiengjai A, Unban K, Nguyen T-H et al (2019) Expression and biochemical characterization of a new alkaline tannase from Lactobacillus pentosus. Protein Expr Purif 157:36–41. https://doi.org/10.1016/j.pep.2019.01.005
Food and Agriculture Organization (FAO) (2019). https://www.fao.org/statistics/en/
Fritsch C, Staebler A, Happel A et al (2017) Processing, valorization and application of bio-waste derived compounds from potato, tomato, olive and cereals: a review. Sustainability 9:1492. https://doi.org/10.3390/su9081492
Galhano dos Santos R, Ventura P, Bordado JC, Mateus MM (2016) Valorizing potato peel waste: an overview of the latest publications. Rev Env Sci Biotechnol 15:585–592. https://doi.org/10.1007/s11157-016-9409-7
Venturi F, Bartolini S, Sanmartin C et al (2019) Potato peels as a source of novel green extracts suitable as antioxidant additives for fresh-cut fruits. Appl Sci 9:2431. https://doi.org/10.3390/app9122431
Singh B, Singh J, Singh JP et al (2020) Phenolic compounds in potato (Solanum tuberosum L.) peel and their health-promoting activities. Int J Food Sci Technol 55:2273–2281
Mushtaq Q, Irfan M, Tabssum F, Iqbal Qazi J (2017) Potato peels: a potential food waste for amylase production. J Food Proc Eng 40:12512. https://doi.org/10.1111/jfpe.12512
Tuysuz E, Gonul-Baltaci N, Omeroglu MA et al (2020) Co-production of amylase and protease by locally isolated thermophilic bacterium Anoxybacillus rupiensis T2 in sterile and non-sterile media using waste potato peels as substrate. Waste Biomass Valorization 11:6793–6802. https://doi.org/10.1007/s12649-020-00936-3
Kumar A, Singh A, Bilal M, Chandra R (2021) Sustainable production of thermostable laccase from agro-residues waste by Bacillus aquimaris AKRC02. In press, Cat Lett. https://doi.org/10.1007/s10562-021-03753-y
Wu C, Zhang F, Li L et al (2018) Novel optimization strategy for tannase production through a modified solid-state fermentation system. Biotechnol Biofuels 11:92. https://doi.org/10.1186/s13068-018-1093-0
Kanpiengjai A, Khanongnuch C, Lumyong S et al (2020) Co-production of gallic acid and a novel cell-associated tannase by a pigment-producing yeast Sporidiobolus ruineniae A45.2. Microb Cell Fact 19:95. https://doi.org/10.1186/s12934-020-01353-w
Sharma S, Bhat TK, Dawra RK (2000) A spectrophotometric method for assay of tannase using Rhodanine. Anal Biochem 279:85–89. https://doi.org/10.1006/abio.1999.4405
Liu TPSL, Brandão Costa RMP, de Vasconcelos Freitas DJ et al (2017) Tannase from Aspergillus melleus improves the antioxidant activity of green tea: purification and biochemical characterisation. Int J Food Sci Technol 52:652–661. https://doi.org/10.1111/ijfs.13318
Mansor A, Ramli MS, Abdul Rashid NY et al (2019) Evaluation of selected agri-industrial residues as potential substrates for enhanced tannase production via solid-state fermentation. Biocatal Agric Biotechnol 20:101216. https://doi.org/10.1016/j.bcab.2019.101216
Kumar M, Mugunthan M (2018) Evaluation of three DNA extraction methods from fungal cultures. Med J Armed Forces India 74:333–336. https://doi.org/10.1016/j.mjafi.2017.07.009
Oduro-Mensah D, Ocloo A, Lowor ST et al (2018) Isolation and characterisation of theobromine-degrading filamentous fungi. Microbiol Res 206:16–24. https://doi.org/10.1016/j.micres.2017.09.006
Gafar AA, Khayat ME, Ahmad SA et al (2020) Response surface methodology for the optimization of keratinase production in culture medium containing feathers by Bacillus sp UPM-AAG1. Catalysts 10:848. https://doi.org/10.3390/catal10080848
Xiao A, Huang Y, Ni H et al (2015) Statistical optimization for tannase production by Aspergillus tubingensis in solid-state fermentation using tea stalks. Electron J Biotechnol 18:143–147. https://doi.org/10.1016/j.ejbt.2015.02.001
Aharwar A, Parihar DK (2021) Talaromyces verruculosus tannase immobilization, characterization, and application in tea infusion treatment. Biomass Conv Bioref 1:12. https://doi.org/10.1007/s13399-020-01162-6
Al-Mraai STY, Al-Fekaiki DF, Al-Manhel AJA (2019) Purification and characterization of tannase from the local isolate of Aspergillus niger. J Appl Biol Biotechnol 7:29–34. https://doi.org/10.7324/JABB.2019.70106
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Hu G, Heitmann JA, Rojas OJ et al (2010) Monitoring cellulase protein adsorption and recovery using SDS-PAGE. Ind Eng Chem Res 49:8333–8338. https://doi.org/10.1021/ie100731b
Shao Y, Zhang Y-H, Zhang F et al (2020) Thermostable tannase from Aspergillus niger and its application in the enzymatic extraction of green tea. Molecules 25:952. https://doi.org/10.3390/molecules25040952
de Lima JS, Cabrera MP, Casazza AA et al (2018) Immobilization of Aspergillus ficuum tannase in calcium alginate beads and its application in the treatment of boldo (Peumus boldus) tea. Int J Biol Macromol 118:1989–1994. https://doi.org/10.1016/j.ijbiomac.2018.07.084
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42. https://doi.org/10.2307/3001478
Lekha PK, Lonsane BK (1997) Production and application of tannin acyl hydrolase: state of the art. In Advances in Applied Microbiology 44:215–260. https://doi.org/10.1016/S0065-2164(08)70463-5
Lekshmi R, Arif Nisha S, Kaleeswaran B, Alfarhan AH (2020) Pomegranate peel is a low-cost substrate for the production of tannase by Bacillus velezensis TA3 under solid state fermentation. J King Saud Univ Sci 32:1831–1837. https://doi.org/10.1016/j.jksus.2020.01.022
Thiyonila B, Kannan M, Paulin Reneeta N et al (2020) Influence of tannase from Serratia marcescens strain IMBL5 on enhancing antioxidant properties of green tea. Biocatal Agric Biotechnol 27:101675. https://doi.org/10.1016/j.bcab.2020.101675
Selvaraj S, Natarajan K, Nowak A, Murty VR (2021) Mathematical modeling and simulation of newly isolated bacillus cereus M1GT for tannase production through semi-solid state fermentation with agriculture residue triphala. South Afric J Chem Eng 35:89–97. https://doi.org/10.1016/j.sajce.2020.10.001
Lima JS de, Cruz R, Fonseca JC, et al (2014) Production, characterization of tannase from Penicillium montanense URM 6286 under SSF using agroindustrial wastes, and application in the clarification of grape juice (Vitis vinifera L.). Sci World J 182025. https://doi.org/10.1155/2014/182025
Saeed S, Bibi I, Mehmood T et al (2020) Valorization of locally available waste plant leaves for production of tannase and gallic acid by solid-state fermentation. In press, Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00989-3
Lei J, Zhang Y, Ni X et al (2021) Degradation of epigallocatechin and epicatechin gallates by a novel tannase TanHcw from Herbaspirillum camelliae. Microb Cell Fact 20:197. https://doi.org/10.1186/s12934-021-01685-1
Andrade PML, Baptista L, Bezerra CO et al (2021) Immobilization and characterization of tannase from Penicillium rolfsii CCMB 714 and its efficiency in apple juice clarification. J Food Measurement Characterization 15:1005–1013. https://doi.org/10.1007/s11694-020-00705-9
Mahmoud AE, Fathy SA, Rashad MM et al (2018) Purification and characterization of a novel tannase produced by Kluyveromyces marxianus using olive pomace as solid support, and its promising role in gallic acid production. Int J Biol Macromol 107:2342–2350. https://doi.org/10.1016/j.ijbiomac.2017.10.117
Govindarajan RK, Krishnamurthy M, Neelamegam R et al (2019) Purification, structural characterization and biotechnological potential of tannase enzyme produced by Enterobacter cloacae strain 41. Process Biochem 77:37–47. https://doi.org/10.1016/j.procbio.2018.10.013
Koseki T, Ichikawa K, Sasaki K, Shiono Y (2018) Characterization of a novel Aspergillus oryzae tannase expressed in Pichia pastoris. J Biosci Bioeng 126:553–558. https://doi.org/10.1016/j.jbiosc.2018.05.010
Tomás-Cortázar J, Plaza-Vinuesa L, de las Rivas B et al (2018) Identification of a highly active tannase enzyme from the oral pathogen Fusobacterium nucleatum subsp polymorphum. Microb Cell Fact 17:33. https://doi.org/10.1186/s12934-018-0880-4
Abdel-Naby MA, El-Tanash AB, Sherief ADA (2016) Structural characterization, catalytic, kinetic and thermodynamic properties of Aspergillus oryzae tannase. Int J Biol Macromol 92:803–811. https://doi.org/10.1016/j.ijbiomac.2016.06.098
Gorgulu TY, Ozdemir OD, Kipcak AS et al (2016) The effect of lemon on the essential element concentrations of herbal and fruit teas. Appl Biol Chem 59:425–431. https://doi.org/10.1007/s13765-016-0161-z
Li J, Xiao Q, Huang Y et al (2017) Tannase application in secondary enzymatic processing of inferior Tieguanyin oolong tea. Elect J Biotechnol 28:87–94. https://doi.org/10.1016/j.ejbt.2017.05.010
Acknowledgements
The author thanks Dr. Heba Mohamad for the assistance in phylogenetic tree drawing.
Author information
Authors and Affiliations
Contributions
Investigation, visualization, and writing—review and editing: HSM.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: During the production process, the arrangement of Table 3 columns are wrong.
Rights and permissions
About this article
Cite this article
Mostafa, H.S. Potato peels for tannase production from Penicillium commune HS2, a high tannin-tolerant strain, and its optimization using response surface methodology. Biomass Conv. Bioref. 13, 16765–16778 (2023). https://doi.org/10.1007/s13399-021-02205-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02205-2